Identification and Classification of Long Non-Coding RNAs in the Mammary Gland of the Holstein Cow
Abstract
:1. Introduction
2. Results
2.1. Characterization and Identification of Long Non-Coding RNAs
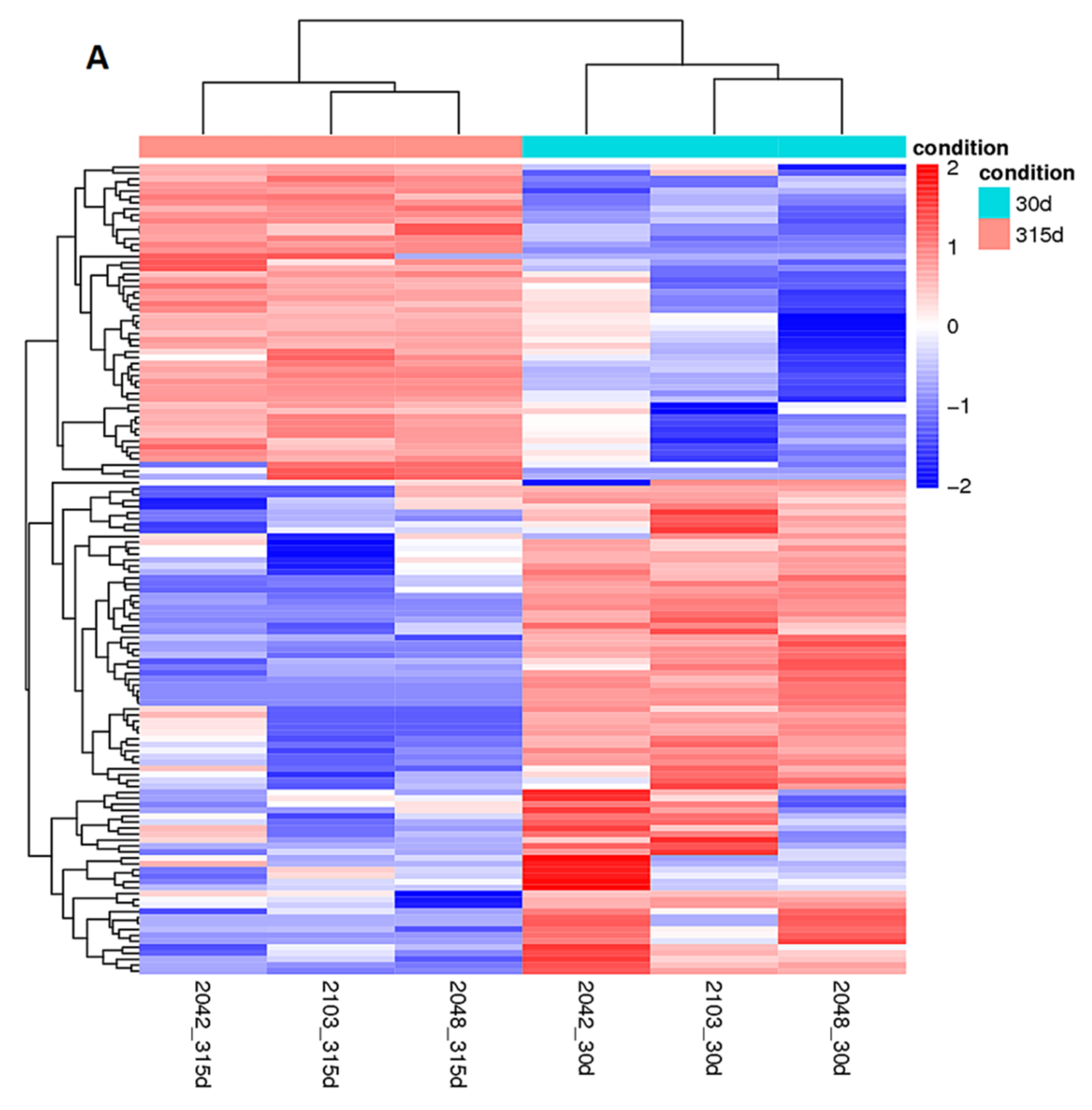
2.2. Analysis of Different lncRNA Expression
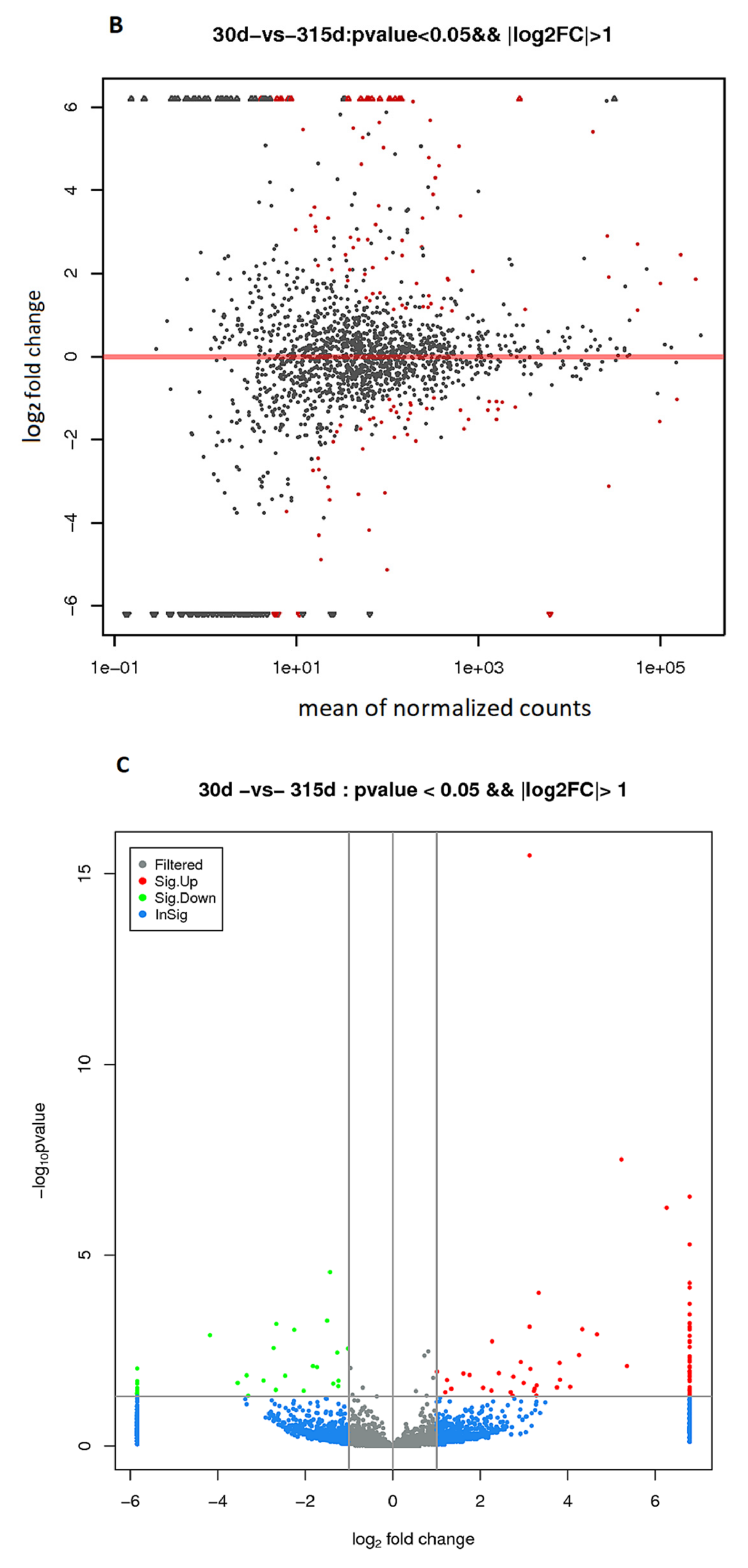
2.3. Differential Expression of Long Non-Coding RNA Genes in Holstein Cows during Lactation
2.4. Differentially Expressed lncRNA and mRNA Co-Expression Analysis
2.5. Differentially Expressed lncRNA Target Genes and Functional Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Ethics Statement
4.2. Animal Sample Collection
4.3. RNA Extraction and Sequencing
4.4. Identification and Expression Level of lncRNAs
4.5. Analysis of the Co-Expression of Differentially Expressed mRNAs and lncRNAs
4.6. Prediction of the Target Gene and Functional Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| lncRNAs | long non-coding RNAs |
| ncRNAs | non-coding RNA |
| MAPK4 | Mitogen-activated protein kinases4 |
| TLR2 | Toll-like receptor 2 |
| AMPK | activated protein kinase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| PCA | principal component analysis |
| WTSS | whole transcriptome sequencing studies |
| mRNAs | protein-coding messenger RNAs |
References
- Strucken, E.M.; Laurenson, Y.C.S.M.; Brockmann, G.A. Go with the Flow—Biology and Genetics of the Lactation Cycle. Front. Genet. 2015, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.R.; Hernandez, L.L. Autocrine-Paracrine Regulation of the Mammary Gland. J. Dairy Sci. 2016, 99, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gao, Q.; Wang, H.; Guo, M.; Arbab, A.A.I.; Nazar, M.; Li, M.; Yang, Z.; Karrow, N.A.; Mao, Y. Identification and Characterization of Circular RNAs in Mammary Tissue from Holstein Cows at Early Lactation and Non-Lactation. Biomolecules 2022, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sahana, G.; Su, G.; Yu, Y.; Zhang, S.; Lund, M.S.; Sørensen, P. Integrating Sequence-Based GWAS and RNA-Seq Provides Novel Insights into the Genetic Basis of Mastitis and Milk Production in Dairy Cattle. Sci. Rep. 2017, 7, 45560. [Google Scholar] [CrossRef]
- Wang, X.; Ma, P.; Liu, J.; Zhang, Q.; Zhang, Y.; Ding, X.; Jiang, L.; Wang, Y.; Zhang, Y.; Sun, D.; et al. Genome-Wide Association Study in Chinese Holstein Cows Reveal Two Candidate Genes for Somatic Cell Score as an Indicator for Mastitis Susceptibility. BMC Genet. 2015, 16, 111. [Google Scholar] [CrossRef]
- Bakhtiarizadeh, M.R.; Mirzaei, S.; Norouzi, M.; Sheybani, N.; Vafaei Sadi, M.S. Identification of Gene Modules and Hub Genes Involved in Mastitis Development Using a Systems Biology Approach. Front. Genet. 2020, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.M.; Ibeagha, A.E.; Messier, S.; Zhao, X. Proteomics, Genomics, and Pathway Analyses of Escherichia Coli and Staphylococcus Aureus Infected Milk Whey Reveal Molecular Pathways and Networks Involved in Mastitis. J. Proteome Res. 2010, 9, 4604–4619. [Google Scholar] [CrossRef]
- Jørgensen, H.B.H.; Buitenhuis, B.; Røntved, C.M.; Jiang, L.; Ingvartsen, K.L.; Sørensen, P. Transcriptional Profiling of the Bovine Hepatic Response to Experimentally Induced E. coli Mastitis. Physiol. Genom. 2012, 44, 595–606. [Google Scholar] [CrossRef]
- Barrett, D.J.; Healy, A.M.; Leonard, F.C.; Doherty, M.L. Prevalence of Pathogens Causing Subclinical Mastitis in 15 Dairy Herds in the Republic of Ireland. Ir. Vet. J. 2005, 58, 333–337. [Google Scholar] [CrossRef]
- Al-harbi, H.; Ranjbar, S.; Moore, R.J.; Alawneh, J.I. Bacteria Isolated from Milk of Dairy Cows with and without Clinical Mastitis in Different Regions of Australia and Their AMR Profiles. Front. Vet. Sci. 2021, 8, 743725. [Google Scholar] [CrossRef]
- Kabir, M.H.; Ershaduzzaman, M.; Giasuddin, M.; Nazir, K.H.M.N.H.; Mahmud, M.M.; Islam, M.R.; Islam, M.S.; Karim, M.R.; Yousuf, M.A.; Rahman, S.M.; et al. Prevalence and Molecular Detection of the Causal Agents of Sub-Clinical Mastitis in Dairy Cows in Sirajganj and Pabna Districts, Bangladesh. J. Adv. Vet. Anim. Res. 2017, 4, 378–384. [Google Scholar] [CrossRef]
- Acharya, K.R.; Brankston, G.; Slavic, D.; Greer, A.L. Spatio-Temporal Variation in the Prevalence of Major Mastitis Pathogens Isolated from Bovine Milk Samples between 2008 and 2017 in Ontario, Canada. Front. Vet. Sci. 2021, 8, 742696. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Xu, H.; Zhang, C.; Chen, S.; Liu, F.; Guan, S.; Zhang, S.; Zhu, K.; Wu, C. The Prevalence of Pathogens Causing Bovine Mastitis and Their Associated Risk Factors in 15 Large Dairy Farms in China: An Observational Study. Vet. Microbiol. 2020, 247, 108757. [Google Scholar] [CrossRef]
- Mbindyo, C.M.; Gitao, G.C.; Mulei, C.M. Prevalence, Etiology, and Risk Factors of Mastitis in Dairy Cattle in Embu and Kajiado Counties, Kenya. Vet. Med. Int. 2020, 2020, 8831172. [Google Scholar] [CrossRef]
- Ali, T.; Kamran; Raziq, A.; Wazir, I.; Ullah, R.; Shah, P.; Ali, M.I.; Han, B.; Liu, G. Prevalence of Mastitis Pathogens and Antimicrobial Susceptibility of Isolates from Cattle and Buffaloes in Northwest of Pakistan. Front. Vet. Sci. 2021, 8, 746755. [Google Scholar] [CrossRef]
- Pascu, C.; Herman, V.; Iancu, I.; Costinar, L. Etiology of Mastitis and Antimicrobial Resistance in Dairy Cattle Farms in the Western Part of Romania. Antibiotics 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Q.; Zhu, W.-J.; Gao, P. New Insights into Long Non-Coding RNAs in Breast Cancer: Biological Functions and Therapeutic Prospects. Exp. Mol. Pathol. 2021, 120, 104640. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique Features of Long Non-Coding RNA Biogenesis and Function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y. Regulation of Oxidative Stress by Long Non-Coding RNAs in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 931704. [Google Scholar] [CrossRef] [PubMed]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: LncRNA Localization and Function. J. Cell Biol. 2021, 220, 202009045. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Egranov, S.D.; Yang, L.; Lin, C. Molecular Mechanisms of Long Noncoding RNAs-mediated Cancer Metastasis. Genes, Chromosom. Cancer 2019, 58, 200–207. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The Functions and Unique Features of Long Intergenic Non-Coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Mattick, J.S. Structure and Function of Long Noncoding RNAs in Epigenetic Regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Dieci, G.; Fiorino, G.; Castelnuovo, M.; Teichmann, M.; Pagano, A. The Expanding RNA Polymerase III Transcriptome. Trends Genet. 2007, 23, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kapranov, P.; Cheng, J.; Dike, S.; Nix, D.A.; Duttagupta, R.; Willingham, A.T.; Stadler, P.F.; Hertel, J.; Hackermüller, J.; Hofacker, I.L.; et al. RNA Maps Reveal New RNA Classes and a Possible Function for Pervasive Transcription. Science 2007, 316, 1484–1488. [Google Scholar] [CrossRef]
- Yin, Q.-F.; Yang, L.; Zhang, Y.; Xiang, J.-F.; Wu, Y.-W.; Carmichael, G.G.; Chen, L.-L. Long Noncoding RNAs with SnoRNA Ends. Mol. Cell 2012, 48, 219–230. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Fang, S.; Kang, Y.; Wu, W.; Hao, Y.; Li, Z.; Bu, D.; Sun, N.; Zhang, M.Q.; et al. NONCODE 2016: An Informative and Valuable Data Source of Long Non-Coding RNAs. Nucleic Acids Res. 2016, 44, D203–D208. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Zhou, Z.; Wang, L.; Liu, Y.; Liu, Y. ALDB: A Domestic-Animal Long Noncoding RNA Database. PLoS ONE 2015, 10, e0124003. [Google Scholar] [CrossRef]
- Weikard, R.; Hadlich, F.; Kuehn, C. Identification of Novel Transcripts and Noncoding RNAs in Bovine Skin by Deep next Generation Sequencing. BMC Genom. 2013, 14, 789. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.; Do, D.; Dudemaine, P.-L.; Fomenky, B.; Bissonnette, N. Integration of LncRNA and MRNA Transcriptome Analyses Reveals Genes and Pathways Potentially Involved in Calf Intestinal Growth and Development during the Early Weeks of Life. Genes 2018, 9, 142. [Google Scholar] [CrossRef]
- Do, D.N.; Dudemaine, P.-L.; Fomenky, B.; Ibeagha-Awemu, E.M. Transcriptome Analysis of Non-Coding RNAs in Livestock Species: Elucidating the Ambiguity. In Applications of RNA-Seq and Omics Strategies—From Microorganisms to Human Health; InTech: London, UK, 2017. [Google Scholar]
- Koufariotis, L.T.; Chen, Y.-P.P.; Chamberlain, A.; Vander Jagt, C.; Hayes, B.J. A Catalogue of Novel Bovine Long Noncoding RNA across 18 Tissues. PLoS ONE 2015, 10, e0141225. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Z.; Jiang, H. MicroRNAs in Farm Animals. Animal 2013, 7, 1567–1575. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Jin, J.; Xia, A.; Wang, C.; Cui, Y.; Qu, B.; Li, Q.; Sheng, C. Comparative Transcriptome Analysis to Investigate the Potential Role of MiRNAs in Milk Protein/Fat Quality. Sci. Rep. 2018, 8, 6250. [Google Scholar] [CrossRef]
- Suravajhala, P.; Benso, A. Prioritizing Single-Nucleotide Polymorphisms and Variants Associated with Clinical Mastitis. Adv. Appl. Bioinform. Chem. 2017, 10, 57–64. [Google Scholar] [CrossRef]
- Huang, W.; Long, N.; Khatib, H. Genome-wide identification and initial characterization of bovine long non-coding RNAs from EST data. Anim. Genet. 2012, 43, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.; Li, R.; Dudemaine, P.-L.; Do, D.; Bissonnette, N. Transcriptome Analysis of Long Non-Coding RNA in the Bovine Mammary Gland Following Dietary Supplementation with Linseed Oil and Safflower Oil. Int. J. Mol. Sci. 2018, 19, 3610. [Google Scholar] [CrossRef]
- Zheng, X.; Ning, C.; Zhao, P.; Feng, W.; Jin, Y.; Zhou, L.; Yu, Y.; Liu, J. Integrated Analysis of Long Noncoding RNA and MRNA Expression Profiles Reveals the Potential Role of Long Noncoding RNA in Different Bovine Lactation Stages. J. Dairy Sci. 2018, 101, 11061–11073. [Google Scholar] [CrossRef]
- Jin, Y.; Ouyang, Y.; Fan, X.; Huang, J.; Guo, W.; Miao, Y. Genome-Wide Identification of Long Noncoding RNA Genes and Their Potential Association with Mammary Gland Development in Water Buffalo. Anim. Biosci. 2022, 35, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Peng, X.; Shen, C. Identification and Validation of Immune-Related LncRNA Prognostic Signature for Breast Cancer. Genomics 2020, 112, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, X.; Huang, W.; Ma, Y.; Ke, H.; Zou, L.; Yang, Q.; Jiao, B. Single-Cell Profiling of Long Noncoding RNAs and Their Cell Lineage Commitment Roles via RNA-DNA-DNA Triplex Formation in Mammary Epithelium. Stem Cells 2020, 38, 1594–1611. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, J.; Wang, Z.; Yao, X.; Zhao, J.; Zhao, X.; Wang, F.; Zhang, Y. Genome-Wide Analysis and Function Prediction of Long Noncoding RNAs in Sheep Pituitary Gland Associated with Sexual Maturation. Genes 2020, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, L.; Wang, L.; Liu, X.; Gao, H.; Hou, X.; Zhao, F.; Yan, H.; Cai, W.; Wang, L. Identifying Long Non-Coding RNAs and Characterizing Their Functional Roles in Swine Mammary Gland from Colostrogenesis to Lactogenesis. Anim. Biosci. 2022, 35, 814–825. [Google Scholar] [CrossRef]
- Zhibin, J.; Tianle, C.; Zhaohua, L.; Lei, H.; Jin, W.; Aili, W.; Jie, Z.; Rong, X.; Guizhi, W.; Jianmin, W. Genome-wide integrated analysis demonstrates widespread functions of lncRNAs in mammary gland development and lactation in dairy goats. BMC Genom. 2020, 21, 254. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, H.; Chen, M.; Liang, W.; Yang, J.; Deng, G.; Guo, M. Transcriptional Profiling of Exosomes Derived from Staphylococcus Aureus-Infected Bovine Mammary Epithelial Cell Line MAC-T by RNA-Seq Analysis. Oxid. Med. Cell. Longev. 2021, 2021, 8460355. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, M.; Wang, H.; Liu, H. Suppression of LncRNA MALAT1 Reduces Pro-Inflammatory Cytokines Production by Regulating MiR-150-5p/ZBTB4 Axis through JAK/STAT Signal Pathway in Systemic Juvenile Idiopathic Arthritis. Cytokine 2021, 138, 155397. [Google Scholar] [CrossRef]
- Kang, M.; Jeong, W.; Bae, H.; Lim, W.; Bazer, F.W.; Song, G. Bifunctional Role of Ephrin A1-Eph System in Stimulating Cell Proliferation and Protecting Cells from Cell Death through the Attenuation of ER Stress and Inflammatory Responses in Bovine Mammary Epithelial Cells. J. Cell. Physiol. 2018, 233, 2560–2571. [Google Scholar] [CrossRef]
- Kim, S.M.; Vetrivel, P.; Ha, S.E.; Kim, H.H.; Kim, J.-A.; Kim, G.S. Apigetrin Induces Extrinsic Apoptosis, Autophagy and G2/M Phase Cell Cycle Arrest through PI3K/AKT/MTOR Pathway in AGS Human Gastric Cancer Cell. J. Nutr. Biochem. 2020, 83, 108427. [Google Scholar] [CrossRef]
- Li, C.; Cai, W.; Zhou, C.; Yin, H.; Zhang, Z.; Loor, J.J.; Sun, D.; Zhang, Q.; Liu, J.; Zhang, S. RNA-Seq Reveals 10 Novel Promising Candidate Genes Affecting Milk Protein Concentration in the Chinese Holstein Population. Sci. Rep. 2016, 6, 26813. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the Protein-Coding Potential of Transcripts Using Sequence Features and Support Vector Machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing Sequence Intrinsic Composition to Classify Protein-Coding and Long Non-Coding Transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Sonnhammer, E.L.L.; Eddy, S.R.; Birney, E.; Bateman, A.; Durbin, R. Pfam: Multiple Sequence Alignments and HMM-Profiles of Protein Domains. Nucleic Acids Res. 1998, 26, 320–322. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Zhou, Z. PLEK: A Tool for Predicting Long Non-Coding RNAs and Messenger RNAs Based on an Improved k-Mer Scheme. BMC Bioinform. 2014, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level–the DESeq Package. DESq Man. 2012, 10, f1000research. [Google Scholar]
- Bhat, B.A.; Singh, G.; Sharma, R.; Yaseen, M.; Ganai, N.A. Biological Networks: Tools, Methods, and Analysis. In Essentials of Bioinformatics, Volume I; Springer: Cham, Switzerland, 2019; pp. 255–286. [Google Scholar] [CrossRef]
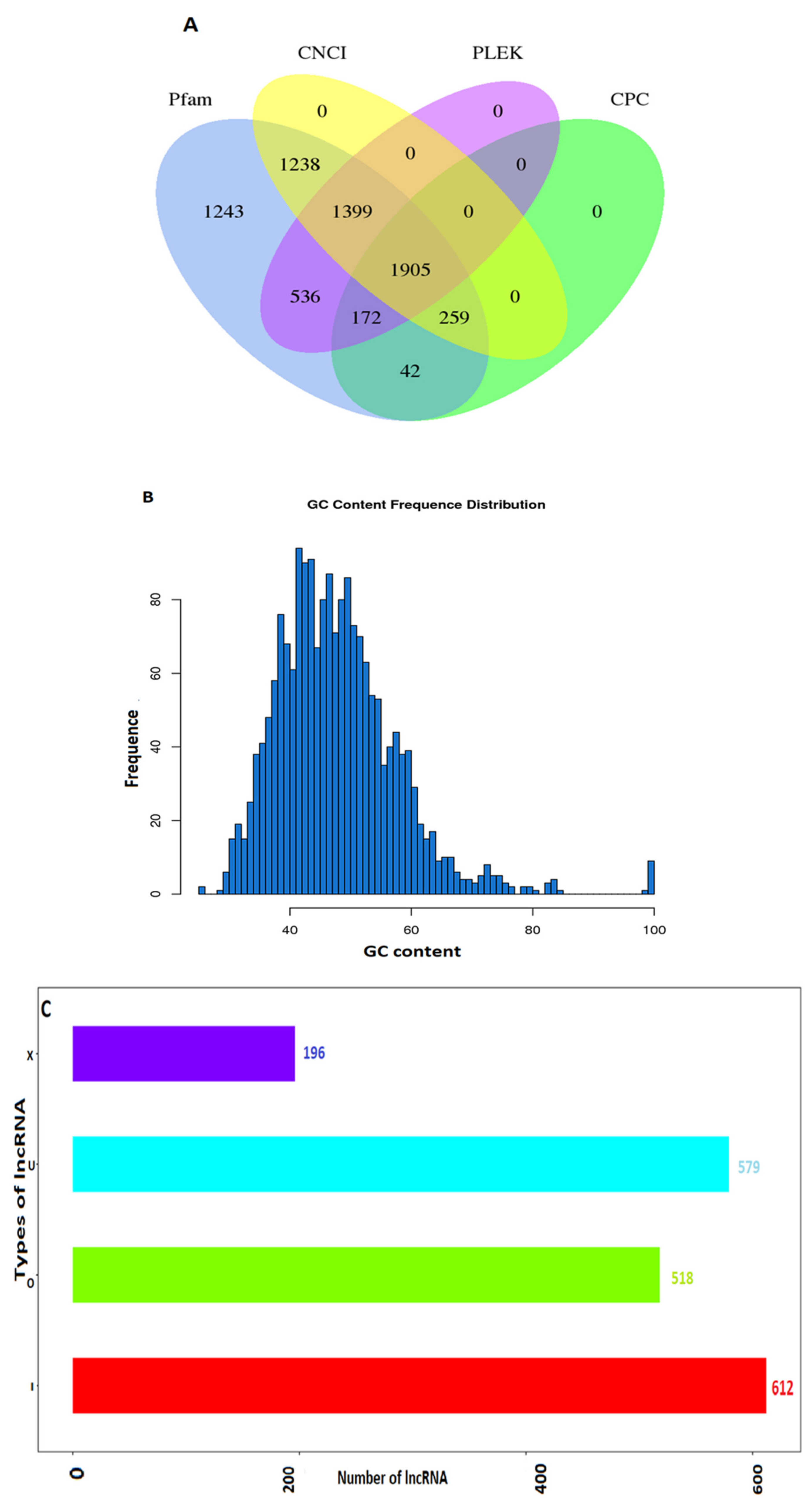
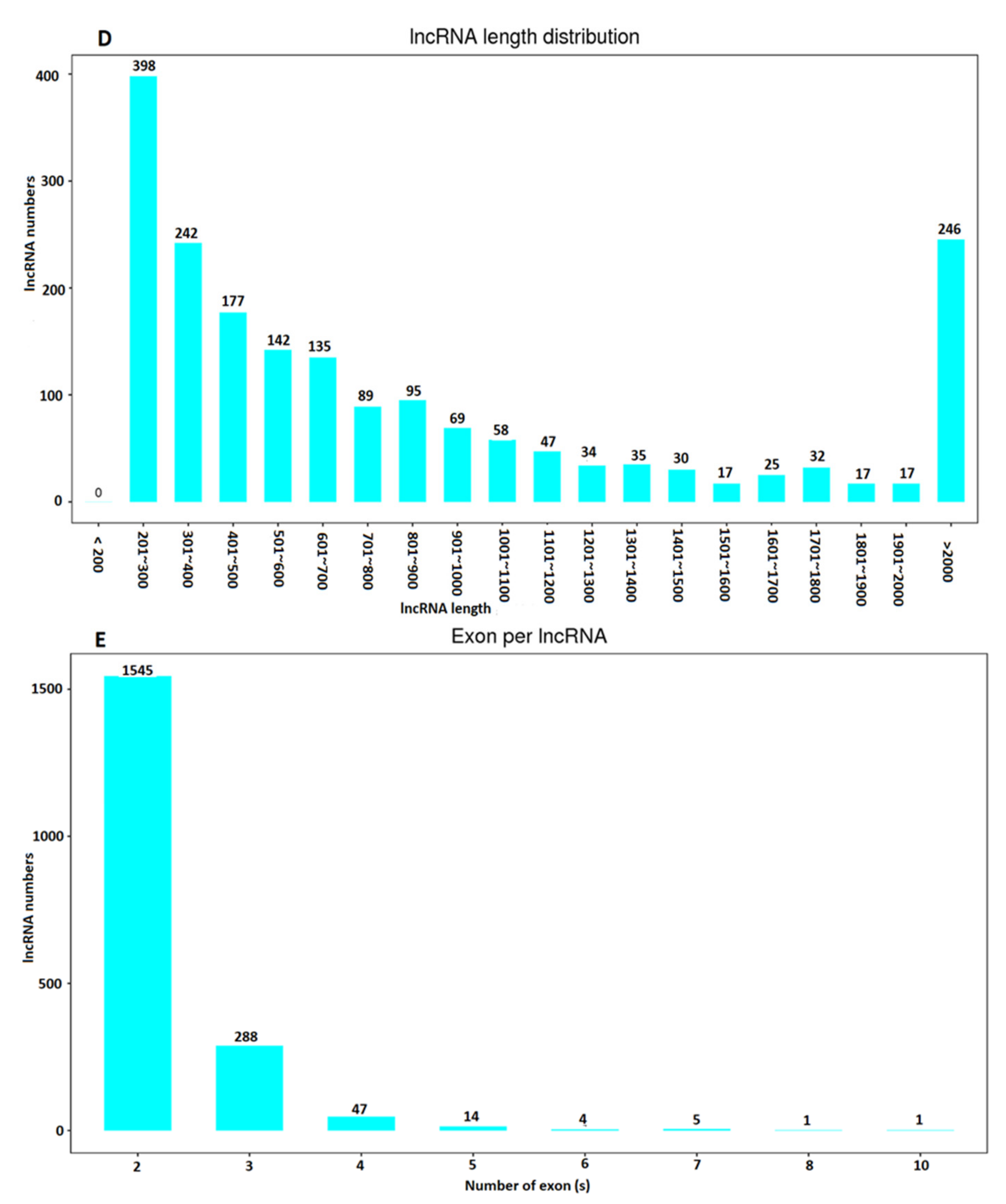

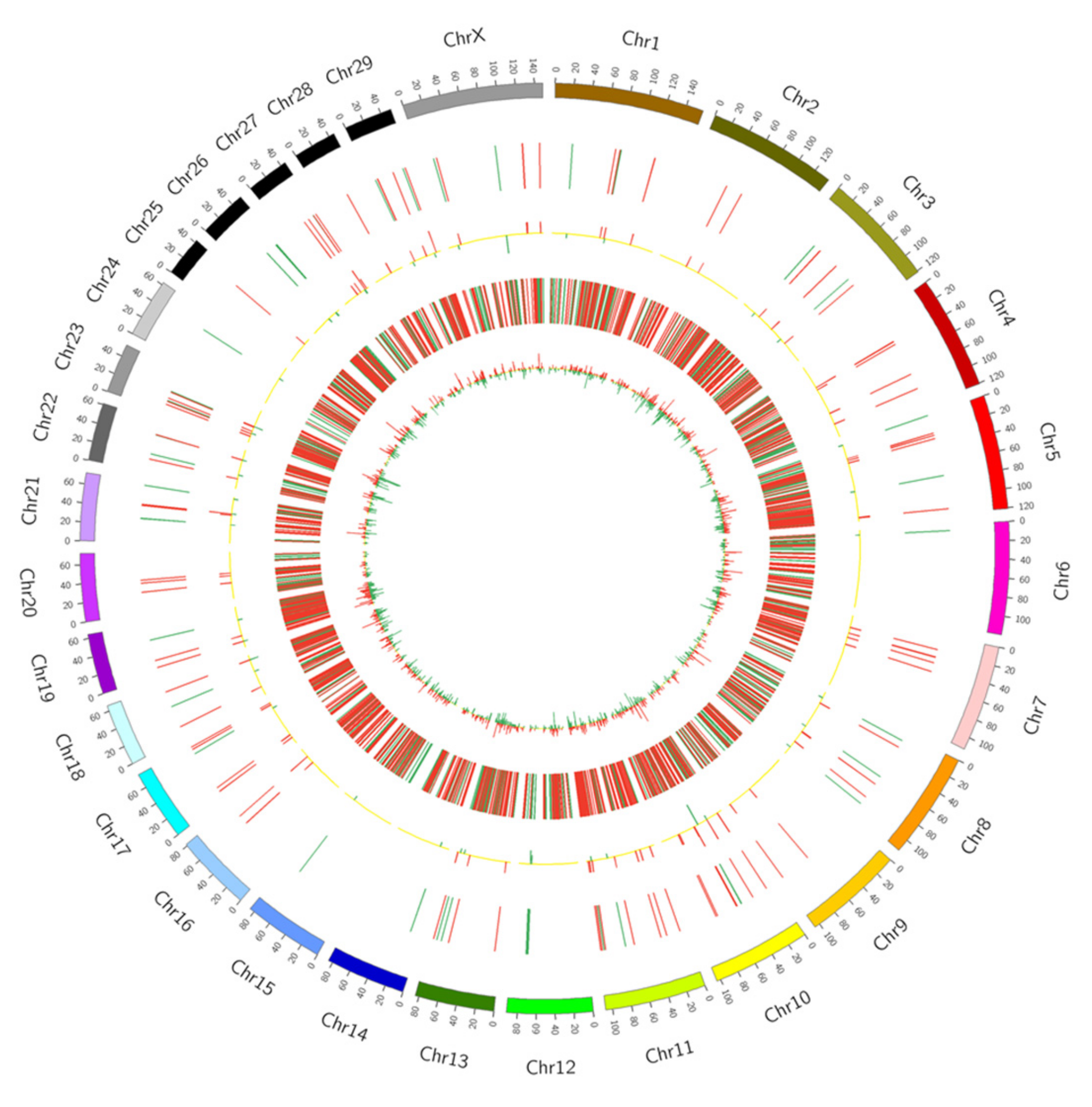

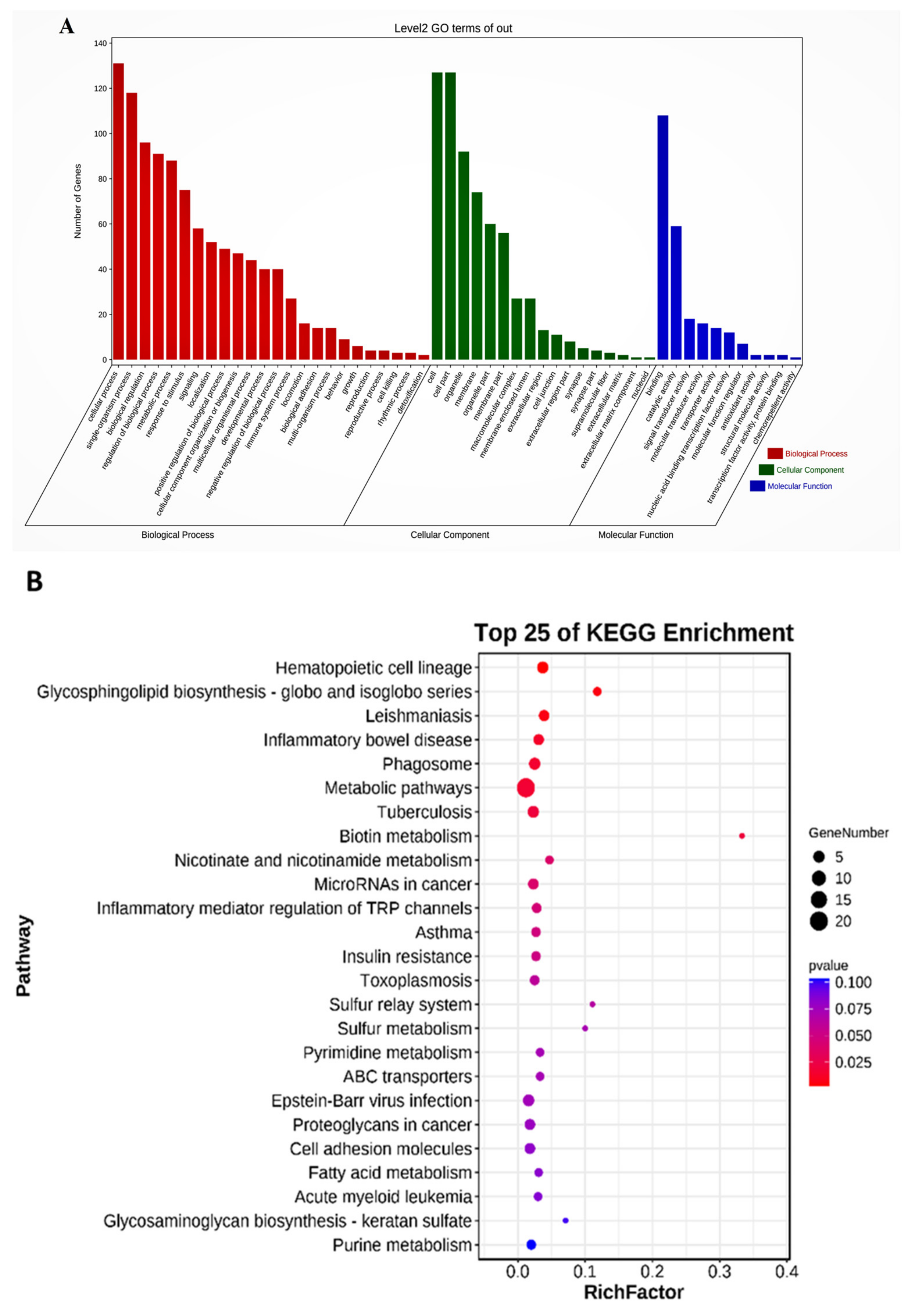
| Term | All 1 | ≥200 bp 2 | ≥500 bp 3 | ≥1000 bp 4 | N50 5 | Total_Length 6 | Max_Length 7 | Min_Length 8 | Average_Length 9 |
|---|---|---|---|---|---|---|---|---|---|
| lncRNA | 1905 | 1905 | 1091 | 559 | 1833 | 2,028,835 | 18449 | 201 | 1065.01 |
| lncRNAs ID | Fold Change | log2Fold Change | p-Value | Up/ Down Change 2 | Gene ID | BaseMean Control 3 315 d | BaseMean Case 4 30 d |
|---|---|---|---|---|---|---|---|
| TCONS_00000127 | 0.3159 | −1.662 | 0.0484 | Down | XLOC_000078 | 46.65 | 14.73 |
| TCONS_00000573 | 0.2427 | −2.042 | 0.00009 | Down | XLOC_000422 | 332.4 | 80.71 |
| TCONS_00000892 | 7.237 | 2.855 | 0.045 | Up | XLOC_000639 | 9.581 | 69.34 |
| TCONS_00001928 | 32.77 | 5.034 | 0.0055 | Up | XLOC_001382 | 5.348 | 175.29 |
| TCONS_00002045 | 2.2491 | 1.169 | 0.0164 | Up | XLOC_001477 | 243.6 | 548.04 |
| TCONS_00004577 | 2.4189 | 1.27 | 0.0203 | Up | XLOC_003131 XLOC_003235 | 177.6 | 429.74 |
| TCONS_00004705 | Inf 1 | Inf | 0.0038 | Up | 0 | 16.12 | |
| TCONS_00006010 | 6.1316 | 2.6162 | 0.0020 | Up | XLOC_004109 | 11.86 | 72.74 |
| TCONS_00007339 | 0.4084 | −1.291 | 0.00090 | Down | XLOC_004984 | 912.2 | 372.57 |
| TCONS_00007734 | 2.306 | 1.2059 | 0.0090 | Up | XLOC_005241 | 149.6 | 345.11 |
| TCONS_00007858 | 0.2149 | −2.217 | 0.0484 | Down | XLOC_005316 | 88.74 | 19.07 |
| TCONS_00007979 | Inf | Inf | 0.04212 | Up | XLOC_005396 | 0 | 10.26 |
| TCONS_00008771 | 81.063 | 6.340 | 0.0278 | Up | XLOC_006002 | 1.640 | 132.96 |
| TCONS_00008773 | 33.52 | 5.067 | 0.0098 | Up | XLOC_006003 | 35.85 | 1201.8 |
| TCONS_00010180 | 19.627 | 4.294 | 4.2221 | Up | XLOC_007129 | 32.80 | 643.83 |
| TCONS_00010615 | Inf | Inf | 0.041 | Up | XLOC_007443 | 0 | 8.11 |
| TCONS_00010780 | 3.5306 | 1.819 | 0.013 | Up | XLOC_007571 | 16.12 | 56.94 |
| TCONS_00011358 | 2.1697 | 1.117 | 0.0048 | Up | XLOC_008032 | 35,441.9 | 76,899.5 |
| TCONS_00012030 | 0.0753 | −3.729 | 0.047 | Down | XLOC_008500 | 14.55 | 1.097 |
| TCONS_00012647 | 0.2409 | −2.053 | 0.043 | Down | XLOC_008902 | 41.19 | 9.925 |
| TCONS_00012998 | 10.057 | 3.330 | 0.0454 | Up | XLOC_009211 | 4.028 | 40.51 |
| TCONS_00013114 | 12.2699 | 3.6170 | 0.0206 | Up | XLOC_009282 | 12.10 | 148.56 |
| TCONS_00014214 | 120.43 | 6.912 | 0.0002 | Up | XLOC_010015 | 0.556 | 67.073 |
| TCONS_00014380 | 0.447 | −1.161 | 0.0309 | Down | XLOC_010130 | 250.66 | 112.06 |
| TCONS_00017362 | 5.416 | 2.4372 | 0.0081 | Up | XLOC_012269 | 45.81 | 248.16 |
| TCONS_00017554 | 6.236 | 2.6408 | 0.00088 | Up | XLOC_012384 | 65.91 | 411.13 |
| TCONS_00018805 | 5.469 | 2.451 | 0.0030 | Up | XLOC_013313 | 10.77 | 58.95 |
| TCONS_00019123 | 2.2016 | 1.1385 | 0.016 | Up | XLOC_013518 | 2072.96 | 4563.8 |
| TCONS_00020026 | 0.499 | −1.001 | 0.045 | Down | XLOC_014135 | 436.85 | 218.17 |
| TCONS_00020595 | 2.532 | 1.340 | 0.032 | Up | XLOC_014531 | 36.46 | 92.34 |
| TCONS_00021189 | 44.226 | 5.4668 | 0.010 | Up | XLOC_014971 | 0.526 | 23.28 |
| TCONS_00021586 | 0.337 | −1.567 | 0.00003 | Down | XLOC_015283 | 149,600.4 | 50,464.8 |
| TCONS_00022016 | Inf | Inf | 0.007 | Up | XLOC_015571 | 0 | 12.20 |
| TCONS_00022067 | 0.0338 | −4.88 | 0.013 | Down | XLOC_015611 | 35.93 | 1.215 |
| TCONS_00023829 | 10.066 | 3.331 | 0.0035 | Up | XLOC_016897 | 44.15 | 444.5 |
| TCONS_00024356 | 5.110 | 2.353 | 0.039 | Up | XLOC_017317 | 31.69 | 161.98 |
| TCONS_00024512 | 110.983 | 6.794 | 2.214 | Up | XLOC_017449 | 1.104 | 122.56 |
| TCONS_00024513 | 44.96 | 5.490 | 0.003 | Up | XLOC_017449 | 1.842 | 82.85 |
| TCONS_00024834 | 5.4621 | 2.449 | 0.0009 | Up | XLOC_017667 | 51,842.6 | 283,172.0 |
| TCONS_00024835 | 0.490 | −1.0277 | 0.004 | Down | XLOC_017667 | 205,345.5 | 100,714.3 |
| TCONS_00024837 | 7.4177 | 2.890 | 0.0009 | Up | XLOC_017667 | 6292.51 | 46,676.4 |
| TCONS_00024971 | 0.2988 | −1.742 | 0.020 | Down | XLOC_017757 | 78.287 | 23.39 |
| TCONS_00024975 | 0.05063 | −4.303 | 0.018 | Down | XLOC_017757 | 33.67 | 1.704 |
| TCONS_00024978 | 0.0286 | −5.123 | 7.275 | Down | XLOC_017757 | 193.08 | 5.537 |
| TCONS_00024979 | 0 | Inf | 0.012 | Down | XLOC_017757 | 12.54 | 0 |
| TCONS_00024982 | 0.1028 | −3.281 | 0.010 | Down | XLOC_017757 | 169.87 | 17.47 |
| TCONS_00025065 | 27.54 | 4.783 | 0.012 | Up | XLOC_017811 | 20.204 | 556.5 |
| TCONS_00025067 | 12.063 | 3.592 | 0.037 | Up | XLOC_017811 | 2.43 | 29.33 |
| TCONS_00026548 | 78.218 | 6.289 | 0.016 | Up | XLOC_018845 | 0.83 | 65.34 |
| TCONS_00026777 | 2.355 | 1.235 | 0.011 | Up | XLOC_019017 | 87.54 | 206.19 |
| TCONS_00026788 | 69.85 | 6.289 | 2.475 | Up | XLOC_019024 | 5.409 | 377.87 |
| TCONS_00027914 | 0 | Inf | 0.016 | Down | XLOC_019812 | 21.38 | 0 |
| TCONS_00028242 | 0 | Inf | 0.023 | Down | XLOC_020028 | 12.286 | 0 |
| TCONS_00028308 | 15.025 | 3.909 | 0.035 | Up | XLOC_020075 | 39.73 | 597.08 |
| TCONS_00028334 | 2.2416 | 1.164 | 0.011 | Up | XLOC_020089 | 98.86 | 221.61 |
| TCONS_00028551 | 10.535 | 3.397 | 0.009 | Up | XLOC_020238 | 2.506 | 26.40 |
| TCONS_00028770 | 2.270 | 1.183 | 0.007 | Up | XLOC_020344 | 173.28 | 393.48 |
| TCONS_00028872 | Inf | Inf | 0.024 | Up | XLOC_020430 | 0 | 13.47 |
| TCONS_00029140 | 7.055 | 2.818 | 0.034 | Up | XLOC_020626 | 11.87 | 83.7 |
| TCONS_00029471 | 4.2468 | 2.086 | 0.009 | Up | XLOC_020853 | 14.83 | 62.99 |
| Items | Content (%) |
|---|---|
| Alfalfa hay | 8.39 |
| Oat hay | 6.43 |
| Whole corn silage | 52.18 |
| Corn | 8.1 |
| Soya bean meal | 10.8 |
| Cotton seed meal | 6.8 |
| DDGS 1 | 4.8 |
| Premix | 2.5 |
| Total | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghulam Mohyuddin, S.; Liang, Y.; Xia, Y.; Wang, M.; Zhang, H.; Li, M.; Yang, Z.; A. Karrow, N.; Mao, Y. Identification and Classification of Long Non-Coding RNAs in the Mammary Gland of the Holstein Cow. Int. J. Mol. Sci. 2023, 24, 13585. https://doi.org/10.3390/ijms241713585
Ghulam Mohyuddin S, Liang Y, Xia Y, Wang M, Zhang H, Li M, Yang Z, A. Karrow N, Mao Y. Identification and Classification of Long Non-Coding RNAs in the Mammary Gland of the Holstein Cow. International Journal of Molecular Sciences. 2023; 24(17):13585. https://doi.org/10.3390/ijms241713585
Chicago/Turabian StyleGhulam Mohyuddin, Sahar, Yan Liang, Yuxin Xia, Mengqi Wang, Huimin Zhang, Mingxun Li, Zhangping Yang, Niel A. Karrow, and Yongjiang Mao. 2023. "Identification and Classification of Long Non-Coding RNAs in the Mammary Gland of the Holstein Cow" International Journal of Molecular Sciences 24, no. 17: 13585. https://doi.org/10.3390/ijms241713585






