Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Effectiveness of Hemoglobin Glutathionylation during Incubation with Oxidized Glutathione (GSSG)
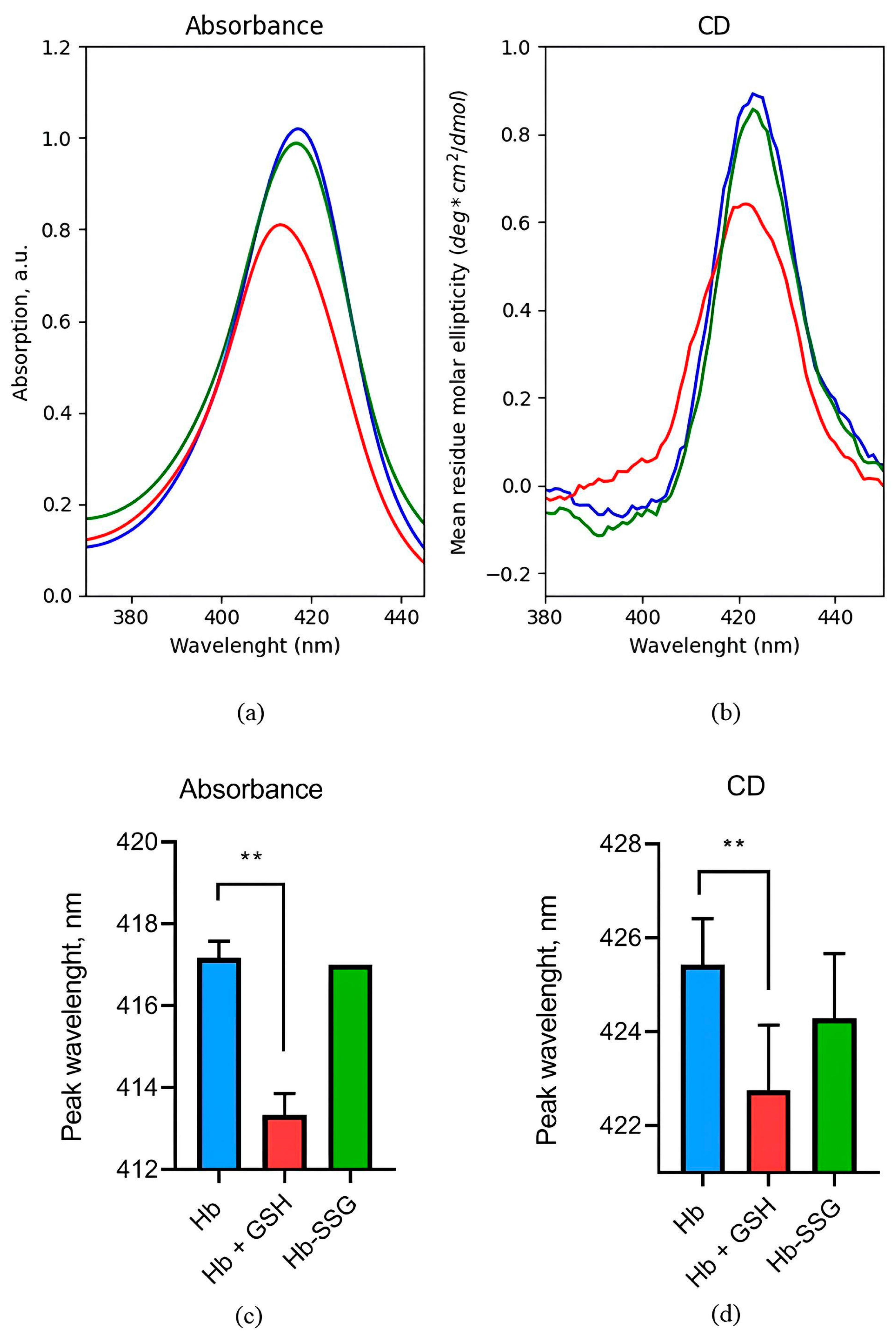
2.2. Evaluation of Changes in the Circular Dichroism Spectrum in the Region of the Soret Band during the Glutathionylation of Hb and the Formation of a NonCovalent Complex of Hb with GSH
2.3. Evaluation of Secondary Structure Changes during Hemoglobin Glutathionylation and the Formation of Noncovalent Complex Hemoglobin with GSH
2.4. Evaluation of Changes in Tryptophan Fluorescence Spectra during Hb Glutathionylation and the Formation of a Complex Hemoglobin with GSH
2.5. Modeling of Tryptophan Availability
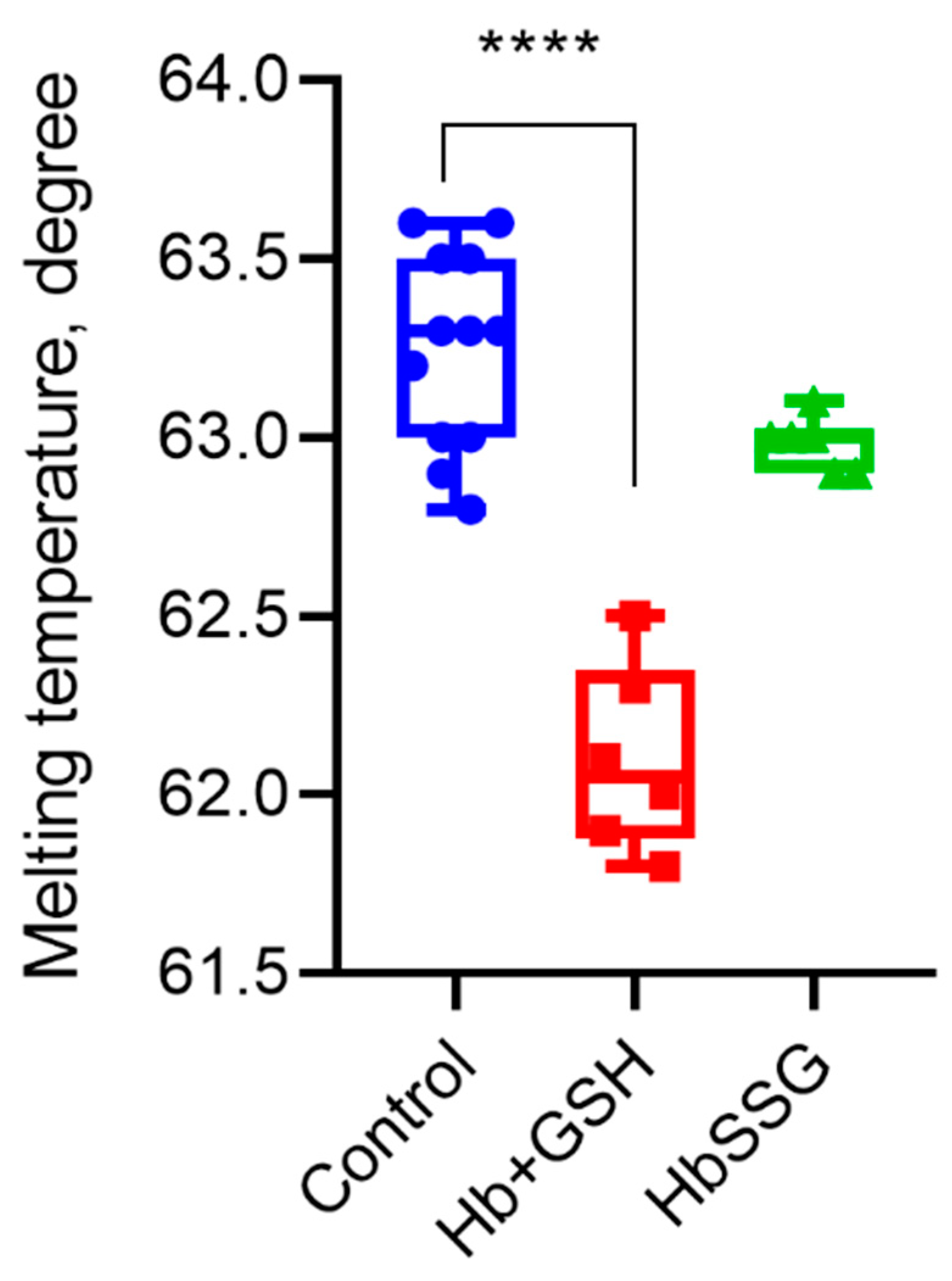
2.6. Effect of Hb Glutathionylation and Formation of Hb Complex with GSH on Protein Thermal Stability
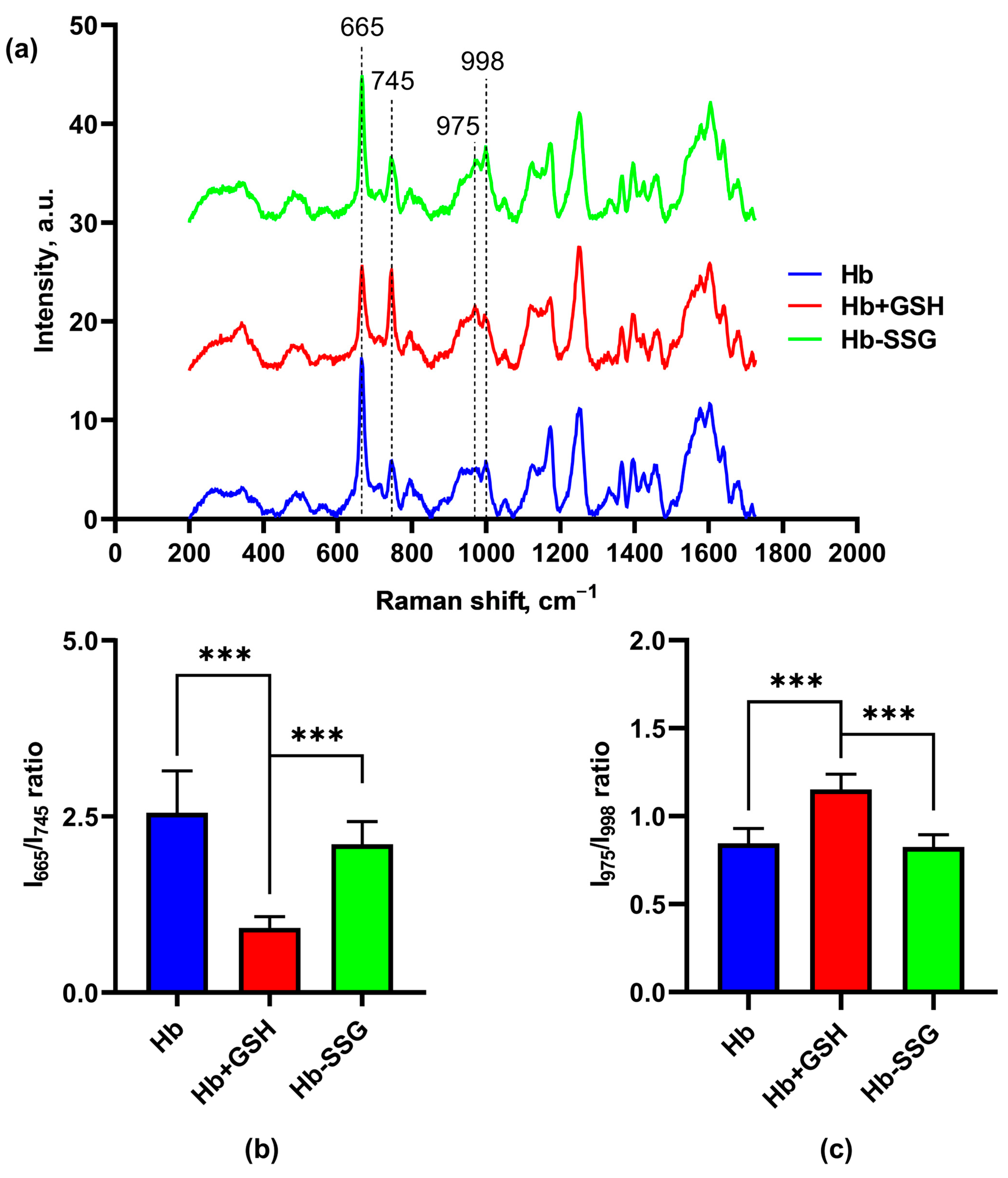
2.7. Effect of Hb Glutathionylation and Hb Complex Formation with GSH on the Conformation of the Hemoporphyrin Macrocycle of the Heme of Hemoglobin
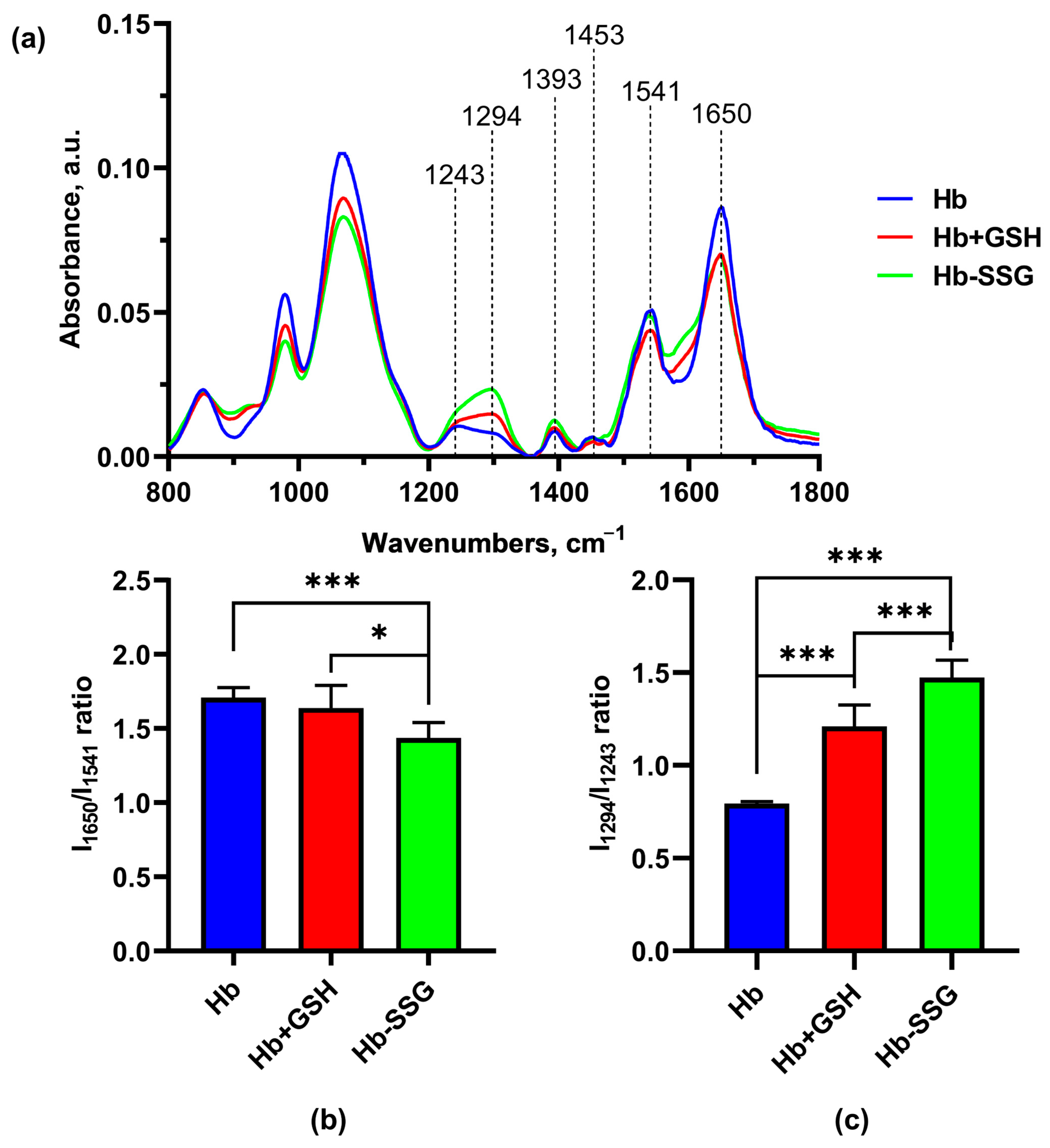
2.8. Effect of Hb Glutathionylation and Hb Complex formation with GSH on the Infrared Spectrum of Hemoglobin
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Circular Dichroism (CD) Studies
4.3. Absorption Spectrum Measurements
4.4. Fluorescence Spectroscopy Measurements
4.5. Thermal Stability Measurement
4.6. Western Blotting Assay
4.7. Raman Spectroscopy Measurement
4.8. IR Spectroscopy Measurement
4.9. Modeling
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Perutz, M.F. Stereochemistry of Cooperative Effects in Haemoglobin: Haem–Haem Interaction and the Problem of Allostery. Nature 1970, 228, 726–734. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Fenk, S.; Melnikova, E.V.; Anashkina, A.A.; Poluektov, Y.M.; Zaripov, P.I.; Mitkevich, V.A.; Tkachev, Y.V.; Kaestner, L.; Minetti, G.; Mairbäurl, H.; et al. Hemoglobin Is an Oxygen-Dependent Glutathione Buffer Adapting the Intracellular Reduced Glutathione Levels to Oxygen Availability. Redox Biol. 2022, 58, 102535. [Google Scholar] [CrossRef] [PubMed]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular Mechanisms and Clinical Implications of Reversible Protein S -Glutathionylation. Antioxid. Redox Signal. 2008, 10, 1941–1988. [Google Scholar] [CrossRef]
- Oppong, D.; Schiff, W.; Shivamadhu, M.C.; Ahn, Y.-H. Chemistry and Biology of Enzymes in Protein Glutathionylation. Curr. Opin. Chem. Biol. 2023, 75, 102326. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P. Protein Glutathionylation in Health and Disease. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 3165–3172. [Google Scholar] [CrossRef]
- Nonaka, K.; Kume, N.; Urata, Y.; Seto, S.; Kohno, T.; Honda, S.; Ikeda, S.; Muroya, T.; Ikeda, Y.; Ihara, Y.; et al. Serum Levels of S-Glutathionylated Proteins as a Risk-Marker for Arteriosclerosis Obliterans. Circ. J. 2007, 71, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Woodi, M.; Sood, V.; Krishnaswamy, P.R.; Rao, A.; Ballal, S.; Balaram, P. Quantitation and Characterization of Glutathionyl Haemoglobin as an Oxidative Stress Marker in Chronic Renal Failure by Mass Spectrometry. Clin. Biochem. 2007, 40, 986–994. [Google Scholar] [CrossRef]
- Zaripov, P.I.; Kuleshova, I.D.; Poluektov, Y.M.; Sidorenko, S.V.; Kvan, O.K.; Maksimov, G.V.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Metabolic Stress of Red Blood Cells Induces Glutathioylation of Hemoglobin. Mol. Biol. 2023, 58. in press. [Google Scholar]
- Craescu, C.T.; Poyart, C.; Schaefferll, C.; Garel, M.-C.; Kisterp, J.; Beuzard, Y. Covalent Binding of Glutathione to Hemoglobin. II. Functional Consequences and Structural Changes Reflected in NMR Spectra. J. Biol. Chem. 1986, 261, 14710–14716. [Google Scholar] [PubMed]
- Mitra, G.; Muralidharan, M.; Narayanan, S.; Pinto, J.; Srinivasan, K.; Mandal, A.K. Glutathionylation Induced Structural Changes in Oxy Human Hemoglobin Analyzed by Backbone Amide Hydrogen/Deuterium Exchange and MALDI-Mass Spectrometry. Bioconj. Chem. 2012, 23, 2344–2353. [Google Scholar] [CrossRef]
- Collins, J.-A.; Rudenski, A.; Gibson, J.; Howard, L.; O’Driscoll, R. Relating Oxygen Partial Pressure, Saturation and Content: The Haemoglobin-Oxygen Dissociation Curve. Breathe 2015, 11, 194–201. [Google Scholar] [CrossRef]
- Cruz-Landeira, A.; Bal, M.J.; Quintela, O.; Lopez-Rivadulla, M. Determination of Methemoglobin and Total Hemoglobin in Toxicological Studies by Derivative Spectrophotometry. J. Anal. Toxicol. 2002, 26, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.E. Hemoglobin Fluorescence. In Hemoglobin Disorders; Humana Press: Totowa, NJ, USA, 2003; Volume 82, pp. 133–154. ISBN 978-1-59259-373-6. [Google Scholar]
- Meyers, R.A. Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Radmilović, M.D.; Drvenica, I.T.; Rabasović, M.D.; Ilić, V.L.; Pavlović, D.; Oasa, S.; Vukojević, V.; Perić, M.; Nikolić, S.N.; Krmpot, A.J. Interactions of Ultrashort Laser Pulses with Hemoglobin: Photophysical Aspects and Potential Applications. Int. J. Biol. Macromol. 2023, 244, 125312. [Google Scholar] [CrossRef]
- Fraczkiewicz, R.; Braun, W. Exact and Efficient Analytical Calculation of the Accessible Surface Areas and Their Gradients for Macromolecules. J. Comput. Chem. 1998, 19, 319–333. [Google Scholar] [CrossRef]
- Wood, B.R.; Tait, B.; McNaughton, D. Micro-Raman Characterisation of the R to T State Transition of Haemoglobin within a Single Living Erythrocyte. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2001, 1539, 58–70. [Google Scholar] [CrossRef]
- Wood, B.R.; Kochan, K.; Marzec, K.M. Resonance Raman Spectroscopy of Hemoglobin in Red Blood Cells. In Vibrational Spectroscopy in Protein Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–414. ISBN 978-0-12-818610-7. [Google Scholar]
- Ye, S.; Ruan, P.; Yong, J.; Shen, H.; Liao, Z.; Dong, X. The Impact of the HbA1c Level of Type 2 Diabetics on the Structure of Haemoglobin. Sci. Rep. 2016, 6, 33352. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Huang, P.; Caughey, W.S. Protein Secondary Structures in Water from Second-Derivative Amide I Infrared Spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef]
- Lorenz-Fonfria, V.A. Infrared Difference Spectroscopy of Proteins: From Bands to Bonds. Chem. Rev. 2020, 120, 3466–3576. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen Types Analysis and Differentiation by FTIR Spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining Information about Protein Secondary Structures in Aqueous Solution Using Fourier Transform IR Spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the Secondary Structure of Proteins by Deconvolved FTIR Spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Almeida, I.; Rebelo, S.; Magalhães, S.; Nunes, A. Age-Related Fourier-Transform Infrared Spectroscopic Changes in Protein Conformation in an Aging Model of Human Dermal Fibroblasts. Spectrosc. J. 2023, 1, 37–48. [Google Scholar] [CrossRef]
- Singh, B.R.; DeOliveira, D.B.; Fu, F.-N.; Fuller, M.P. Fourier Transform Infrared Analysis of Amide III Bands of Proteins for the Secondary Structure Estimation; Nafie, L.A., Mantsch, H.H., Eds.; Proceedings Volume 1890, Biomolecular Spectroscopy III; SPIE: Los Angeles, CA, USA, 1993; pp. 47–55. [Google Scholar]
- Metere, A.; Iorio, E.; Scorza, G.; Camerini, S.; Casella, M.; Crescenzi, M.; Minetti, M.; Pietraforte, D. Carbon Monoxide Signaling in Human Red Blood Cells: Evidence for Pentose Phosphate Pathway Activation and Protein Deglutathionylation. Antioxid. Redox Signal. 2014, 20, 403–416. [Google Scholar] [CrossRef]
- Nagai, M.; Sugita, Y.; Yoneyama, Y. Circular Dichroism of Hemoglobin and Its Subunits in the Soret Region. J. Biol. Chem. 1969, 244, 1651–1653. [Google Scholar] [CrossRef]
- Quds, R.; Hashmi, M.A.; Iqbal, Z.; Mahmood, R. Interaction of Mancozeb with Human Hemoglobin: Spectroscopic, Molecular Docking and Molecular Dynamic Simulation Studies. Spectrochim. Acta Mol. Biomol. Spectrosc. 2022, 280, 121503. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhang, H.-M.; Zhou, Q.-H. Studies on the Interaction of Caffeine with Bovine Hemoglobin. Eur. J. Med. Chem. 2009, 44, 2100–2105. [Google Scholar] [CrossRef]
- Hellmann, N.; Schneider, D. Hands On: Using Tryptophan Fluorescence Spectroscopy to Study Protein Structure. In Protein Supersecondary Structures; Kister, A.E., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1958, pp. 379–401. ISBN 978-1-4939-9160-0. [Google Scholar]
- De, S.; Girigoswami, A. A Fluorimetric and Circular Dichroism Study of Hemoglobin—Effect of PH and Anionic Amphiphiles. J. Colloid Interface Sci. 2006, 296, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Manconi, B.; De Rosa, M.C.; Cappabianca, M.P.; Olianas, A.; Alinovi, C.C.; Mastropietro, F.; Ponzini, D.; Amato, A.; Pellegrini, M. A New β-Chain Haemoglobin Variant with Increased Oxygen Affinity: Hb Roma [Β115(G17)Ala→Val]. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2010, 1800, 327–335. [Google Scholar] [CrossRef]
- Rubino, F.M. The Redox Potential of the β-93-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments. Molecules 2021, 26, 2528. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.C.; Lin, W.-P.; Chiu, S.-D.; Fan, C.-H. Multistage Mass Spectrometric Analysis of Human Hemoglobin Glutathionylation: Correlation with Cigarette Smoking. Chem. Res. Toxicol. 2014, 27, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Kukulage, D.; Don, N.N.J.M.; Ahn, Y.-H. Emerging Chemistry and Biology in Protein Glutathionylation. Curr. Opin. Chem. Biol. 2022, 71, 102221. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sikdar, J.; Seal, P.; Haldar, R. Cigarette Smokers Develop Structurally Modified Hemoglobin: A Possible Way of Increasing Oxidative Stress. Inhal. Toxicol. 2015, 27, 300–307. [Google Scholar] [CrossRef]
- Toth, K.M.; Berger, E.M.; Beehler, C.J.; Repine, J.E. Erythrocytes from Cigarette Smokers Contain More Glutathione and Catalase and Protect Endothelial Cells from Hydrogen Peroxide Better than Do Erythrocytes from Nonsmokers. Am. Rev. Respir. Dis. 1986, 134, 281–284. [Google Scholar]
- Drvenica, I.T.; Stančić, A.Z.; Maslovarić, I.S.; Trivanović, D.I.; Ilić, V.L. Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects. Biomolecules 2022, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Do, L.H. Taming Glutathione Potentiates Metallodrug Action. Curr. Opin. Chem. Biol. 2022, 71, 102213. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.; Aviles, M.V.; Chen, Y.-L.; Latunde-Dada, G.O. The Role of GSH in Intracellular Iron Trafficking. Int. J. Mol. Sci. 2021, 22, 1278. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.E.; Louie, S.; Halliwell, B. Oxidants, Antioxidants, and Respiratory Tract Nin Uids. Environ. Health Perspect. 1994, 102, 185–191. [Google Scholar]
- Sotgia, S.; Fois, A.G.; Paliogiannis, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Methodological Fallacies in the Determination of Serum/Plasma Glutathione Limit Its Translational Potential in Chronic Obstructive Pulmonary Disease. Molecules 2021, 26, 1572. [Google Scholar] [CrossRef]
- Böhm, G.; Muhr, R.; Jaenicke, R. Quantitative Analysis of Protein Far UV Circular Dichroism Spectra by Neural Networks. Protein Eng. Des. Sel. 1992, 5, 191–195. [Google Scholar] [CrossRef]



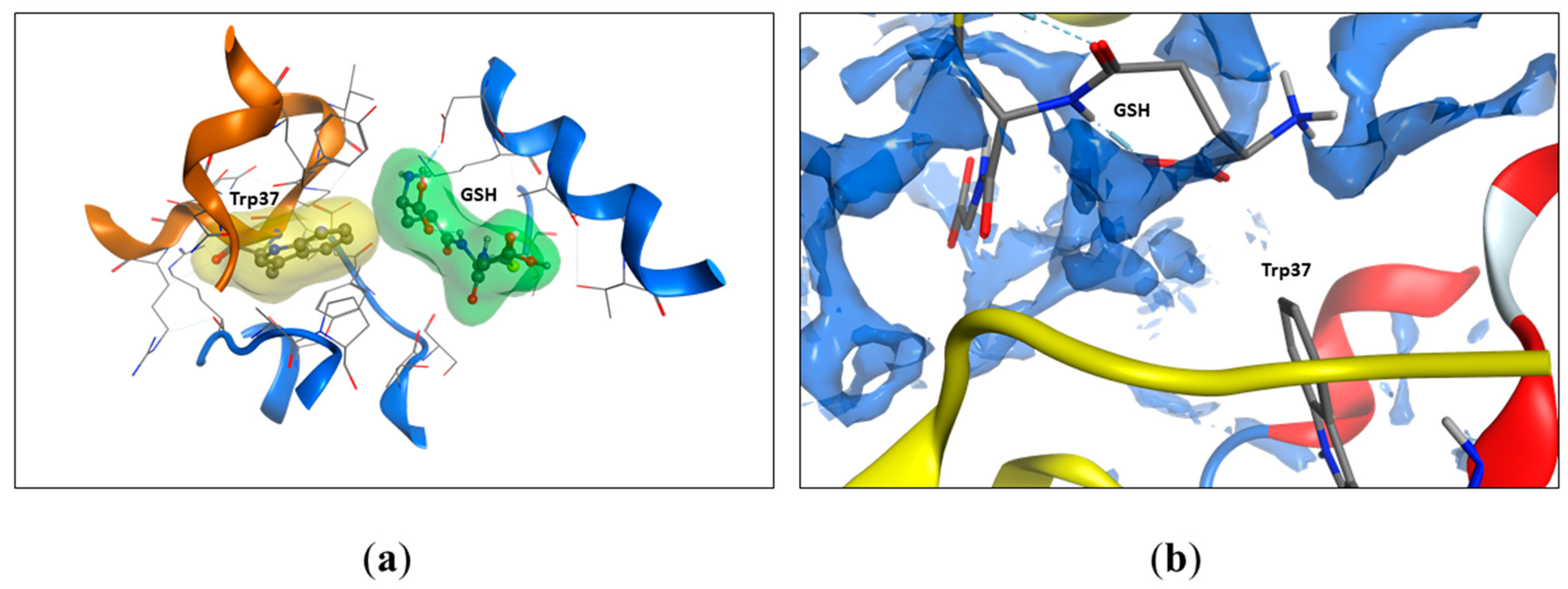

| Residue | Total | Apolar | Backbone | Sidechain | Ratio (%) | Availability In/Out |
|---|---|---|---|---|---|---|
| Complex of oxyhemoglobin with four GSH molecules | ||||||
| Trp14.α1 | 141.50 | 134.76 | 7.48 | 134.02 | 59.7 | Out |
| Trp15.β1 | 27.67 | 23.53 | 2.76 | 24.91 | 11.1 | In |
| Trp37.β1 | 18.91 | 18.12 | 0.93 | 17.97 | 8.0 | In |
| Trp14.α2 | 141.50 | 134.76 | 7.48 | 134.02 | 59.7 | Out |
| Trp15.β2 | 27.67 | 13.53 | 2.76 | 24.91 | 11.1 | In |
| Trp37.β2 | 17.76 | 17.76 | 0.25 | 17.50 | 7.8 | In |
| Structure of oxyhemoglobin (1R1X) | ||||||
| Trp14.α | 141.51 | 134.77 | 7.48 | 134.03 | 59.7 | Out |
| Trp15.β | 27.67 | 23.53 | 2.76 | 24.91 | 11.1 | In |
| Trp37.β | 138.37 | 134.70 | 14.98 | 123.38 | 54.9 | Out |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuleshova, I.D.; Zaripov, P.I.; Poluektov, Y.M.; Anashkina, A.A.; Kaluzhny, D.N.; Parshina, E.Y.; Maksimov, G.V.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation. Int. J. Mol. Sci. 2023, 24, 13557. https://doi.org/10.3390/ijms241713557
Kuleshova ID, Zaripov PI, Poluektov YM, Anashkina AA, Kaluzhny DN, Parshina EY, Maksimov GV, Mitkevich VA, Makarov AA, Petrushanko IY. Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation. International Journal of Molecular Sciences. 2023; 24(17):13557. https://doi.org/10.3390/ijms241713557
Chicago/Turabian StyleKuleshova, Iuliia D., Pavel I. Zaripov, Yuri M. Poluektov, Anastasia A. Anashkina, Dmitry N. Kaluzhny, Evgeniia Yu. Parshina, Georgy V. Maksimov, Vladimir A. Mitkevich, Alexander A. Makarov, and Irina Yu. Petrushanko. 2023. "Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation" International Journal of Molecular Sciences 24, no. 17: 13557. https://doi.org/10.3390/ijms241713557
APA StyleKuleshova, I. D., Zaripov, P. I., Poluektov, Y. M., Anashkina, A. A., Kaluzhny, D. N., Parshina, E. Y., Maksimov, G. V., Mitkevich, V. A., Makarov, A. A., & Petrushanko, I. Y. (2023). Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation. International Journal of Molecular Sciences, 24(17), 13557. https://doi.org/10.3390/ijms241713557







