Structural Motifs in Aryl Organogermanium Ge-O Derivatives for Material Design
Abstract
:1. Introduction
2. Results
3. Discussion
| Structural Type | Linear Formula of the Ge-Containing Fragment | CCDS Code | Main Structural Parameters (Average Values) | Reference | ||
|---|---|---|---|---|---|---|
| d(Ge-O), Å | Ge-O-Ge Angle, deg | O-Ge-O Angle, deg | ||||
| A | 1,1′-Fc[cyclo-GeC4Me4]2O | BIPVOL | 1.752 | 136.5 | - | [49] |
| [(PhCH2)3Ge]2O | BZGEOX10 | 1.730 | 180.0 | - | [50] | |
| [Mes2(2-Flu)Ge]2O | KIXWAP | 1.787 | 151.0 | - | [51] | |
| [1,1′-Naphth-2-GeMe2]2O | ROGMAB | 1.789 | 119.6 | - | [52] | |
| [Ph3Ge]2O | TPGERO | 1.752 | 137.9 | - | [53] | |
| [Ph3Ge]2O | TPGERO01 | 1.767 | 135.2 | - | [54] | |
| [(F5C2)3Ge]2O | UKUBAF | 1.742 | 150.2 | - | [31] | |
| [(p-FC6H4)3Ge]2O (1) | - | 1.778 | 132.4 | - | This work | |
| [(p-F3CC6H4)3Ge]2O (2) | - | 1.774 | 133.6 | - | This work | |
| B | trans-cyclo-[DippGe(OH)O]2O | CEDNIJ | 1.752 | 110.8 | - | [55] |
| C | cyclo-[cyclo-((CH2C(SiMe3)2)2Ge)O]2 | EYEBEP | 1.821 | 94.5 | 85.5 | [56] |
| cyclo-[Mes2GeO]2 | MEYPEL | 1.812 | 92.0 | 88.0 | [40] | |
| cyclo-[(2,6-Et2C6H3)2GeO]2 | VALKIC | 1.817 | 92.1 | 87.6 | [39] | |
| trans-cyclo-[Fc(Tip)GeO]2 | WEPDAY | 1.816 | 92.8 | 87.2 | [57] | |
| trans-cyclo-[Mes(Tbt)GeO]2*C6H14 | XIXNUN | 1.813 | 94.2 | 85.7 | [41] | |
| trans-cyclo-[Mes(Tbt)GeO]2 | XIXPAV | 1.810 | 96.0 | 84.0 | [41] | |
| cis-cyclo-[Mes(Tbt)GeO]2 | XIXPEZ | 1.810 | 92.2 | 86.1 | [41] | |
| D | bicyclo-[2,6-Dipp2C6H3Ge(O)O]2 | XAMQIN | 1.802 | 84.2 | 87.1 | [42] |
| E | cyclo-[Ph2GeO]3 | BECHEW | 1.769 | 128.6 | 107.4 | [33] |
| cyclo-[(tBu)2GeO]3 | BUDVIF | 1.781 | 133.2 | 106.8 | [58] | |
| cyclo-[(t-Bu)2GeO]3 | BUDVIF10 | 1.781 | 133.0 | 107.0 | [34] | |
| cyclo-[(Me4Si(O)(CH2)2Ge)O]3 | DUBZEF | 1.758 | 127.2 | 109.4 | [59] | |
| cyclo-[Ph4C4GeO]3 | EGULAT | 1.768 | 132.5 | 107.6 | [35] | |
| cyclo-[(F5C2)2GeO]3*phen a | EKEGOS | 1.735 | 125.7 | 113.5 | [37] | |
| cis-cyclo-[Fc(Ph)GeO]3 | VAHNUO | 1.771 | 127.5 | 107.8 | [36] | |
| trans-cyclo-[Fc(Ph)GeO]3 | VAHPAW | 1.776 | 123.2 | 105.9 | [36] | |
| F | cyclo-[Ph2GeO]4 | CIRBIO | 1.756 | 134.2 | 109.0 | [46] |
| cyclo-[(2,8-Et2C8H2Ge)O]4 | PAFHEM | 1.762 | 126.96 | 105.9 | [23] | |
| cyclo-[(CH2)5GeO]4 | SONHAE | 1.775 | 125.8 | 107.7 | [60] | |
| cyclo-[(p-F3CC6H4)2GeO]4 (3) | - | 1.769 | 124.5 | 109.0 | This work | |
| G | bicyclo-[PhGe(Ph2GeO2)3GePh] | CUPHIE | 1.76 | 130.9 | 109.1 | [43] |
| H* | trans-tricyclo-[(t-BuGe)6O8Cl2] | FOTCAS | 1.76 | 129.7 | 107.7 | [61] |
| trans-tricyclo-[(t-BuGe)6O8Cl2] | FOTCAS01 | 1.76 | 126.5 | 107.0 | [62] | |
| tricyclo-[(Me2NCH2FcGe)6O8(OH)2] | MUSYUU | 1.766 | 125.6 | 108.2 | [45] | |
| I | tetracyclo-[(t-BuGe)6O9] | CATFEI | 1.750 | 132.7 | 108.3 | [63] |
| tetracyclo-[(MesGe)6O9] | GIHSAR | 1.751 | 131.0 | 107.8 | [64] | |
| tetracyclo-[(i-PrGe)6O9] | GIHSEV | 1.760 | 129.7 | 108.4 | [64] | |
| tetracyclo-[(i-PrGe)6O9] | GIHSEV01 | 1.755 | 130.5 | 108.0 | [65] | |
| tetracyclo-[(i-PrGe)6O9] | GIHSEV02 | 1.756 | 131.0 | 108.0 | [62] | |
| tetracyclo-[(CyGe)6O9] | GIHSIZ | 1.748 | 131.9 | 108.5 | [64] | |
| tetracyclo-[(CyGe)6O9] | GIHSIZ01 | 1.758 | 133.3 | 108.6 | [62] | |
| J | pentacyclo-[(VinSiMe2OGeO)8O4]*Et4NF | VOFZIC | 1.721 | 133.8 | 113.9 | [12] |
| K | [(p-F3CC6H4)3GeO]4Ge (4) | - | 1.757 | 133.3 | 110.6 | This work |
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazurek, P.; Vudayagiri, S.; Skov, A.L. How to tailor flexible silicone elastomers with mechanical integrity: A tutorial review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Gutacker, A.; Mejía, E. Industrial synthesis of reactive silicones: Reaction mechanisms and processes. Org. Chem. Front. 2020, 7, 4108–4120. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes—Silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289. [Google Scholar] [CrossRef]
- Kamino, B.A.; Bender, T.P. The use of siloxanes, silsesquioxanes, and silicones in organic semiconducting materials. Chem. Soc. Rev. 2013, 42, 5119–5130. [Google Scholar] [CrossRef] [PubMed]
- John, Ł.; Ejfler, J. A Brief Review on Selected Applications of Hybrid Materials Based on Functionalized Cage-like Silsesquioxanes. Polymers 2023, 15, 1452. [Google Scholar] [CrossRef]
- Skoczeń, A.; Frąckowiak, D.; Przekop, R.E.; Frydrych, M.; Kasperkowiak, M.; Jeleń, P.; Sitarz, M.; Marciniec, B. New Ceramics Precursors Containing Si and Ge Atoms—Cubic Germasilsesquioxanes—Synthesis, Thermal Decomposition and Spectroscopic Analysis. Molecules 2022, 27, 1441. [Google Scholar] [CrossRef]
- Smart, M.M.; McMillen, C.D.; Ivey, K.; Kolis, J.W. Chemistry of Metal Silicates and Germanates: The Largest Metal Polygermanate, K11Mn21Ge32O86(OH)9(H2O), with a 76 Å Periodic Lattice. Inorg. Chem. 2020, 59, 16804–16808. [Google Scholar] [CrossRef]
- Lin, Z.-E.; Yang, G.-Y. Germanate Frameworks Constructed from Oxo Germanium Cluster Building Units. Eur. J. Inorg. Chem. 2010, 2010, 2895–2902. [Google Scholar] [CrossRef]
- Huang, S.; Yue, H.; Chen, Y.; Liu, Y.; Guan, Y.; Zou, X.; Sun, J. Three-Dimensional Open-Framework Germanate Built from a Novel Ge13 Cluster and Containing Two Types of Chiral Layers. Cryst. Growth Des. 2018, 18, 928–933. [Google Scholar] [CrossRef]
- Villaescusa, L.A.; Lightfoot, P.; Morris, R.E. Synthesis and structure of fluoride-containing GeO2 analogues of zeolite double four-ring building units. Chem. Commun. 2002, 2220–2221. [Google Scholar] [CrossRef]
- Sato, N.; Hayashi, T.; Tochigi, K.; Wada, H.; Shimojima, A.; Kuroda, K. Synthesis of Organosilyl-Functionalized Cage-Type Germanoxanes Containing Fluoride Ions. Chem. Eur. J. 2019, 25, 7860–7865. [Google Scholar] [CrossRef]
- Stock, N.; Jargstorff, C.; Wriedt, S. Two New Crystalline Organogermanate-Based Inorganic-Organic Hybrid Compounds. Z. Anorg. Allg. Chem. 2011, 637, 572–577. [Google Scholar] [CrossRef]
- Hayashi, T.; Murase, N.; Sato, N.; Fujino, K.; Sugimura, N.; Wada, H.; Kuroda, K.; Shimojima, A. Fluoride Ion-Encapsulated Germoxane Cages Modified with Organosiloxane Chains as Anionic Components of Ionic Liquids. Organometallics 2022, 41, 1454–1463. [Google Scholar] [CrossRef]
- He, H.; Cao, G.-J.; Zheng, S.-T.; Yang, G.-Y. Lanthanide Germanate Cluster Organic Frameworks Constructed from {Ln8Ge12} or {Ln11Ge12} Cage Cluster Building Blocks. J. Am. Chem. Soc. 2009, 131, 15588–15589. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Sato, N.; Wada, H.; Shimojima, A.; Kuroda, K. Variation of counter quaternary ammonium cations of anionic cage germanoxanes as building blocks of nanoporous materials. Dalton Trans. 2021, 50, 8497–8505. [Google Scholar] [CrossRef]
- Endo, M.; Fugami, K.; Enokido, T.; Sano, H.; Kosugi, M. Palladium-Catalyzed Cross-Coupling Reaction Using Arylgermanium Sesquioxide. Adv. Synth. Catal. 2007, 349, 1025–1027. [Google Scholar] [CrossRef]
- Pitteloud, J.-P.; Zhang, Z.-T.; Liang, Y.; Cabrera, L.; Wnuk, S.F. Fluoride-Promoted Cross-Coupling of Chloro(mono-, di-, or triphenyl)germanes with Aryl Halides in “Moist” Toluene. Multiple Transfer of the Phenyl Group from Organogermane Substrates and Comparison of the Coupling Efficiencies of Chloro(phenyl)germanes with their Corresponding Stannane and Silane Counterparts. J. Org. Chem. 2010, 75, 8199–8212. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shimada, Y.; Sato, K. Bioorganic and Medicinal Organogermanium Chemistry. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; Wiley: Hoboken, NJ, USA, 2023; Volume 1, Chapter 19; pp. 839–865. [Google Scholar] [CrossRef]
- Duverneuil, G.; Mazerolles, P.; Perrier, E. Polygermoxanes suitable for biochemical purposes: II Linear trigermoxanes (high-viscosity oils). Appl. Organomet. Chem. 1995, 9, 37–42. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S. Cagelike metallagermanates and metallagermoxanes: Synthesis, structures and functional properties. Coord. Chem. Rev. 2019, 386, 209–239. [Google Scholar] [CrossRef]
- Liu, J.; Ren, X.; Yan, Y.; Wang, N.; Wang, S.; Zhang, H.; Li, J.; Yu, J. A new two-dimensional layered germanate with in situ embedded carbon dots for optical temperature sensing. Inorg. Chem. Front. 2018, 5, 139–144. [Google Scholar] [CrossRef]
- Ohshita, J.; Nakamura, M.; Ooyama, Y. Preparation and Reactions of Dichlorodithienogermoles. Organometallics 2015, 34, 5609–5614. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Kapranov, A.A.; Churakov, A.V.; Poleshchuk, O.K.; Oprunenko, Y.F.; Tarasevich, B.N.; Zaitseva, G.S.; Karlov, S.S. “Donor–Acceptor” Oligogermanes: Synthesis, Structure, and Electronic Properties. Organometallics 2013, 32, 6500–6510. [Google Scholar] [CrossRef]
- Hemmert, C.; Gornitzka, H. X-ray Crystallography of Organogermanium Compounds. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; Wiley: Hoboken, NJ, USA, 2023; Volume 1, Chapter 16; pp. 667–743. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Oprunenko, Y.F. Reaction of Substituted Group 14 Element Potassium Salts with 1-(Chloromethyl)silatrane: Substitution or Rearrangement? Russ. J. Gen. Chem. 2021, 91, 2385–2390. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Poleshchuk, O.K.; Churakov, A.V. Oligoorganogermanes: Interplay between Aryl and Trimethylsilyl Substituents. Molecules 2022, 27, 2147. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Trubachev, A.D.; Poleshchuk, O.K. Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations. Int. J. Mol. Sci. 2023, 24, 10218. [Google Scholar] [CrossRef]
- Zaitsev, K.V. Organogermanium Compounds of the Main Group Elements. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; Wiley: Hoboken, NJ, USA, 2023; Volume 1, Chapter 2; pp. 103–193. [Google Scholar] [CrossRef]
- Delouche, T.; Roisnel, T.; Dorcet, V.; Hissler, M.; Bouit, P.-A. Mixing Polyaromatic Scaffolds and Main Group Elements: Synthesis, Coordination and Optical Properties of Naphthyl-Fused Heteropines. Eur. J. Inorg. Chem. 2021, 2021, 1082–1089. [Google Scholar] [CrossRef]
- Pelzer, S.; Neumann, B.; Stammler, H.-G.; Ignat’ev, N.; Hoge, B. Synthesis of Tris(pentafluoroethyl)germanes. Chem. Eur. J. 2016, 22, 3327–3332. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Lam, K.; Zhanabil, Z.; Suleimen, Y.; Kharcheva, A.V.; Tafeenko, V.A.; Oprunenko, Y.F.; Poleshchuk, O.K.; Lermontova, E.K.; Churakov, A.V. Oligogermanes Containing Only Electron-Withdrawing Substituents: Synthesis and Properties. Organometallics 2017, 36, 298–309. [Google Scholar] [CrossRef]
- Roß, L.; Dräger, M. Über Germanium-haltige Heterocyclen, IV. 2,2,4,4,6,6-Hexaphenyl-1,3,5-trioxa-2,4,6-trigermacyclohexan, (Ph2GeO)3, eine monoplanare Zwischenstufe im Pseudorotationskreislauf der Twist-Wanne-6-Ring-Konformation. Chem. Ber. 1982, 115, 615–621. [Google Scholar] [CrossRef]
- Puff, H.; Franken, S.; Schuh, W.; Schwab, W. Darstellung und struktur von di-t-butylgermaniumdi-hydroxid und di-t-butylgermaniumoxid. J. Organomet. Chem. 1983, 254, 33–41. [Google Scholar] [CrossRef]
- Godelie, M.C.; Jennings, M.C.; Baines, K.M. The Synthesis and Characterization of the Cyclotrigermoxane: (Ph4C4GeO)3. Main Group Met. Chem. 2001, 24, 823–828. [Google Scholar] [CrossRef]
- Zhang, Y.; Cervantes-Lee, F.; Pannell, K.H. Hydrolysis of tert-Butyl and Phenyl Ferrocenyldichlorogermanes: Characterization of Di-tert-butyldiferrocenyldigermoxanediol and 1,3,5-Triphenyl-1,3,5-triferrocenylcyclotrigermoxane. Organometallics 2003, 22, 510–515. [Google Scholar] [CrossRef]
- Pelzer, S.; Neumann, B.; Stammler, H.-G.; Ignat’ev, N.; Hoge, B. Synthesis of Bis(pentafluoroethyl)germanes. Chem. Eur. J. 2016, 22, 4758–4763. [Google Scholar] [CrossRef] [PubMed]
- Glockling, F.; Houston, R.E. Reactions of organodigermanes. J. Chem. Soc. Dalton Trans. 1973, 1357–1359. [Google Scholar] [CrossRef]
- Masamune, S.; Batcheller, S.A.; Park, J.; Davis, W.M.; Yamashita, O.; Ohta, Y.; Kabe, Y. Oxygenation of digermene derivatives. J. Am. Chem. Soc. 1989, 111, 1888–1889. [Google Scholar] [CrossRef]
- Samuel, M.S.; Jennings, M.C.; Baines, K.M. The addition of oxygen to tetramesityldigermene. J. Organomet. Chem. 2001, 636, 130–137. [Google Scholar] [CrossRef]
- Tokitoh, N.; Kishikawa, K.; Okazaki, R.; Sasamori, T.; Nakata, N.; Takeda, N. Synthesis and characterization of an extremely hindered tetraaryl-substituted digermene and its unique properties in the solid state and in solution. Polyhedron 2002, 21, 563–577. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Olmstead, M.M.; Fettinger, J.C.; Power, P.P. An Unsymmetric Oxo/Imido-Bridged Germanium-Centered Singlet Diradicaloid. J. Am. Chem. Soc. 2009, 131, 14164–14165. [Google Scholar] [CrossRef]
- Häberle, K.; Dräger, M. Germanium-haltige Heterocyclen, VI [1] Ph8Ge5O6, ein Germoxan aus bicyclisch verknüpften (GeO)4-Ringen. Z. Naturforsch. B 1984, 39, 1541–1547. [Google Scholar] [CrossRef]
- Beckmann, J.; Jurkschat, K.; Schürmann, M. On the Hydrolysis of tBu2Ge(OEt)2: Supramolecular Self Assembly in the Solid State of 2 tBu2Ge(OH)2, (tBu2GeOH)2O, and H2O. Eur. J. Inorg. Chem. 2000, 2000, 939–941. [Google Scholar] [CrossRef]
- Lorenz, V.; Jacob, K.; Wagner, C.; Görls, H. Metalloxane des Siliciums und Germaniums mit dem 2-(Dimethylaminomethyl)ferrocenyl-Liganden (FcN): Darstellung und Molekülstrukturen von (FcN)4M4O4(OH)4(M = Si, Ge), (FcN)6Ge6O8(OH)2 und von (FcN)2Si(OH)2. Z. Anorg. Allg. Chem. 2002, 628, 2855–2861. [Google Scholar] [CrossRef]
- Roß, L.; Dräger, M. Formen des Diphenylgermaniumoxids(Ph2GeO)x, x = 3, 4, n/Phases of Diphenylgermanium Oxide(Ph2GeO)x, x = 3, 4, n. Z. Naturforsch. B 1984, 39, 868–875. [Google Scholar] [CrossRef]
- Pérez, J.; García, L.; Kessler, M.; Nolsøe, K.; Pérez, E.; Serrano, J.L.; Martínez, J.F.; Carrascosa, R. Solid state conformational classification of eight-membered rings in copper complexes double bridged by phosphate, phosphonate or phosphinate ligands. Inorg. Chim. Acta 2005, 358, 2432–2436. [Google Scholar] [CrossRef]
- Beckmann, J.; Dakternieks, D.; Lim, A.E.K.; Lim, K.F.; Jurkschat, K. Understanding ring strain and ring flexibility in six- and eight-membered cyclic organometallic group 14 oxides. J. Mol. Struct. THEOCHEM 2006, 761, 177–193. [Google Scholar] [CrossRef]
- Zürcher, S.; Gramlich, V.; Togni, A. Germanium-containing ferrocenes and ferrocenophanes. Potential precursors for ring-opening polymerizations and ‘germaferrocenes’. Inorg. Chim. Acta 1999, 291, 355–364. [Google Scholar] [CrossRef]
- Glidewell, C.; Liles, D.C. The crystal and molecular structure of oxo-bis[tribenzylgermanium(IV)]. J. Organomet. Chem. 1979, 174, 275–279. [Google Scholar] [CrossRef]
- Lazraq, M.; Couret, C.; Escudie, J.; Satge, J.; Draeger, M. Two germaoxetanes from a germene: Formation and structure. Organometallics 1991, 10, 1771–1778. [Google Scholar] [CrossRef]
- Schilling, B.; Kaufmann, D.E.; Kaiser, V. Synthesis and Structure of 2,2′ -Boryl-, Germyl-Silyl-, and Stannyl-Substituted 1,1′-Binaphthyl Systems. Chem. Ber. 1997, 130, 923–932. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Struchkov, Y.T. Crystal structure of bis(triphenylgermyl)ether. Russ. J. Struct. Chem. 1973, 13, 884–887. [Google Scholar] [CrossRef]
- Glidewell, C.; Liles, D.C. The crystal and molecular structure of oxobis[triphenylgermanium(IV)]. Acta Crystallogr. Sect. B 1978, 34, 119–124. [Google Scholar] [CrossRef]
- Cui, C.; Olmstead, M.M.; Fettinger, J.C.; Spikes, G.H.; Power, P.P. Reactions of the Heavier Group 14 Element Alkyne Analogues Ar‘EEAr‘ (Ar‘ = C6H3-2,6(C6H3-2,6-Pri2)2; E = Ge, Sn) with Unsaturated Molecules: Probing the Character of the EE Multiple Bonds. J. Am. Chem. Soc. 2005, 127, 17530–17541. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Masuda, H.; Ishida, S.; Kabuto, C.; Kira, M. Diverse reactions of nitroxide-radical adducts of silylene, germylene, and stannylene. J. Organomet. Chem. 2004, 689, 1337–1341. [Google Scholar] [CrossRef]
- Sasamori, T.; Miyamoto, H.; Sakai, H.; Furukawa, Y.; Tokitoh, N. 1,2-Bis(ferrocenyl)digermene: A d−π Electron System Containing a Ge=Ge Unit. Organometallics 2012, 31, 3904–3910. [Google Scholar] [CrossRef]
- Puff, H.; Franken, S.; Schuh, W.; Schwab, W. Struktur des trimeren Di-t-butylgermaniumoxids. J. Organomet. Chem. 1983, 244, C41–C42. [Google Scholar] [CrossRef]
- Gurkova, S.N.; Gusev, A.I.; Alekseev, N.V.; Gar, T.K.; Khromova, N.Y. Crystal and molecular structure of 1,3,5-trigermatris(spiro-1-germa-3,5-disila-3,3,5,5-tetramethyl-4-oxacyclohexa)-2,4,6-cyclotrioxane. Russ. J. Struct. Chem. 1985, 26, 974–977. [Google Scholar] [CrossRef]
- Ogawa, K.; Tanaka, K.; Yoshimura, S.; Takeuchi, Y.; Chien, J.; Kai, Y.; Kasai, N. Structure of tetraspiro[1,3,5,7-tetraoxa-2,4,6,8-tetragermacyclooctane-2,1′:4,1′′:6,1′′′:8,1′′′′-tetrakisgerminane]. Acta Crystallogr. Sect. C 1991, 47, 2558–2561. [Google Scholar] [CrossRef]
- Puff, H.; Braun, K.; Franken, S.; Kök, T.R.; Schuh, W. Zur chalkogenolyse von monoorganyl-germanium-trichloriden I. Halogenhaltige verbindungen. J. Organomet. Chem. 1987, 335, 167–178. [Google Scholar] [CrossRef]
- Nanjo, M.; Sasage, T.; Mochida, K. Synthesis and characterization of alkylgermasesquioxanes. J. Organomet. Chem. 2003, 667, 135–142. [Google Scholar] [CrossRef]
- Puff, H.; Franken, S.; Schuh, W. Hexa-(t-butylgermanium-sesquioxid), ein neuartiges käfigmolekül. J. Organomet. Chem. 1983, 256, 23–30. [Google Scholar] [CrossRef]
- Puff, H.; Braun, K.; Franken, S.; Kök, T.R.; Schuh, W. Zur Chalkogenolyse von Monoorganyl-germanium-trichloriden: II. Sesquioxide. J. Organomet. Chem. 1988, 349, 293–303. [Google Scholar] [CrossRef]
- Masato, N.; Takaomi, S.; Kunio, M. Synthesis of Hexakis(alkylgermasesquioxane)s from Alkyl(chloro)ethoxygermanes and Their Formation Mechanism. Chem. Lett. 2002, 31, 1124–1125. [Google Scholar] [CrossRef]
- Sala, A.; Pérez-Botella, E.; Jordá, J.L.; Cantín, A.; Rey, F.; Valencia, S. ITQ-69: A Germanium-Containing Zeolite and its Synthesis, Structure Determination, and Adsorption Properties. Angew. Chem. Int. Ed. 2021, 60, 11745–11750. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Kharcheva, A.V.; Lam, K.; Zhanabil, Z.; Issabayeva, G.; Oprunenko, Y.F.; Churakov, A.V.; Zaitseva, G.S.; Karlov, S.S. Donor-acceptor molecular oligogermanes: Novel properties and structural aspects. J. Organomet. Chem. 2018, 867, 228–237. [Google Scholar] [CrossRef]
- Roewe, K.D.; Rheingold, A.L.; Weinert, C.S. A luminescent and dichroic hexagermane. Chem. Commun. 2013, 49, 8380–8382. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Tafeenko, V.A.; Oprunenko, Y.F.; Kharcheva, A.V.; Zhanabil, Z.; Suleimen, Y.; Lam, K.; Zaitsev, V.B.; Zaitseva, A.V.; Zaitseva, G.S.; et al. Molecular Oligogermanes and Related Compounds: Structure, Optical and Semiconductor Properties. Chem. Asian J. 2017, 12, 1240–1249. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Lam, K.; Poleshchuk, O.K.; Bezzubov, S.I.; Churakov, A.V. Aryl Germanes as Ligands for Transition Polymetallic Complexes: Synthesis, Structure and Properties. Eur. J. Inorg. Chem. 2019, 2019, 2750–2760. [Google Scholar] [CrossRef]
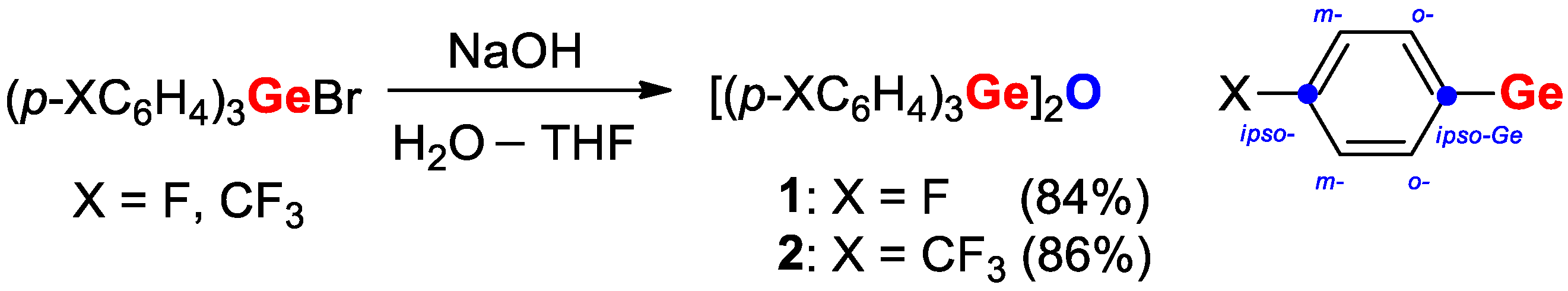
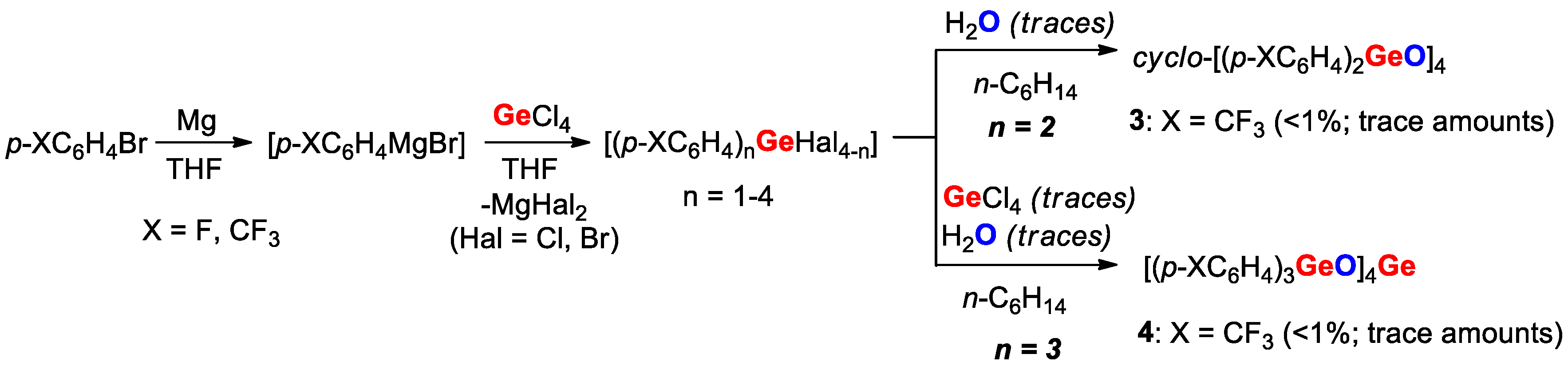
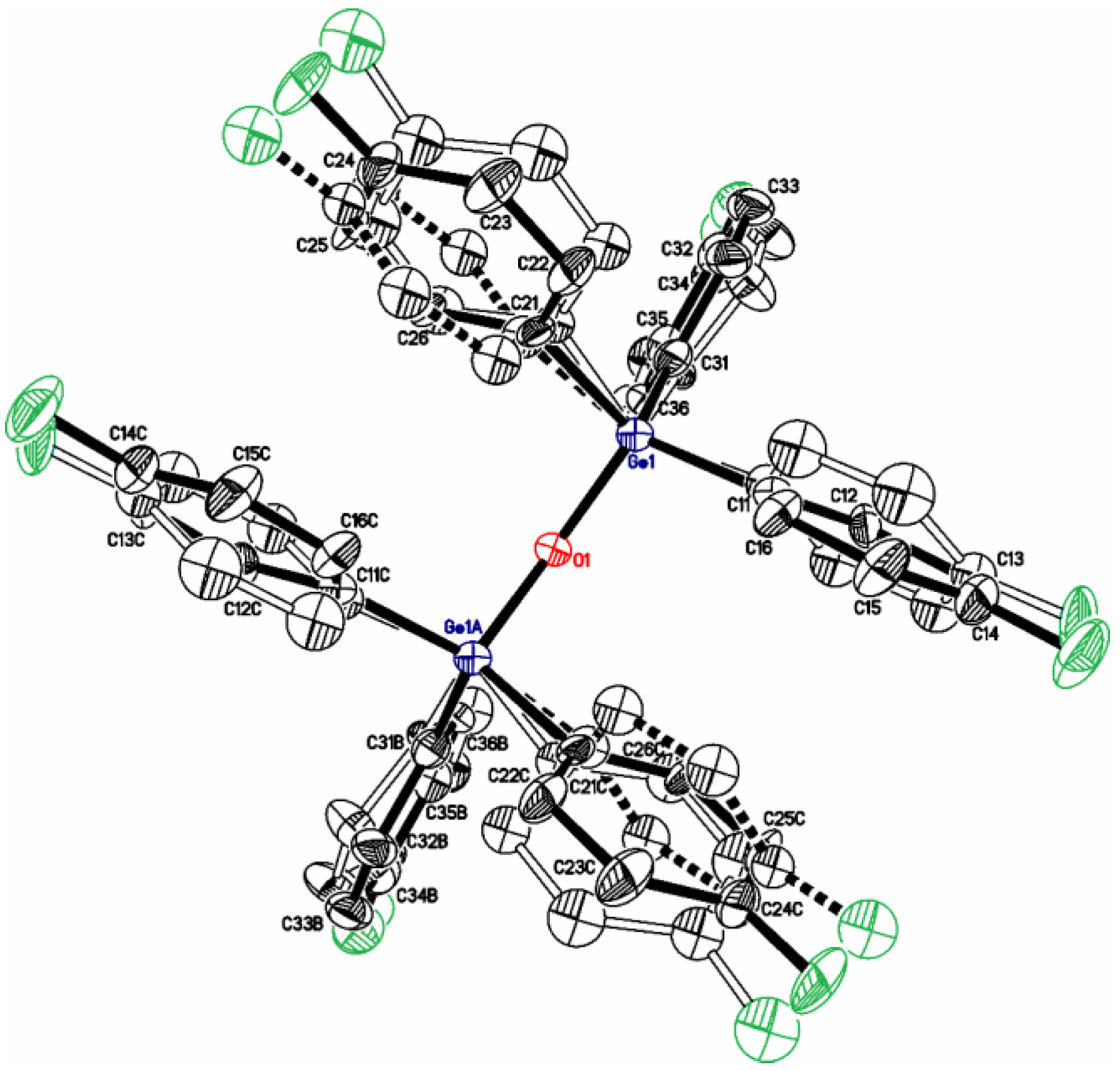
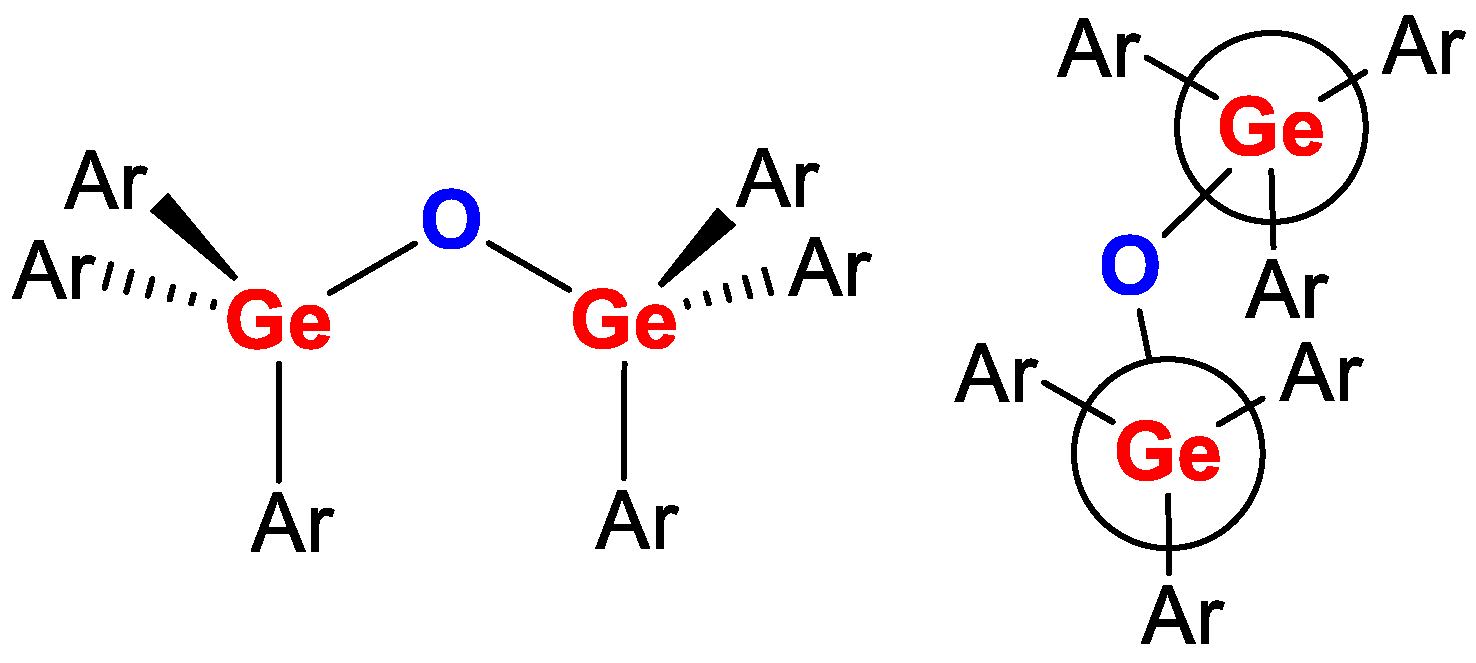

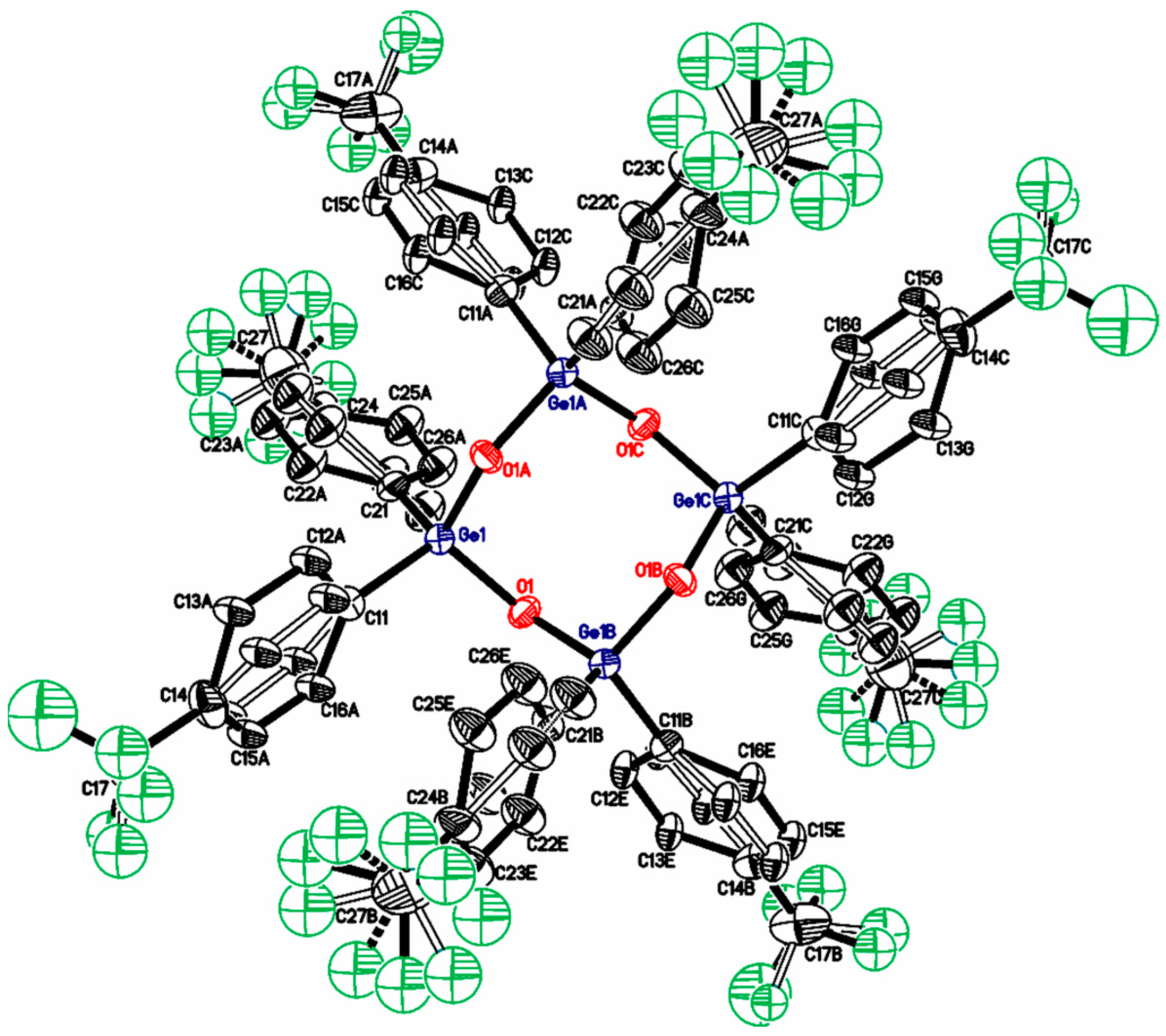
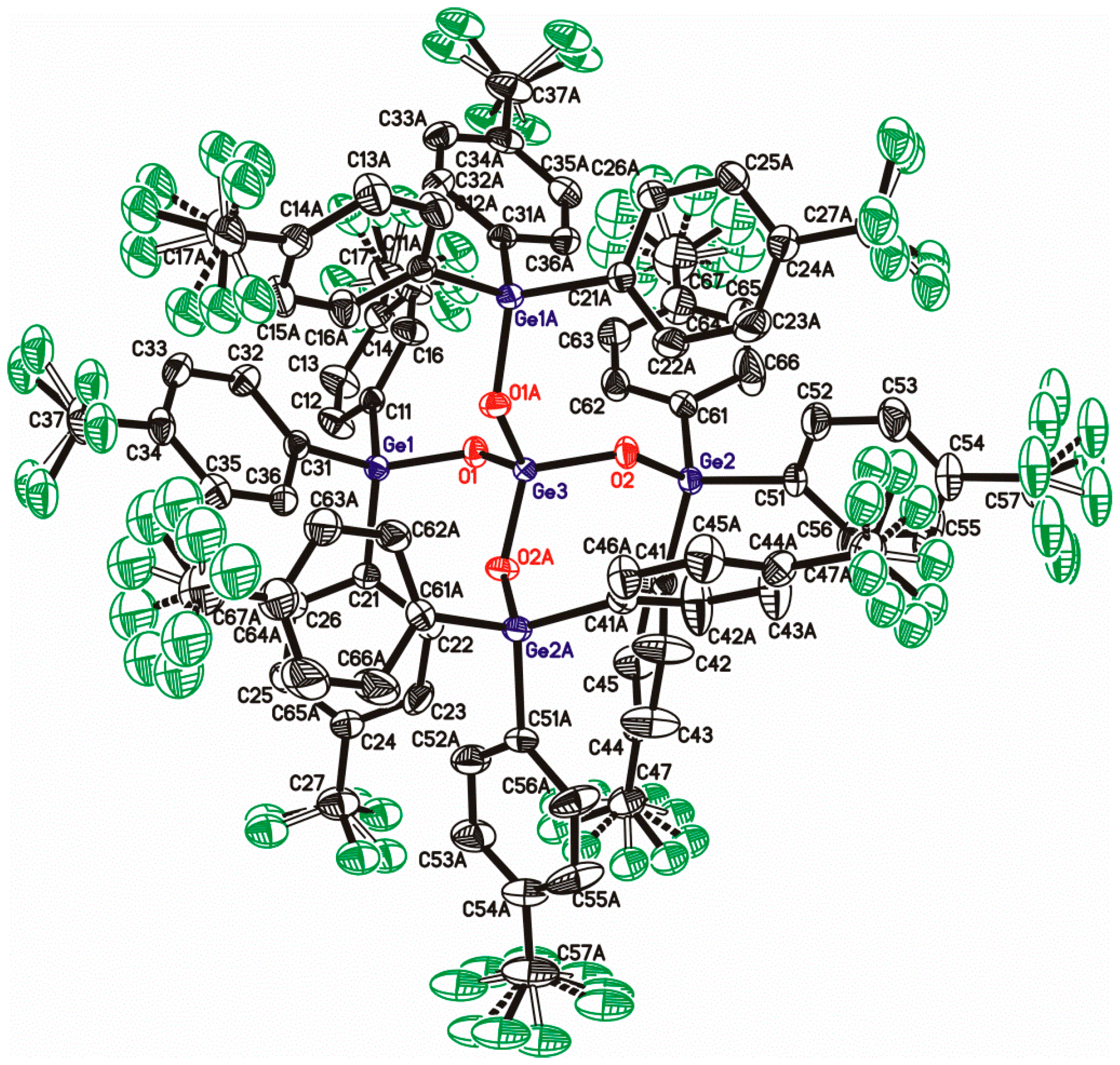
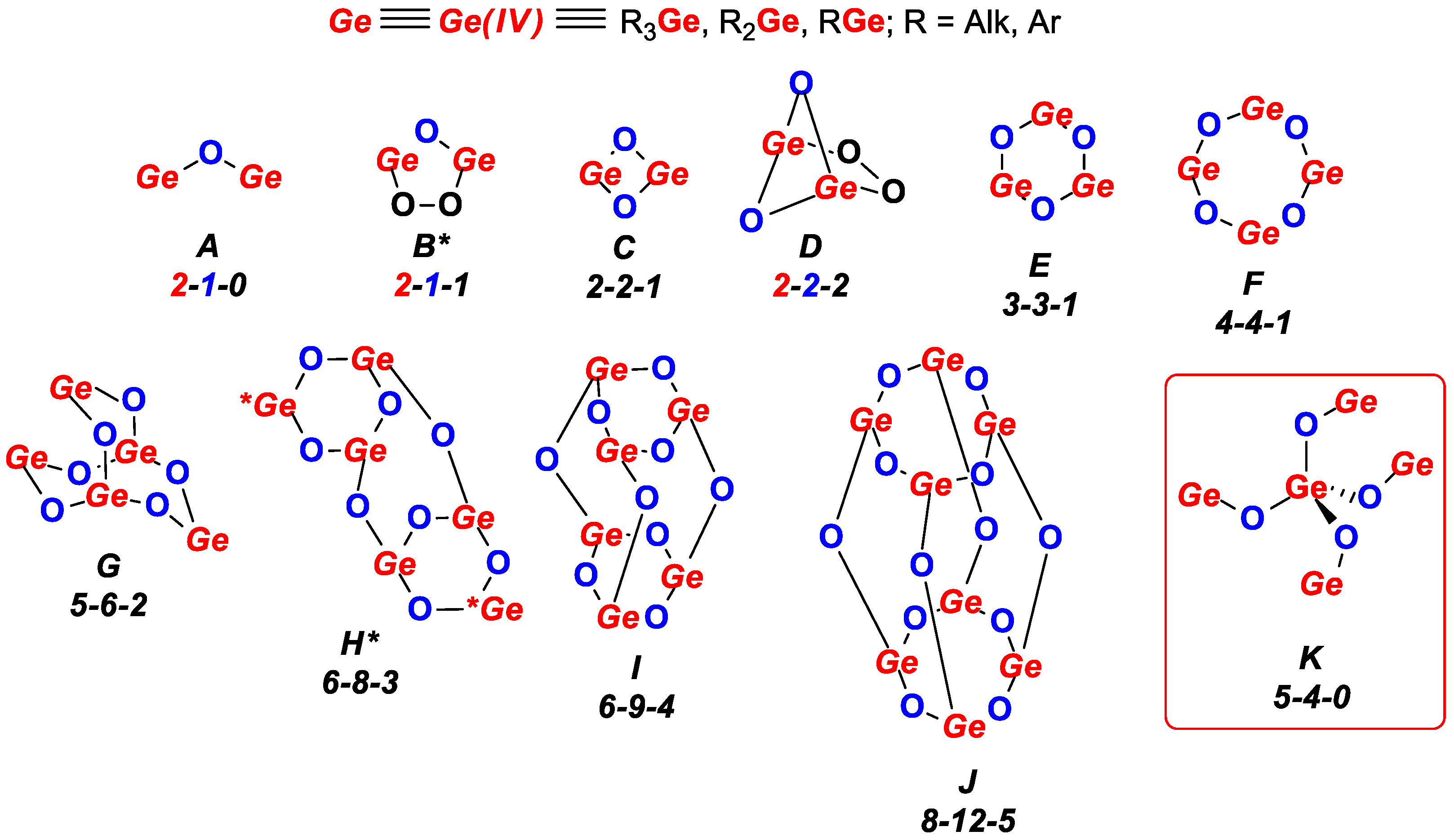
| Compound | X | ipso-ArC | o-ArCH | ipso-ArCGe | m-ArCH |
|---|---|---|---|---|---|
| (p-FC6H4)3GeX | X = Cl | 164.59 (251.0) | 136.00 (8.1) | 129.73 (3.7) | 116.15 (20.8) |
| X = Br | 164.52 (251.0) | 136.11 (8.1) | 129.64 (3.3) | 116.09 (20.5) | |
| X = H | 163.87 (248.7) | 136.75 (7.6) | 130.22 (3.8) | 115.75 (20.6) | |
| [(p-FC6H4)3Ge]2O (1) (this work) | 164.37 (250.3) | 135.93 (7.6) | 130.81 (3.8) | 115.92 (19.8) | |
| (p-F3CC6H4)3GeX | X = Cl | 133.86 (32.8) | 133.13 | 138.74 | 125.76 (3.8) |
| X = Br | 133.11 (32.9) | 134.49 | 137.80 | 125.60 (3.7) | |
| X = H | 131.98 (32.5) | 135.36 | 138.65 | 125.25 (3.7) | |
| [(p-F3CC6H4)3Ge]2O (2) (this work) | 132.59 (32.6) | 134.21 | 139.76 | 125.25 (3.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, K.V.; Makarov, I.S.; Oprunenko, Y.F.; Tafeenko, V.A.; Lermontova, E.K.; Churakov, A.V. Structural Motifs in Aryl Organogermanium Ge-O Derivatives for Material Design. Int. J. Mol. Sci. 2023, 24, 13575. https://doi.org/10.3390/ijms241713575
Zaitsev KV, Makarov IS, Oprunenko YF, Tafeenko VA, Lermontova EK, Churakov AV. Structural Motifs in Aryl Organogermanium Ge-O Derivatives for Material Design. International Journal of Molecular Sciences. 2023; 24(17):13575. https://doi.org/10.3390/ijms241713575
Chicago/Turabian StyleZaitsev, Kirill V., Igor S. Makarov, Yuri F. Oprunenko, Victor A. Tafeenko, Elmira Kh. Lermontova, and Andrei V. Churakov. 2023. "Structural Motifs in Aryl Organogermanium Ge-O Derivatives for Material Design" International Journal of Molecular Sciences 24, no. 17: 13575. https://doi.org/10.3390/ijms241713575
APA StyleZaitsev, K. V., Makarov, I. S., Oprunenko, Y. F., Tafeenko, V. A., Lermontova, E. K., & Churakov, A. V. (2023). Structural Motifs in Aryl Organogermanium Ge-O Derivatives for Material Design. International Journal of Molecular Sciences, 24(17), 13575. https://doi.org/10.3390/ijms241713575









