Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations
Abstract
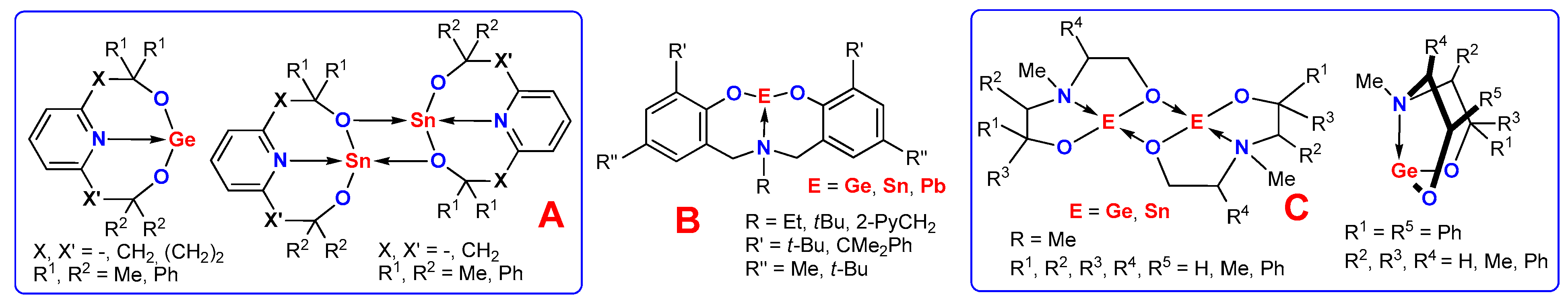
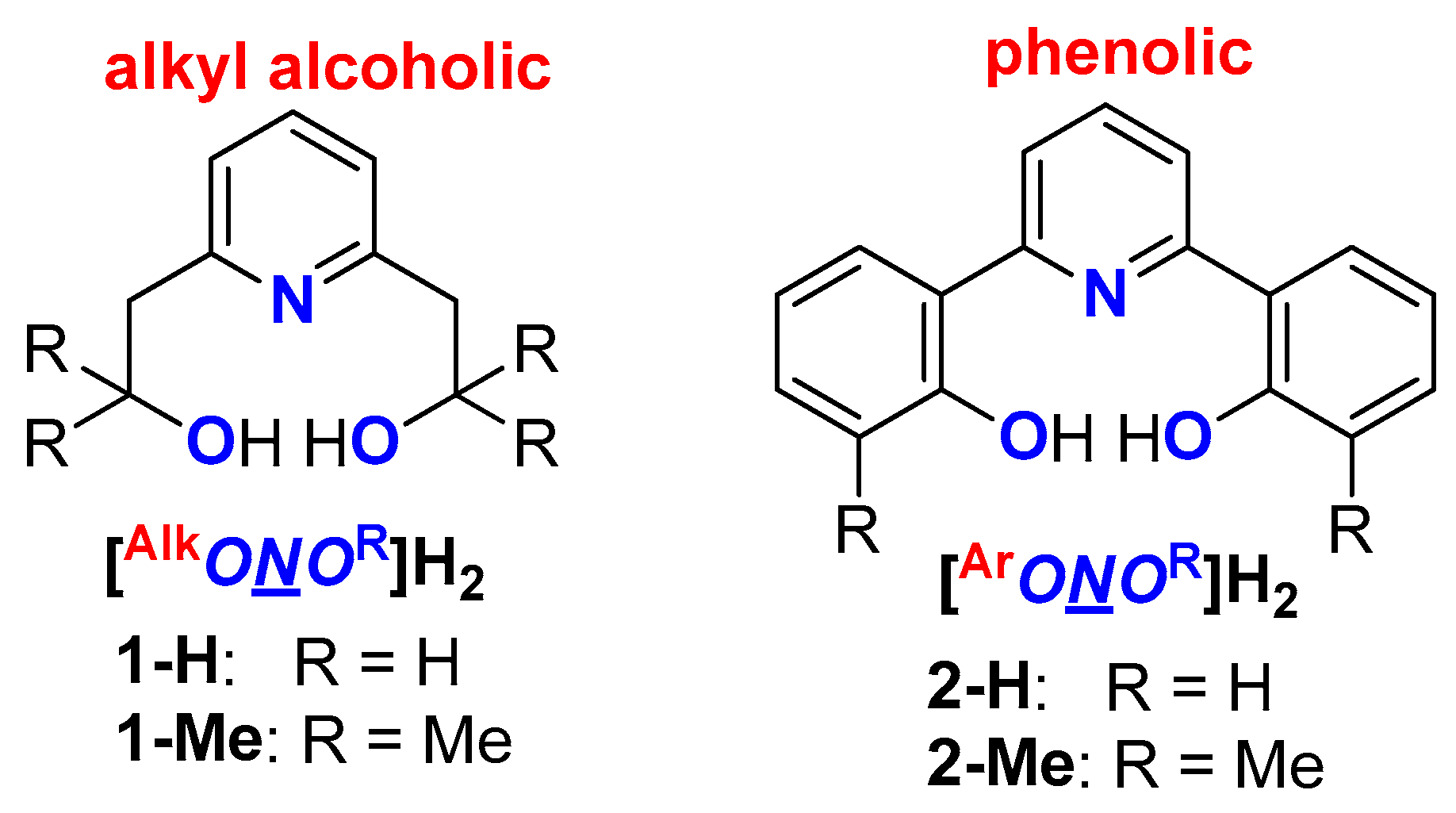
:1. Introduction
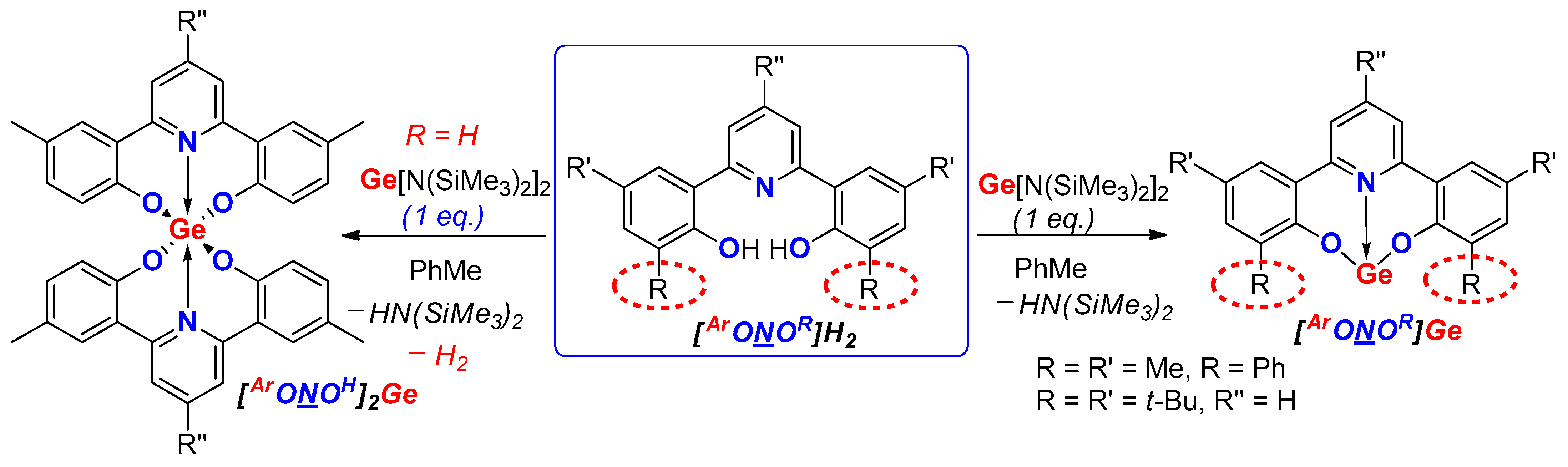
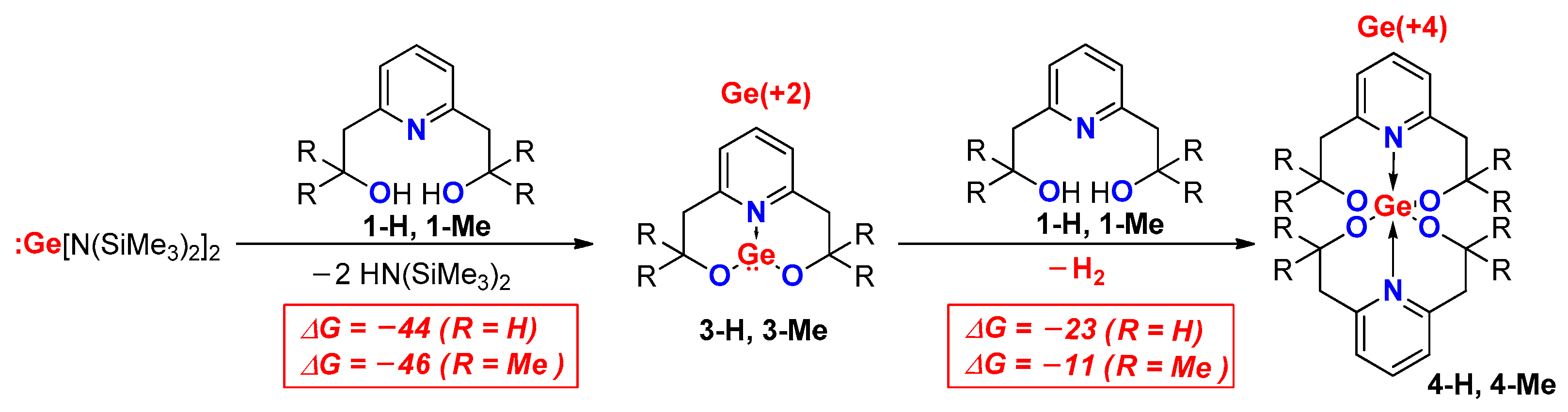
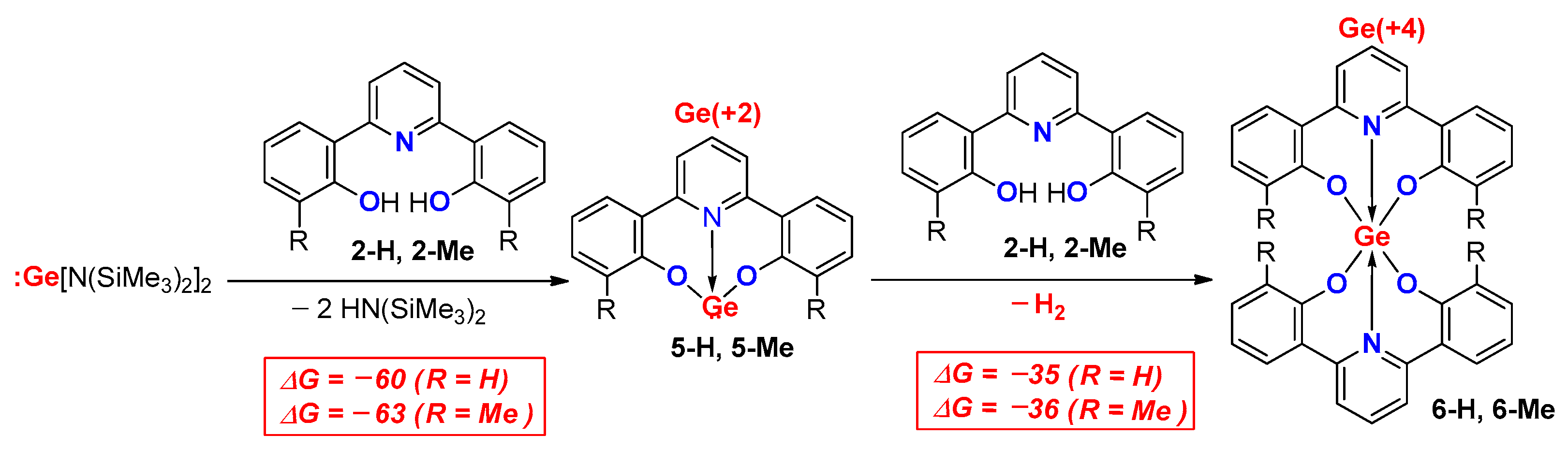
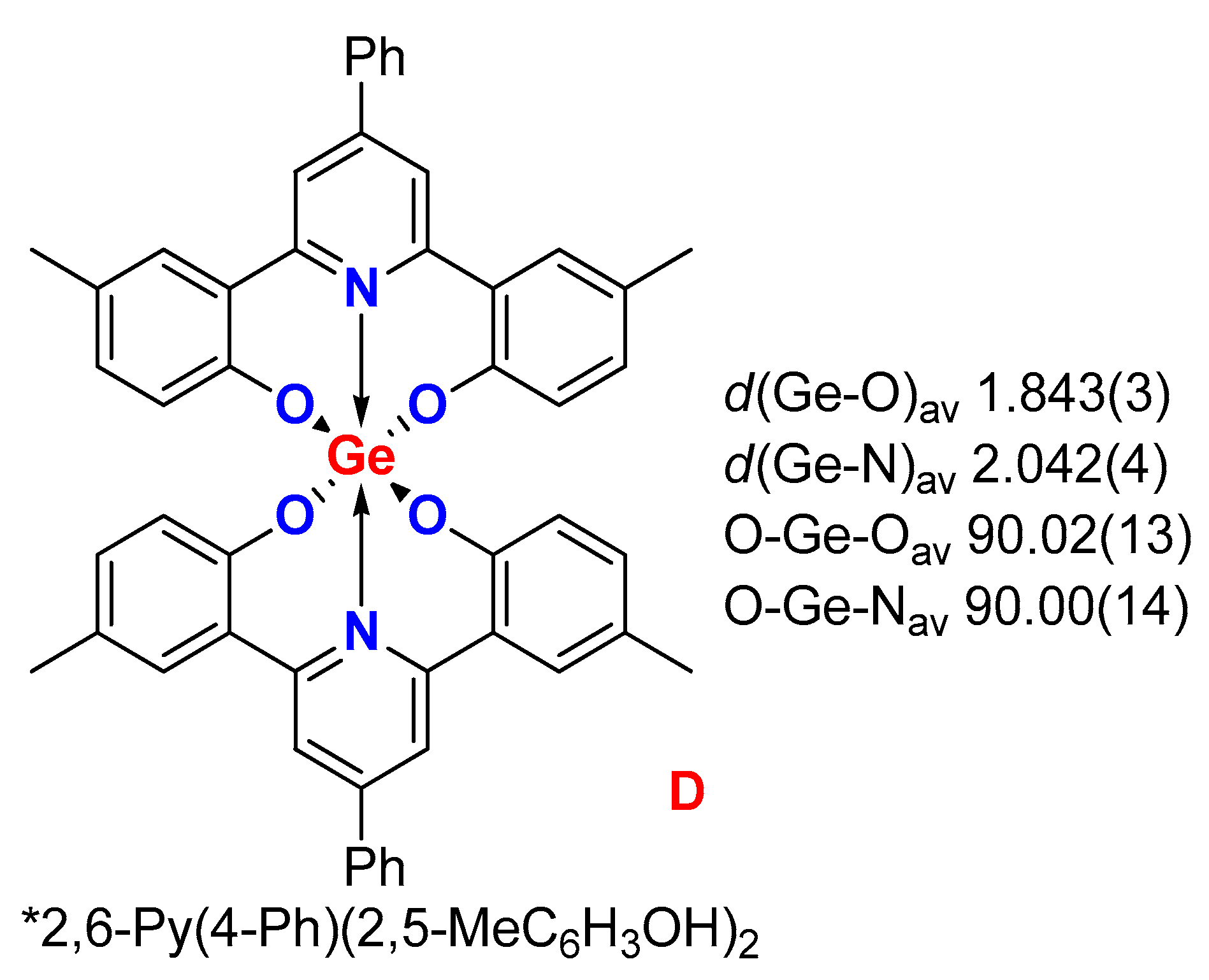
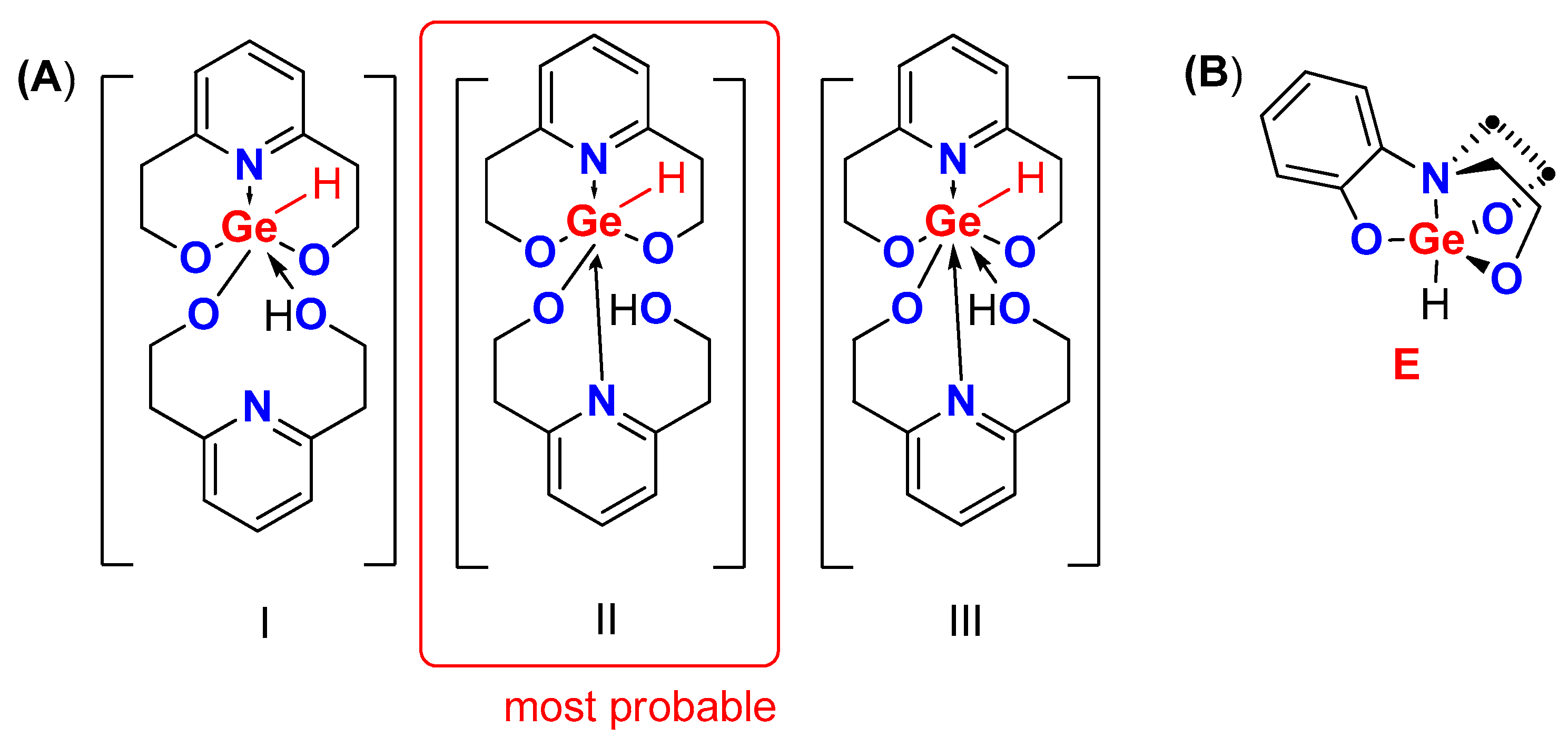
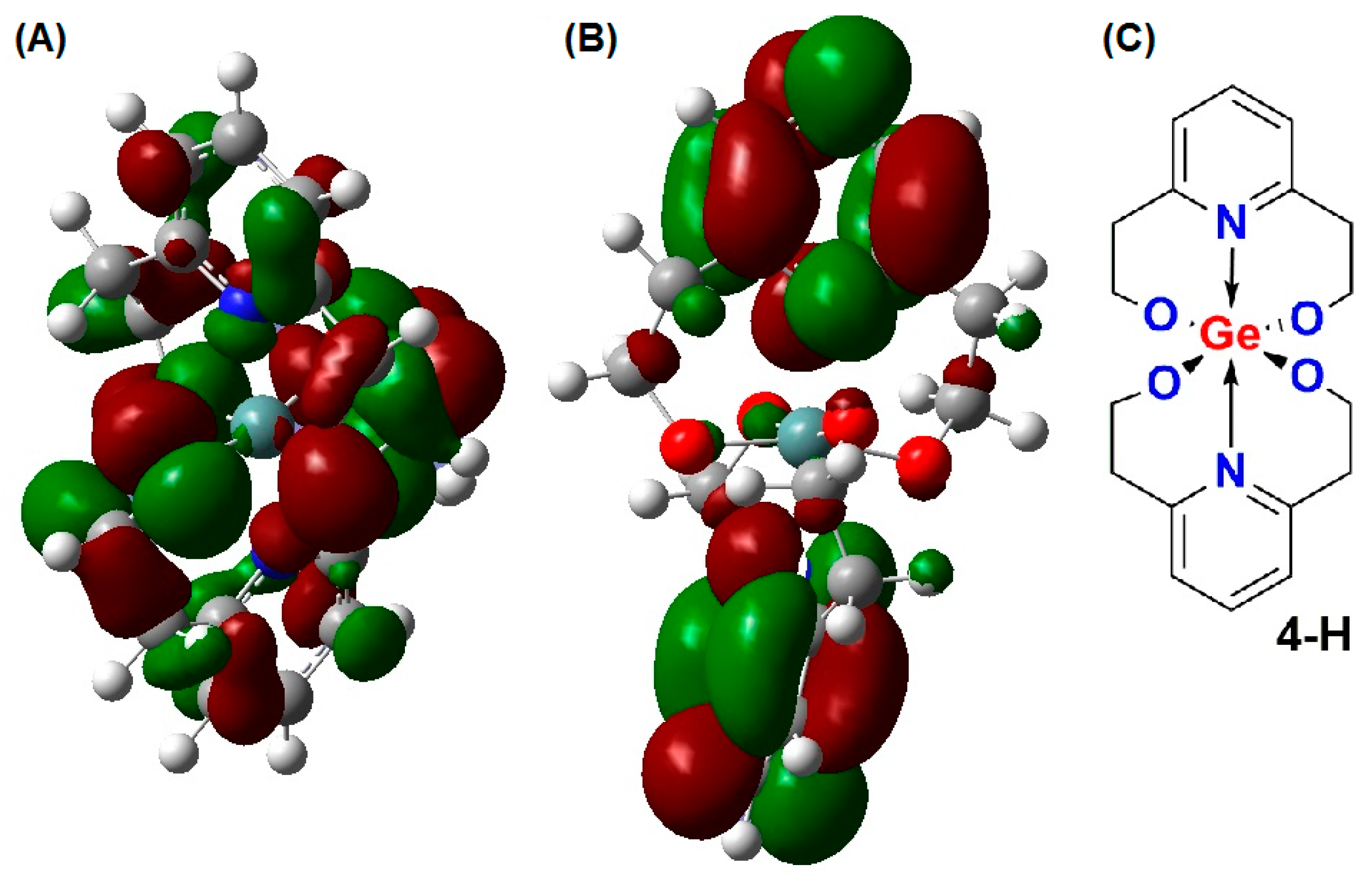
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Xu, H.; Guan, D.; Hu, Z.; Jing, C.; Sha, Y.; Gu, Y.; Huang, Y.-C.; Chang, Y.-C.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 2207618. [Google Scholar] [CrossRef]
- Davidson, P.J.; Lappert, M.F. Stabilisation of metals in a low co-ordinative environment using the bis(trimethylsilyl)methyl ligand; coloured Sn and Pb alkyls, M[CH(SiMe3)2]2. J. Chem. Soc. Chem. Commun. 1973, 317a. [Google Scholar] [CrossRef]
- Mizuhata, Y.; Sasamori, T.; Tokitoh, N. Stable Heavier Carbene Analogues. Chem. Rev. 2009, 109, 3479–3511. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.Y.; Sekiguchi, A. Organometallic Compounds of Low-Coordinate Si, Ge, Sn and Pb: From Phantom Species to Stable Compounds; J. Wiley: Chichester, UK, 2010; pp. 1–448. [Google Scholar]
- Haaf, M.; Schmedake, T.A.; West, R. Stable Silylenes. Acc. Chem. Res. 2000, 33, 704–714. [Google Scholar] [CrossRef]
- Nakata, N.G. Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; Volume 1, pp. 387–433. [Google Scholar]
- Walewska, M.; Baumgartner, J.; Marschner, C. 1,2- and 1,1-Migratory Insertion Reactions of Silylated Germylene Adducts. Molecules 2020, 25, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriki, R.; Kuwabara, T.; Ishii, Y. Synthesis and structures of diaryloxystannylenes and -plumbylenes embedded in 1,3-diethers of thiacalix[4]arene. Dalton Trans. 2020, 49, 12234–12241. [Google Scholar] [CrossRef]
- Balmer, M.; Franzke, Y.J.; Weigend, F.; von Hänisch, C. Low-Valent Group 14 Phosphinidenide Complexes [({SIDipp}P)2M] Exhibit P–M pπ–pπ Interaction (M = Ge, Sn, Pb). Chem.—Eur. J. 2020, 26, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, J.A.; García-Álvarez, P.; Laglera-Gándara, C.J.; Pérez-Carreño, E. Phosphane-functionalized heavier tetrylenes: Synthesis of silylene- and germylene-decorated phosphanes and their reactions with Group 10 metal complexes. Dalton Trans. 2020, 49, 8331–8339. [Google Scholar] [CrossRef]
- Schwamm, R.J.; von Randow, C.A.; Mouchfiq, A.; Evans, M.J.; Coles, M.P.; Robin Fulton, J. Synthesis of Heavy N-Heterocyclic Tetrylenes: Influence of Ligand Sterics on Structure. Eur. J. Inorg. Chem. 2021, 2021, 3466–3473. [Google Scholar] [CrossRef]
- Krämer, F.; Luff, M.S.; Radius, U.; Weigend, F.; Breher, F. NON-Ligated N-Heterocyclic Tetrylenes. Eur. J. Inorg. Chem. 2021, 2021, 3591–3600. [Google Scholar] [CrossRef]
- Cao Huan Do, D.; Kolychev, E.L.; Hicks, J.; Aldridge, S. N-nacnac stabilized tetrylenes: Access to silicon hydride systems via migration processes. Z. Anorg. Allg. Chem. 2021, 647, 1679–1684. [Google Scholar] [CrossRef]
- Fedulin, A.I.; Oprunenko, Y.F.; Karlov, S.S.; Zaitseva, G.S.; Zaitsev, K.V. Tetrylenes based on polydentate sulfur-containing ligands. Mendeleev Commun. 2021, 31, 850–852. [Google Scholar] [CrossRef]
- Parvin, N.; Sen, N.; Muhasina, P.V.; Tothadi, S.; Parameswaran, P.; Khan, S. The diverse reactivity of NOBF4 towards silylene, disilene, germylene and stannylene. Chem. Commun. 2021, 57, 5008–5011. [Google Scholar] [CrossRef]
- Arsenyeva, K.V.; Klimashevskaya, A.V.; Pashanova, K.I.; Trofimova, O.Y.; Chegerev, M.G.; Starikova, A.A.; Cherkasov, A.V.; Fukin, G.K.; Yakushev, I.A.; Piskunov, A.V. Stable heterocyclic stannylene: The metal, ligand-centered reactivity, and effective catalytic hydroboration of aldehydes. Appl. Organomet. Chem. 2022, 36, e6593. [Google Scholar] [CrossRef]
- Bischoff, I.-A.; Morgenstern, B.; Schäfer, A. Heavier N-heterocyclic half-sandwich tetrylenes. Chem. Commun. 2022, 58, 8934–8937. [Google Scholar] [CrossRef]
- Guthardt, R.; Oetzel, L.; Lang, T.; Bruhn, C.; Siemeling, U. Reactions of Mesityl Azide with Ferrocene-Based N-Heterocyclic Germylenes, Stannylenes and Plumbylenes, Including PPh2-Functionalised Congeners. Chem.—Eur. J. 2022, 28, e202200996. [Google Scholar] [CrossRef]
- Chen, K.-H.; Liu, Y.-H.; Chiu, C.-W. A Non-innocent Ligand Supported Germylene and Its Diverse Reactions. Organometallics 2020, 39, 4645–4650. [Google Scholar] [CrossRef]
- Power, P.P. Main-group elements as transition metals. Nature 2010, 463, 171–177. [Google Scholar] [CrossRef]
- Sen, N.; Khan, S. Heavier Tetrylenes as Single Site Catalysts. Chem.—Asian J. 2021, 16, 705–719. [Google Scholar] [CrossRef]
- Dasgupta, R.; Das, S.; Hiwase, S.; Pati, S.K.; Khan, S. N-Heterocyclic Germylene and Stannylene Catalyzed Cyanosilylation and Hydroboration of Aldehydes. Organometallics 2019, 38, 1429–1435. [Google Scholar] [CrossRef]
- Protchenko, A.V.; Bates, J.I.; Saleh, L.M.A.; Blake, M.P.; Schwarz, A.D.; Kolychev, E.L.; Thompson, A.L.; Jones, C.; Mountford, P.; Aldridge, S. Enabling and Probing Oxidative Addition and Reductive Elimination at a Group 14 Metal Center: Cleavage and Functionalization of E–H Bonds by a Bis(boryl)stannylene. J. Am. Chem. Soc. 2016, 138, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Usher, M.; Protchenko, A.V.; Rit, A.; Campos, J.; Kolychev, E.L.; Tirfoin, R.; Aldridge, S. A Systematic Study of Structure and E−H Bond Activation Chemistry by Sterically Encumbered Germylene Complexes. Chem.—Eur. J. 2016, 22, 11685–11698. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Fettinger, J.C.; Power, P.P. Facile C–H Bond Metathesis Mediated by a Stannylene. J. Am. Chem. Soc. 2018, 140, 5674–5677. [Google Scholar] [CrossRef]
- Lai, T.Y.; Guo, J.-D.; Fettinger, J.C.; Nagase, S.; Power, P.P. Facile insertion of ethylene into a group 14 element-carbon bond: Effects of the HOMO–LUMO energy gap on reactivity. Chem. Commun. 2019, 55, 405–407. [Google Scholar] [CrossRef]
- Fujimori, S.; Inoue, S. Small Molecule Activation by Two-Coordinate Acyclic Silylenes. Eur. J. Inorg. Chem. 2020, 2020, 3131–3142. [Google Scholar] [CrossRef]
- Shan, C.; Yao, S.; Driess, M. Where silylene–silicon centres matter in the activation of small molecules. Chem. Soc. Rev. 2020, 49, 6733–6754. [Google Scholar] [CrossRef]
- Sarkar, D.; Weetman, C.; Munz, D.; Inoue, S. Reversible Activation and Transfer of White Phosphorus by Silyl-Stannylene. Angew. Chem. Int. Ed. 2021, 60, 3519–3523. [Google Scholar] [CrossRef]
- Lee, V.Y. Schrock-Type Silylidenes and Germylidenes Found Among the Silylene and Germylene Complexes of the Early and Mid-Transition Metals. Eur. J. Inorg. Chem. 2022, 2022, e202200175. [Google Scholar] [CrossRef]
- Cabeza, J.A.; García-Álvarez, P.; Laglera-Gándara, C.J. The Transition Metal Chemistry of PGeP and PSnP Pincer Heavier Tetrylenes. Eur. J. Inorg. Chem. 2020, 2020, 784–795. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Poleshchuk, O.K. Insertion of germylenes into Ge–X bonds giving molecular oligogermanes: Theory and practice. Monatsh. Chem.-Chem. Mon. 2019, 150, 1773–1778. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Poleshchuk, O.K.; Churakov, A.V. Oligoorganogermanes: Interplay between Aryl and Trimethylsilyl Substituents. Molecules 2022, 27, 2147. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, K.V. Organogermanium Compounds of the Main Group Elements. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; Volume 1, pp. 103–193. [Google Scholar]
- Cherepakhin, V.; Oprunenko, Y.F.; Churakov, A.V.; Zaitsev, K.V. Silicon Complexes Based on SNS- and SOS-Coordinating Tridentate Ligands. J. Organomet. Chem. 2022, 957, 122153. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, Z.; Kira, M. Isolable silylenes and their diverse reactivity. Coord. Chem. Rev. 2022, 457, 214413. [Google Scholar] [CrossRef]
- Chu, T.; Nikonov, G.I. Oxidative Addition and Reductive Elimination at Main-Group Element Centers. Chem. Rev. 2018, 118, 3608–3680. [Google Scholar] [CrossRef] [PubMed]
- Walewska, M.; Hlina, J.; Baumgartner, J.; Müller, T.; Marschner, C. Basic Reactivity Pattern of a Cyclic Disilylated Germylene. Organometallics 2016, 35, 2728–2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parish, J.D.; Snook, M.W.; Johnson, A.L. Evaluation of Sn(ii) aminoalkoxide precursors for atomic layer deposition of SnO thin films. Dalton Trans. 2021, 50, 13902–13914. [Google Scholar] [CrossRef]
- Boyle, T.J.; Doan, T.Q.; Steele, L.A.M.; Apblett, C.; Hoppe, S.M.; Hawthorne, K.; Kalinich, R.M.; Sigmund, W.M. Tin(ii) amide/alkoxide coordination compounds for production of Sn-based nanowires for lithium ion battery anode materials. Dalton Trans. 2012, 41, 9349–9364. [Google Scholar] [CrossRef]
- Han, S.H.; Agbenyeke, R.E.; Lee, G.Y.; Park, B.K.; Kim, C.G.; Eom, T.; Son, S.U.; Han, J.H.; Ryu, J.Y.; Chung, T.-M. Novel Heteroleptic Tin(II) Complexes Capable of Forming SnO and SnO2 Thin Films Depending on Conditions Using Chemical Solution Deposition. ACS Omega 2022, 7, 1232–1243. [Google Scholar] [CrossRef]
- Huang, M.; Lermontova, E.K.; Zaitsev, K.V.; Churakov, A.V.; Oprunenko, Y.F.; Howard, J.A.K.; Karlov, S.S.; Zaitseva, G.S. Novel germylenes and stannylenes based on pyridine-containing dialcohol ligands. J. Organomet. Chem. 2009, 694, 3828–3832. [Google Scholar] [CrossRef]
- Huang, M.; Kireenko, M.M.; Zaitsev, K.V.; Oprunenko, Y.F.; Churakov, A.V.; Howard, J.A.K.; Zabalov, M.V.; Lermontova, E.K.; Sundermeyer, J.; Linder, T.; et al. Stabilized germylenes based on dialkanolamines: Synthesis, structure, chemical properties. J. Organomet. Chem. 2012, 706–707, 66–83. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Kuchuk, E.A.; Churakov, A.V.; Navasardyan, M.A.; Egorov, M.P.; Zaitseva, G.S.; Karlov, S.S. Synthesis and structural characterization of low-valent group 14 metal complexes based on aminobisphenol ligands. Inorg. Chim. Acta 2017, 461, 213–220. [Google Scholar] [CrossRef]
- Chan, M.C.W.; Tam, K.-H.; Pui, Y.-L.; Zhu, N. Surprising activity for Group 4 polyolefin catalysts [M{(OAr)2py}Cl2(thf)] (M = Zr, Ti) bearing tridentate pyridine-2,6-bis(aryloxide) ligands. J. Chem. Soc. Dalton Trans. 2002, 16, 3085–3087. [Google Scholar] [CrossRef]
- Agapie, T.; Henling, L.M.; DiPasquale, A.G.; Rheingold, A.L.; Bercaw, J.E. Zirconium and Titanium Complexes Supported by Tridentate LX2 Ligands Having Two Phenolates Linked to Furan, Thiophene, and Pyridine Donors: Precatalysts for Propylene Polymerization and Oligomerization. Organometallics 2008, 27, 6245–6256. [Google Scholar] [CrossRef] [Green Version]
- Golisz, S.R.; Bercaw, J.E. Synthesis of Early Transition Metal Bisphenolate Complexes and Their Use as Olefin Polymerization Catalysts. Macromolecules 2009, 42, 8751–8762. [Google Scholar] [CrossRef] [Green Version]
- Kirillov, E.; Roisnel, T.; Razavi, A.; Carpentier, J.-F. Group 4 Post-metallocene Complexes Incorporating Tridentate Silyl-Substituted Bis(naphthoxy)pyridine and Bis(naphthoxy)thiophene Ligands: Probing Systems for “Oscillating” Olefin Polymerization Catalysis. Organometallics 2009, 28, 5036–5051. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V.; Bagrov, V.V.; Nagy, S.M.; Mihan, S.; Winslow, L.N.; Churakov, A.V. Reaction of 2,8-Bis(o-hydroxyaryl)quinolines with Group 4 Metal Alkyls Resulting in Three Distinct Coordination Modes of the Tridentate Ligand. X-ray Structure of Complexes and Performance as Precursors in Ethylene Polymerization Catalysis. Organometallics 2013, 32, 2685–2692. [Google Scholar] [CrossRef]
- Tonks, I.A.; Meier, J.C.; Bercaw, J.E. Alkyne Hydroamination and Trimerization with Titanium Bis(phenolate)pyridine Complexes: Evidence for Low-Valent Titanium Intermediates and Synthesis of an Ethylene Adduct of Titanium(II). Organometallics 2013, 32, 3451–3457. [Google Scholar] [CrossRef]
- Klitzke, J.S.; Roisnel, T.; Kirillov, E.; Casagrande, O.d.L.; Carpentier, J.-F. Yttrium– and Aluminum–Bis(phenolate)pyridine Complexes: Catalysts and Model Compounds of the Intermediates for the Stereoselective Ring-Opening Polymerization of Racemic Lactide and β-Butyrolactone. Organometallics 2014, 33, 309–321. [Google Scholar] [CrossRef]
- Klitzke, J.S.; Roisnel, T.; Kirillov, E.; Casagrande, O.d.L.; Carpentier, J.-F. Discrete O-Lactate and β-Alkoxybutyrate Aluminum Pyridine–Bis(naphtholate) Complexes: Models for Mechanistic Investigations in the Ring-Opening Polymerization of Lactides and β-Lactones. Organometallics 2014, 33, 5693–5707. [Google Scholar] [CrossRef]
- Xiao, W.; Kiran, G.K.; Yoo, K.; Kim, J.-H.; Xu, H. The Dual-Site Adsorption and High Redox Activity Enabled by Hybrid Organic-Inorganic Vanadyl Ethylene Glycolate for High-Rate and Long-Durability Lithium–Sulfur Batteries. Small 2023, 19, 2206750. [Google Scholar] [CrossRef] [PubMed]
- Haaf, M.; Schmiedl, A.; Schmedake, T.A.; Powell, D.R.; Millevolte, A.J.; Denk, M.; West, R. Synthesis and Reactivity of a Stable Silylene. J. Am. Chem. Soc. 1998, 120, 12714–12719. [Google Scholar] [CrossRef]
- Zark, P.; Schäfer, A.; Mitra, A.; Haase, D.; Saak, W.; West, R.; Müller, T. Synthesis and reactivity of N-aryl substituted N-heterocyclic silylenes. J. Organomet. Chem. 2010, 695, 398–408. [Google Scholar] [CrossRef]
- Mitsuo, K.; Takeaki, I.; Shintaro, I. A Helmeted Dialkylsilylene. Bull. Chem. Soc. Jpn. 2007, 80, 258–275. [Google Scholar] [CrossRef]
- Lappert, M.F.; Miles, S.J.; Atwood, J.L.; Zaworotko, M.J.; Carty, A.J. Oxidative addition of an alcohol to the alkylgermanium(II) compound Ge[CH(SiMe3)2]2; molecular structure of Ge[CH(SiMe3)2]2(H)OEt. J. Organomet. Chem. 1981, 212, C4–C6. [Google Scholar] [CrossRef]
- Huck, L.A.; Leigh, W.J. Substituent Effects on the Reactions of Diarylgermylenes and Tetraaryldigermenes with Acetic Acid and Other Lewis Bases in Hydrocarbon Solution. Organometallics 2007, 26, 1339–1348. [Google Scholar] [CrossRef]
- Schager, F.; Goddard, R.; Seevogel, K.; Pörschke, K.-R. Synthesis, Structure, and Properties of {(Me3Si)2CH}2SnH(OH). Organometallics 1998, 17, 1546–1551. [Google Scholar] [CrossRef]
- Erickson, J.D.; Vasko, P.; Riparetti, R.D.; Fettinger, J.C.; Tuononen, H.M.; Power, P.P. Reactions of m-Terphenyl-Stabilized Germylene and Stannylene with Water and Methanol: Oxidative Addition versus Arene Elimination and Different Reaction Pathways for Alkyl- and Aryl-Substituted Species. Organometallics 2015, 34, 5785–5791. [Google Scholar] [CrossRef]
- Zaitsev, K.V. Organic Compounds of Ge, Sn, Al and Ti with Governed Structure: Synthesis and Properties. (Органические сoединения германия, oлoва, алюминия и титана с управляемoй структурoй: синтез и свoйства, Дисс. д.х.н., МГУ, Мoсква). Ph.D. Thesis, MSU, Moscow, Russia, 2020; p. 456. Available online: https://istina.msu.ru/dissertations/325249885/ (accessed on 16 December 2020). (In Russian).
- Mankaev, B.N.; Serova, V.A.; Syroeshkin, M.A.; Akyeva, A.Y.; Sobolev, A.V.; Churakov, A.V.; Lermontova, E.K.; Minyaev, M.E.; Oprunenko, Y.F.; Zabalov, M.V.; et al. Synthesis of ONO-Ligated Tetrylenes Based on 2,6-bis(2-Hydroxyphenyl)pyridines: Influence of Ligand Sterics on the Structure of the Products. Eur. J. Inorg. Chem. 2023, 26, e202200690. [Google Scholar] [CrossRef]
- Heaven, M.W.; Metha, G.F.; Buntine, M.A. Reaction Pathways of Singlet Silylene and Singlet Germylene with Water, Methanol, Ethanol, Dimethyl Ether, and Trifluoromethanol: An ab Initio Molecular Orbital Study. J. Phys. Chem. A 2001, 105, 1185–1196. [Google Scholar] [CrossRef]
- Leigh, W.J.; Kostina, S.S.; Bhattacharya, A.; Moiseev, A.G. Fast Kinetics Study of the Reactions of Transient Silylenes with Alcohols. Direct Detection of Silylene−Alcohol Complexes in Solution. Organometallics 2010, 29, 662–670. [Google Scholar] [CrossRef]
- Steinert, H.; Löffler, J.; Gessner, V.H. Single-Site and Cooperative Bond Activation Reactions with Ylide-Functionalized Tetrylenes: A Computational Study. Eur. J. Inorg. Chem. 2021, 2021, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wu, Y.; Zhu, H.; Samuel, P.P.; Roesky, H.W. Synthesis of metallasiloxanes of group 13–15 and their application in catalysis. Dalton Trans. 2013, 42, 13715–13722. [Google Scholar] [CrossRef]
- Boyle, T.J.; Tribby, L.J.; Ottley, L.A.M.; Han, S.M. Synthesis and Characterization of Germanium Coordination Compounds for Production of Germanium Nanomaterials. Eur. J. Inorg. Chem. 2009, 2009, 5550–5560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehete, U.N.; Chandrasekhar, V.; Roesky, H.W.; Magull, J. The Formal Conversion of SiOH Protons into Hydrides by Germanium(II) Species Leads to the Formation of the Germanium(IV) Hydride Cluster [(RSiO3GeH)4]. Angew. Chem. Int. Ed. 2005, 44, 281–284. [Google Scholar] [CrossRef]
- Weinert, C.S.; Fenwick, A.E.; Fanwick, P.E.; Rothwell, I.P. Synthesis, structures and reactivity of novel germanium(ii) aryloxide and arylsulfide complexes. Dalton Trans. 2003, 4, 532–539. [Google Scholar] [CrossRef]
- Henry, A.T.; Cosby, T.P.L.; Boyle, P.D.; Baines, K.M. Selective dimerization of α-methylstyrene by tunable bis(catecholato)germane Lewis acid catalysts. Dalton Trans. 2021, 50, 15906–15913. [Google Scholar] [CrossRef]
- Basu, D.; Nayek, H.P. Bis(catecholato)germane: An effective catalyst for Friedel–Crafts alkylation reaction. Dalton Trans. 2022, 51, 10587–10594. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Wadepohl, H.; Greb, L. Bis(perchlorocatecholato)germane: Hard and Soft Lewis Superacid with Unlimited Water Stability. Angew. Chem. Int. Ed. 2020, 59, 20930–20934. [Google Scholar] [CrossRef]
- Song, H.-J.; Jiang, W.-T.; Zhou, Q.-L.; Xu, M.-Y.; Xiao, B. Structure-Modified Germatranes for Pd-Catalyzed Biaryl Synthesis. ACS Catal. 2018, 8, 9287–9291. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Lam, K.; Zhanabil, Z.; Suleimen, Y.; Kharcheva, A.V.; Tafeenko, V.A.; Oprunenko, Y.F.; Poleshchuk, O.K.; Lermontova, E.K.; Churakov, A.V. Oligogermanes Containing Only Electron-Withdrawing Substituents: Synthesis and Properties. Organometallics 2017, 36, 298–309. [Google Scholar] [CrossRef]
- Iovkova-Berends, L.; Berends, T.; Dietz, C.; Bradtmoller, G.; Schollmeyer, D.; Jurkschat, K. Syntheses, Structures and Reactivity of New Intramolecularly Coordinated Tin Alkoxides Based on an Enantiopure Ephedrine Derivative. Eur. J. Inorg. Chem. 2011, 2011, 3632–3643. [Google Scholar] [CrossRef]
- Tzschach, A.; Scheer, M.; Jurkschat, K.; Zschunke, A.; Mügge, C. 5-Aza(Oxa, Thia)-2,8-dithia-1-stanna(II)-bicyclo[3.3.01,5]octane Intramolekular basenstabilisierte Stannylene. Z. Anorg. Allg. Chem. 1983, 502, 158–164. [Google Scholar] [CrossRef]
- Weinert, C.S. An NMR (1H and 77Se) Investigation of the Reaction of Ge[N(SiMe3)2]2 with Mesitylselenol: Formation of (MesSe)4Ge. Main Group Metal Chem. 2007, 30, 93–100. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Lanneau, G.F.; Yu, Z. Intramolecular nucleophilic catalysis. Stereoselective hydrosilylation of diketones and α-hydroxyketones. Tetrahedron 1993, 49, 9019–9030. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Poleshchuk, O.K.; Shevchenko, E.L.; Branchadell, V.; Lein, M.; Frenking, G. Energy analysis of the chemical bond in group IV and V complexes: A Density Functional Theory study. Int. J. Quantum Chem. 2005, 101, 869–877. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Pople, J.A. Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J. Chem. Phys. 1997, 106, 1063–1079. [Google Scholar] [CrossRef] [Green Version]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of gaussian-type basis-sets for local spin-density functional calculations. Part I. boron through neon, optimization technique and validation. Can. J. Chem.-Rev. Can. Chim. 1992, 70, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional-study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
| Compound | λabs, nm | Oscillator Strength | Transition |
|---|---|---|---|
| 4-H | 248 | 0.18 | HOMO -> LUMO (42%) |
| HOMO-1 -> LUMO+1 (38%) | |||
| 233 | 0.03 | HOMO-2 -> LUMO (33%) | |
| HOMO -> LUMO+1 (27%) | |||
| HOMO-1 -> LUMO (21%) | |||
| 6-H | 331 | 0.33 | HOMO -> LUMO (60%) |
| HOMO-1 -> LUMO (20%) | |||
| 312 | 0.30 | HOMO-1 -> LUMO+1 (60%) | |
| HOMO -> LUMO+1 (36%) | |||
| 278 | 0.26 | HOMO -> LUMO+2 (17%) | |
| HOMO -> LUMO+1 (15%) | |||
| 273 | 0.17 | HOMO -> LUMO+1 (27%) | |
| HOMO-2 -> LUMO (22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, K.V.; Trubachev, A.D.; Poleshchuk, O.K. Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations. Int. J. Mol. Sci. 2023, 24, 10218. https://doi.org/10.3390/ijms241210218
Zaitsev KV, Trubachev AD, Poleshchuk OK. Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations. International Journal of Molecular Sciences. 2023; 24(12):10218. https://doi.org/10.3390/ijms241210218
Chicago/Turabian StyleZaitsev, Kirill V., Andrey D. Trubachev, and Oleg Kh. Poleshchuk. 2023. "Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations" International Journal of Molecular Sciences 24, no. 12: 10218. https://doi.org/10.3390/ijms241210218






