Tetrahydrobiopterin as a Trigger for Vitiligo: Phototransformation during UV Irradiation
Abstract
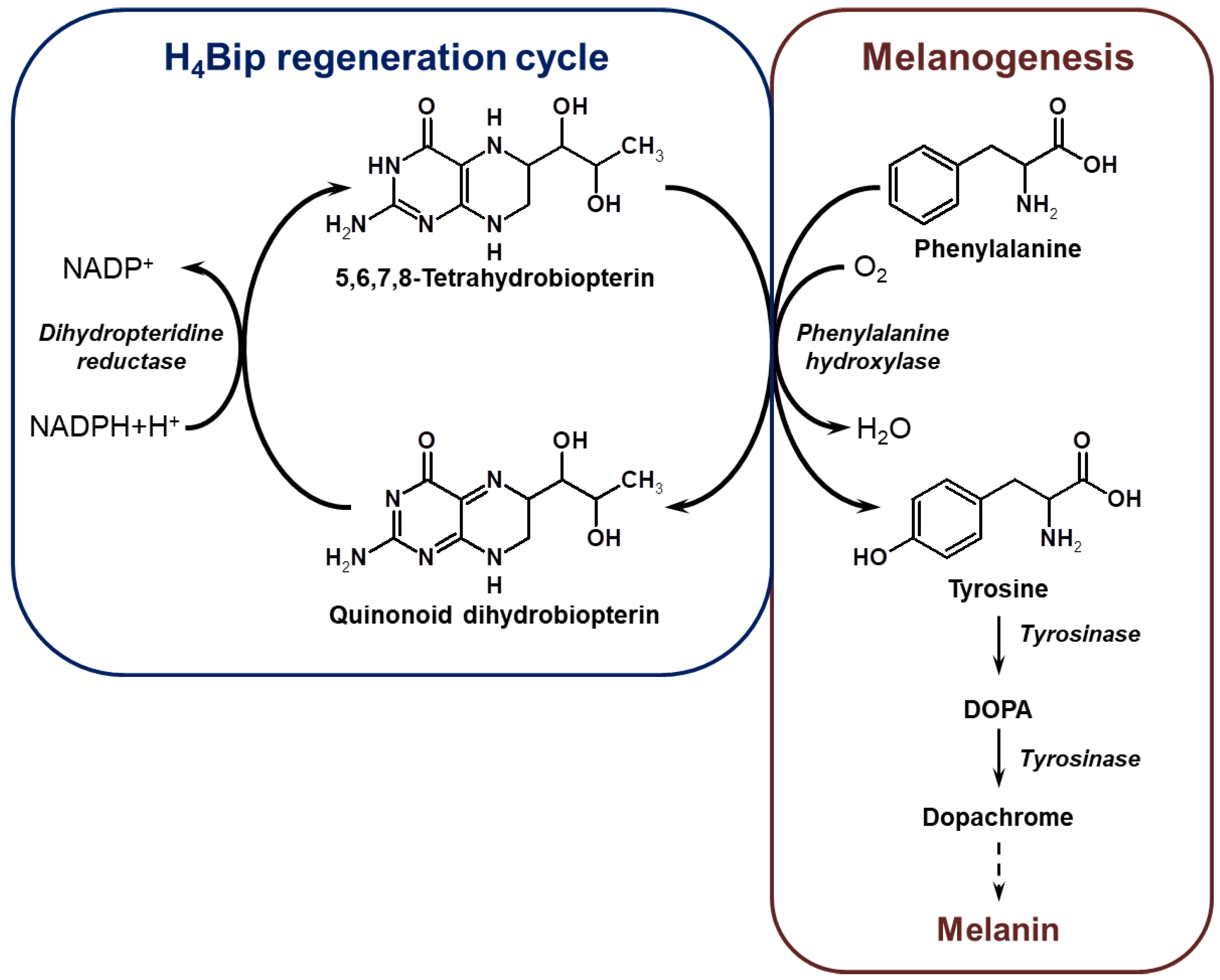
:1. Introduction
2. Results
2.1. Quantum Chemical Calculations: Conformational Analysis, Geometry Optimization, and Hessian Calculation
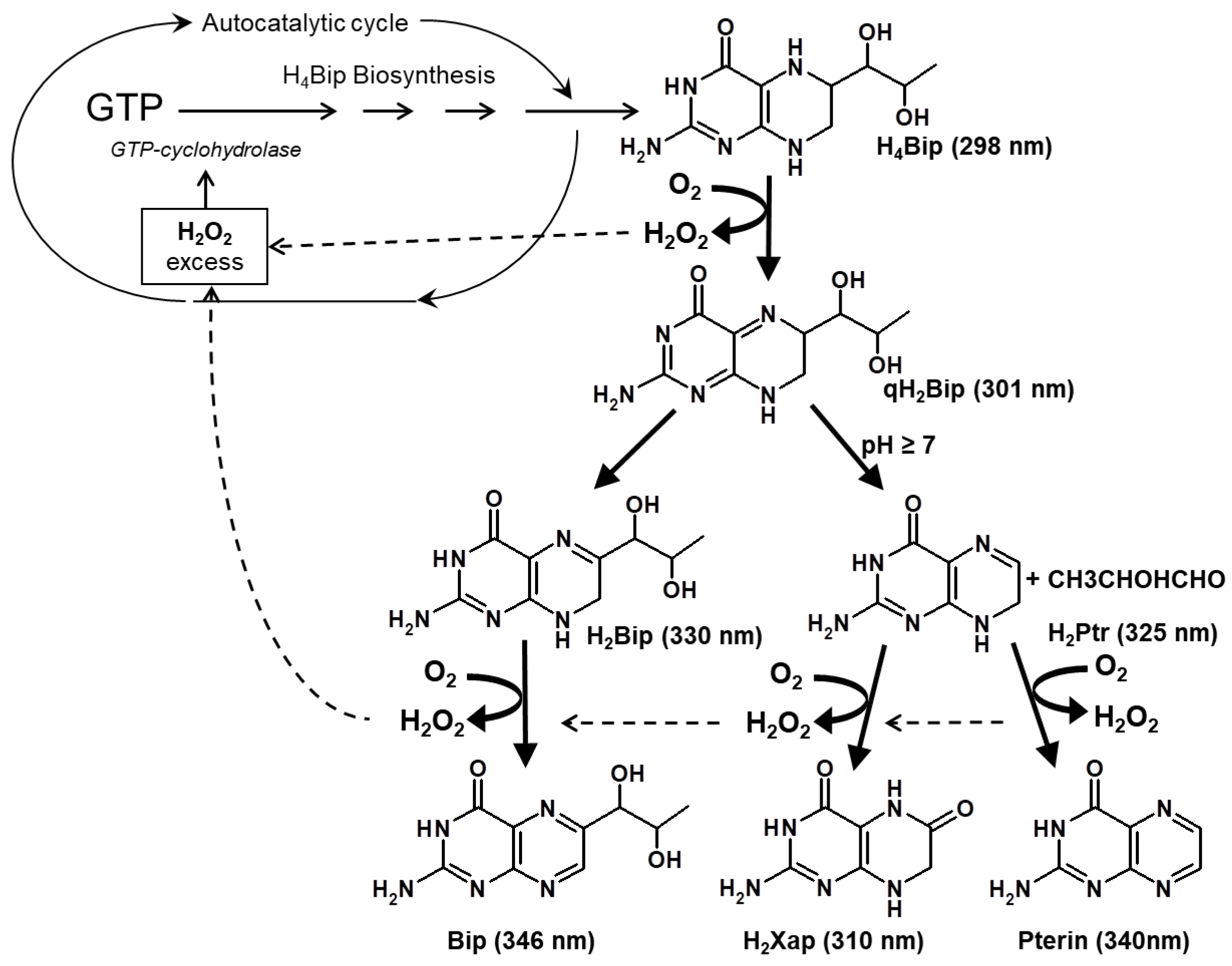
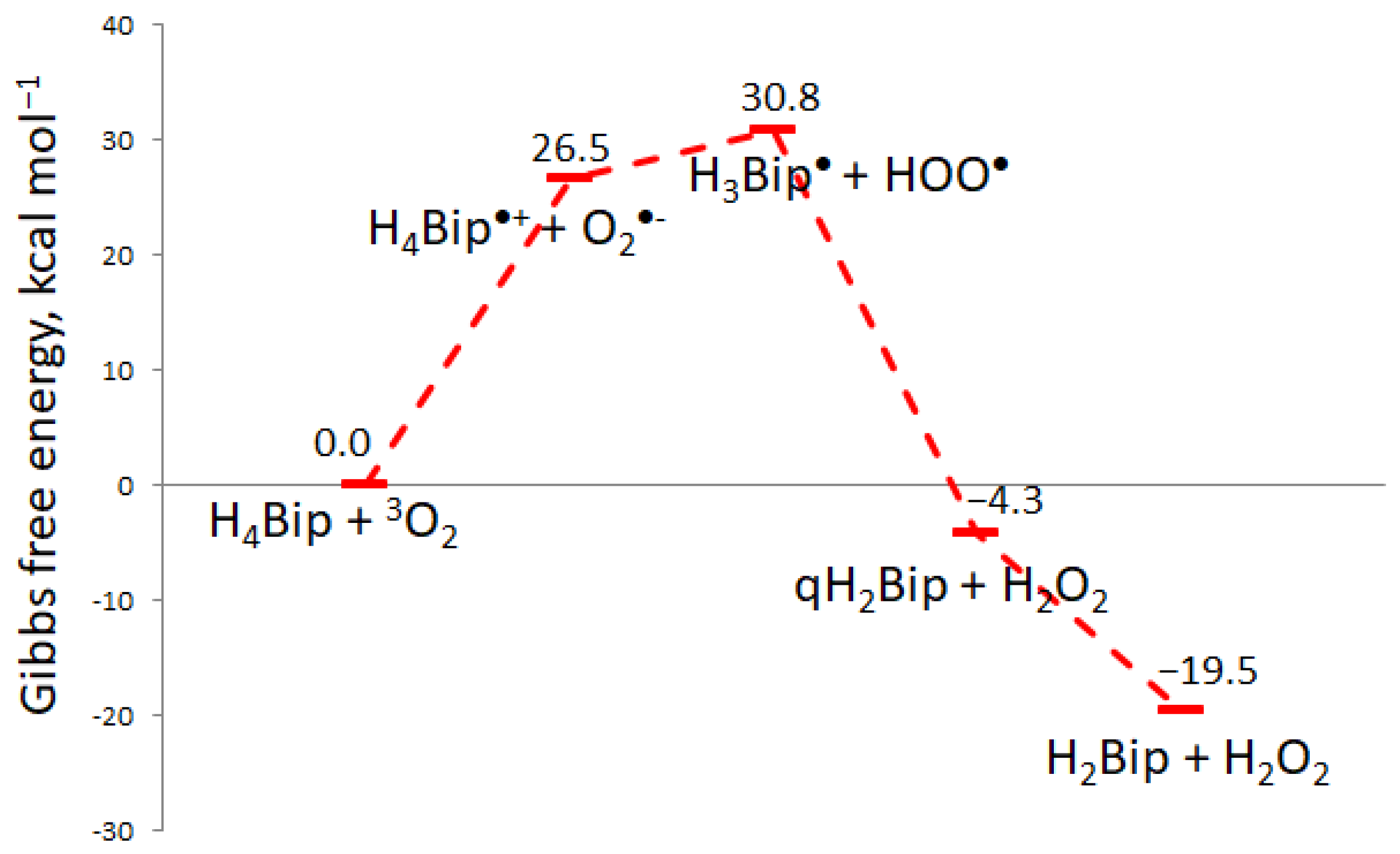
2.1.1. H4Bip Autoxidation
2.1.2. Type-I H4Bip Photooxidation
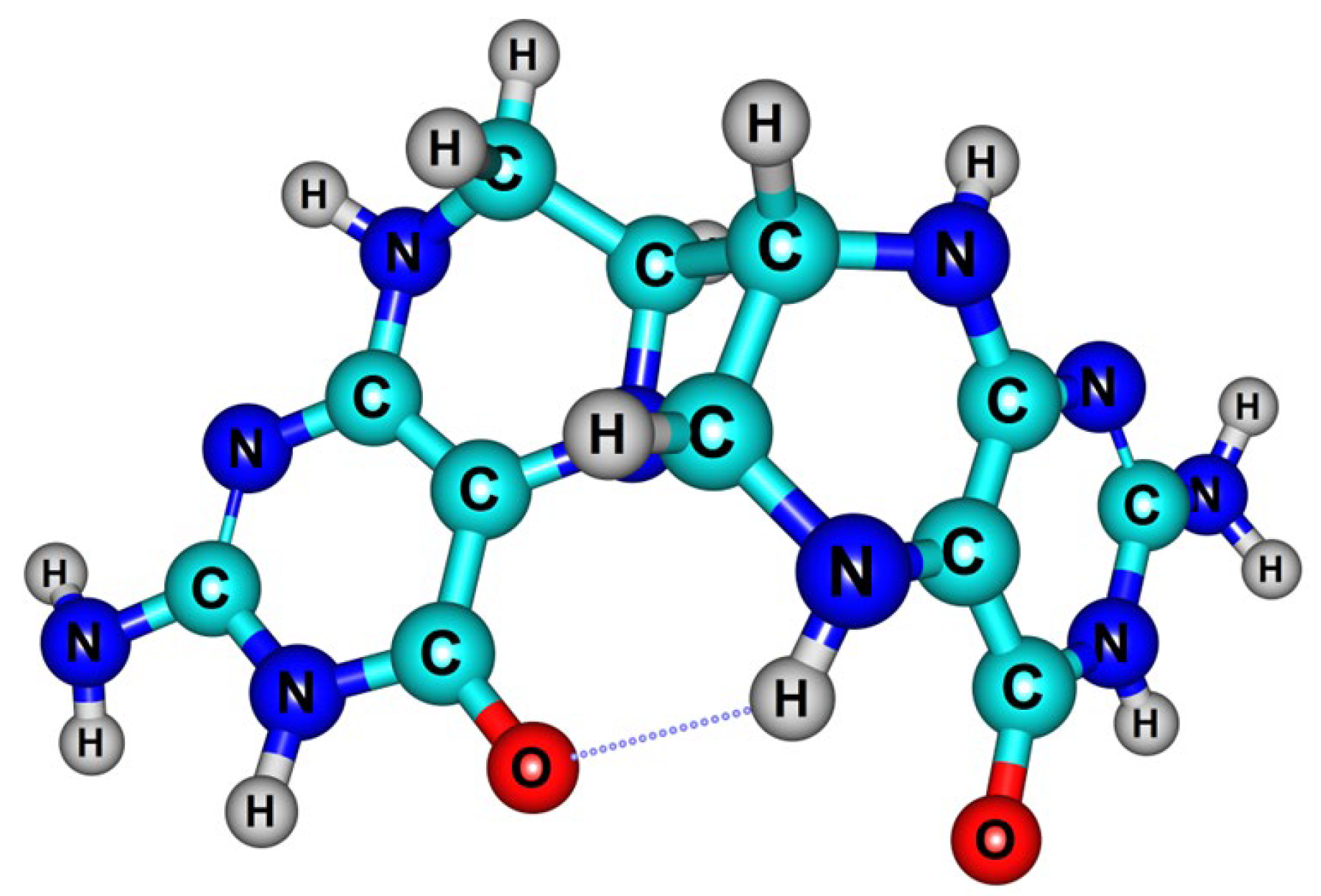
2.1.3. Dihydropterin Dimer Formation
2.2. Oxidative Modifications of HSA in the Presence of H4Bip
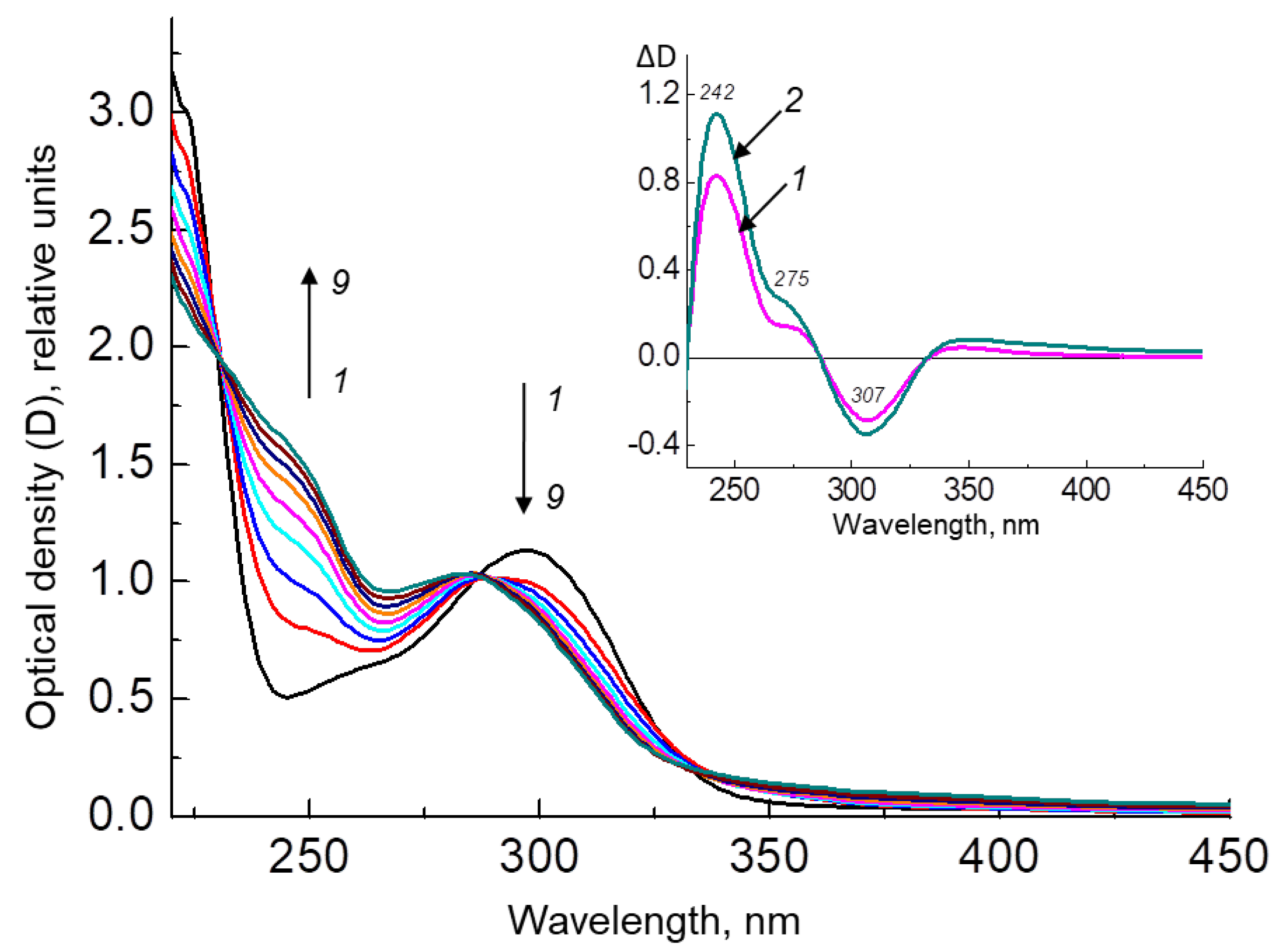
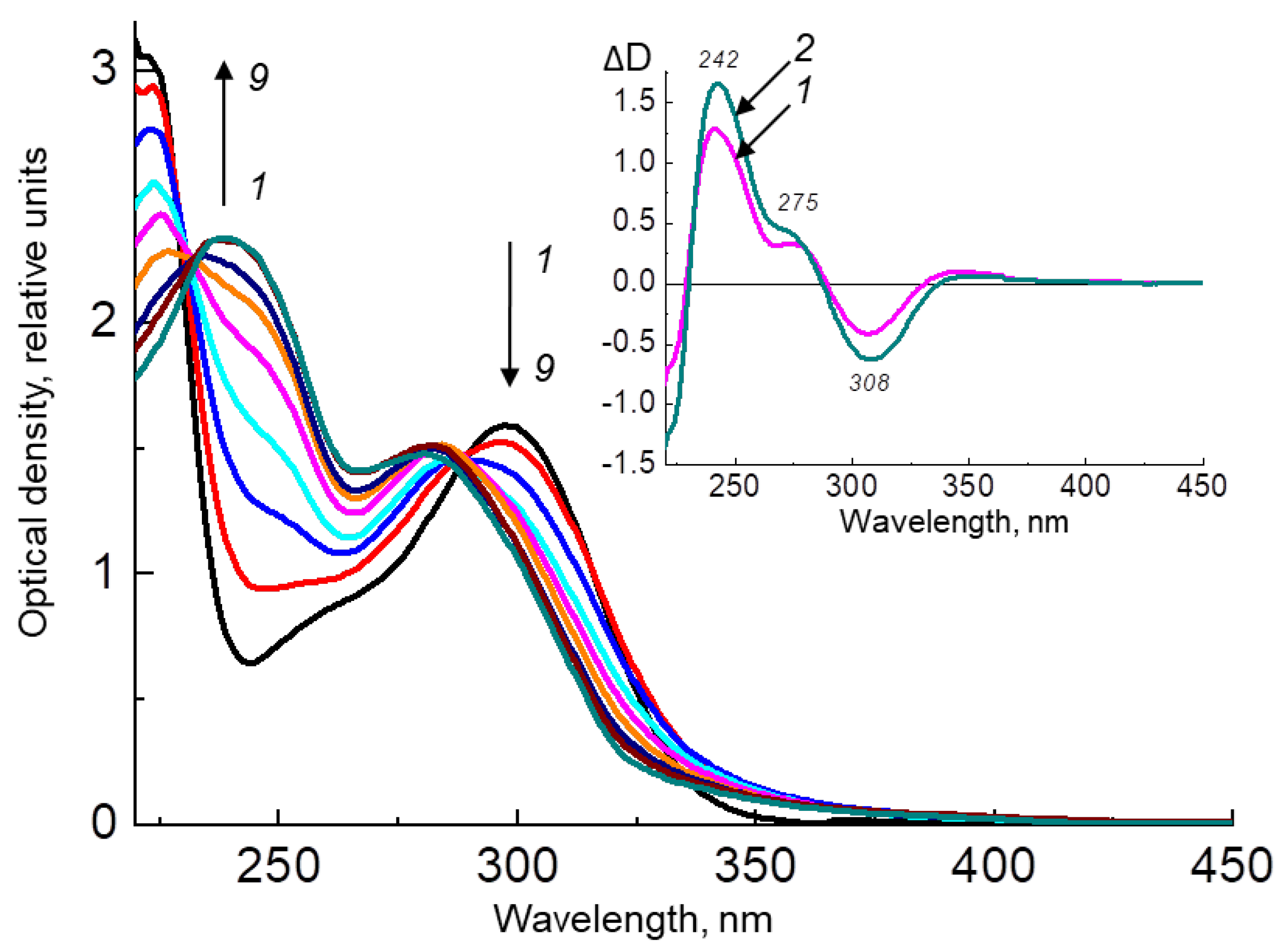
2.2.1. H4Bip Irradiation in the Presence and Absence of HSA
2.2.2. Study of HSA Oxidative Modification with Fluorescence
2.2.3. Dynamic Light Scattering Analysis of HSA Aggregates
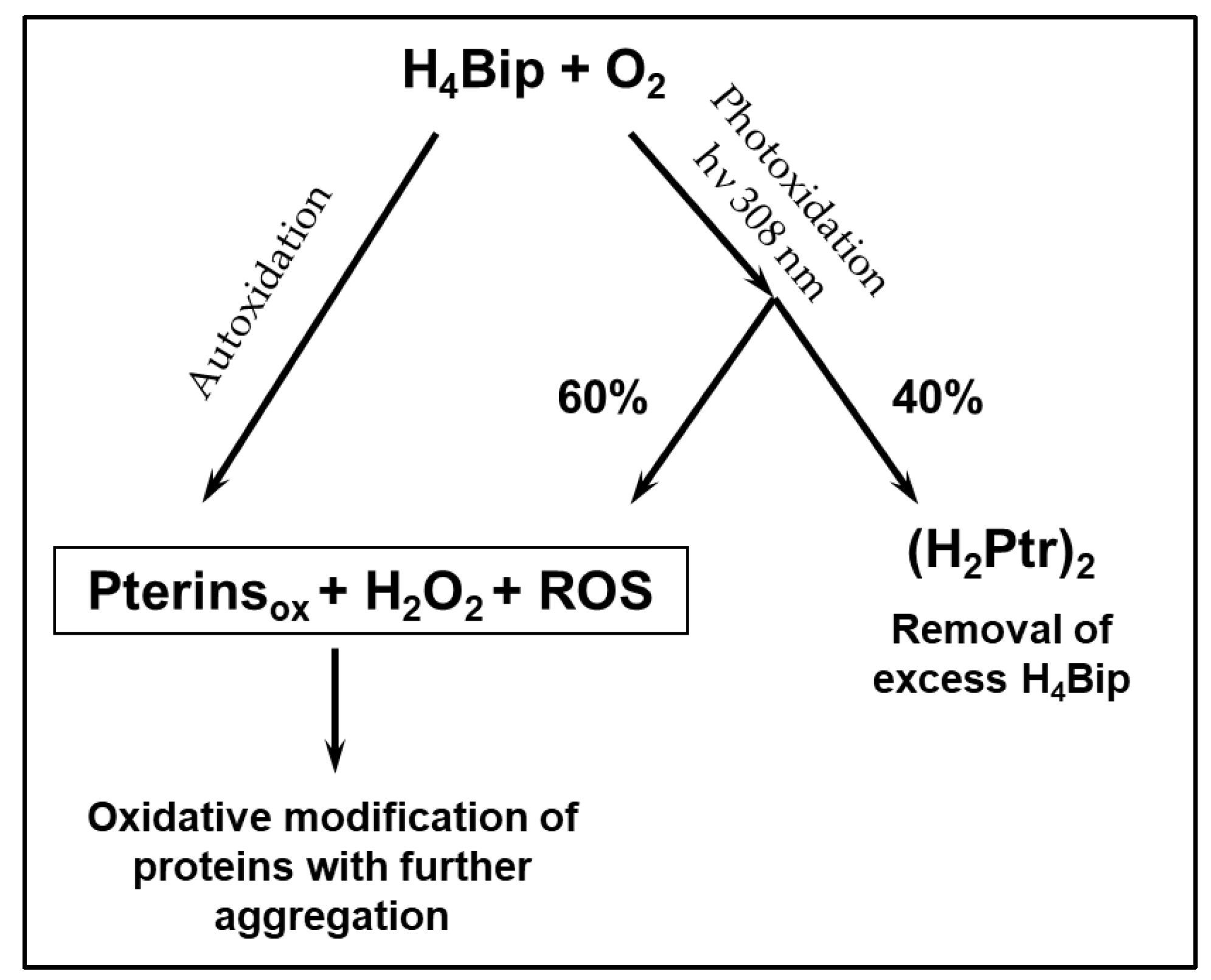
3. Discussion
4. Materials and Methods
4.1. Quantum Chemical Calculations: Conformational Analysis, Geometry Optimization, and Hessian Calculation
4.2. Oxidative Modifications of HSA in the Presence of H4Bip
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schallreuter, K.U.; Wood, J.M.; Pittelkow, M.R.; Gutlich, M.; Lemke, K.R.; Rödl, W.; Swanson, N.N.; Hitzemann, K.; Ziegler, I. Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science 1994, 263, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Peters, E.M.; Marles, L.K.; Behrens-Williams, S.C.; Dummer, R.; Blau, N.; Thony, B. Epidermal H2O2 accumulation alters tetrahydrobiopterin (6BH4) recycling in vitiligo: Identification of a general mechanism in regulation of all 6BH4-dependent processes? J. Investig. Dermatol. 2001, 116, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bidaki, R.; Majidi, N.; Moghadam Ahmadi, A.; Bakhshi, H.; Sadr Mohammadi, R.; Mostafavi, S.A.; Kazemi Arababadi, M.; Hadavi, M.; Mirzaei, A. Vitiligo and social acceptance. Clin. Cosmet. Investig. Dermatol. 2018, 11, 383–386. [Google Scholar] [CrossRef]
- Cho, H.K.; Eun, L.Y.; Song, J.S.; Kang, W.H.; Ro, B.I. A Case of Vitiligo after Kawasaki's Disease. Ann. Dermatol. 2009, 21, 75–77. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, H.J.; Bae, B.G.; Park, Y.K. A case of concurrent vitiligo and psoriasis. Ann. Dermatol. 2009, 21, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, K.; Karbakhsh, M.; Ghiasi, M.; Goodarzi, A.; Fakour, Y.; Akbari, Z.; Ghayoumi, A.; Ghandi, N. Quality of life in patients with vitiligo: A cross-sectional study based on Vitiligo Quality of Life index (VitiQoL). Health Qual. Life Outcomes 2016, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, M.A.; Vargas-Salinas, M.; Peralta-Pedrero, M.L.; Olguín-García, M.G.; Jurado-Santa Cruz, F. Impact of Vitiligo on Quality of Life. Actas Dermosifiliogr. 2017, 108, 637–642. [Google Scholar] [CrossRef]
- Kowlessur, D.; Citron, B.A.; Kaufman, S. Recombinant human phenylalanine hydroxylase: Novel regulatory and structural properties. Arch. Biochem. Biophys. 1996, 333, 85–95. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Schulz-Douglas, V.; Bünz, A.; Beazley, W.; Körner, C. Pteridines in the control of pigmentation. J. Investig. Dermatol. 1997, 109, 31–35. [Google Scholar] [CrossRef]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347, 1–16. [Google Scholar] [CrossRef]
- Werner, E.R.; Blau, N.; Thöny, B. Tetrahydrobiopterin: Biochemistry and pathophysiology. Biochem. J. 2011, 438, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, L.; Wang, J.; Li, H.; Li, Y.; Lv, Y.; Lu, C. BmPAH catalyzes the initial melanin biosynthetic step in Bombyx mori. PLoS ONE 2013, 8, e71984. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shu, R.; Jiang, S.; Xu, M.; Cai, Y.; Qin, H.; Zhang, D.; Feng, M.; Gao, J.; Meng, Y. Exploring the Mystery of the Tetrahydrobiopterin Synthetic Defect Lethal Mutant leml from Birth to Death in the Silkworm Bombyx mori. Int. J. Mol. Sci. 2022, 23, 12083. [Google Scholar] [CrossRef]
- Ze, L.J.; Xu, P.; Wu, J.J.; Jin, L.; Ali Anjum, A.; Li, G.Q. Disruption of tetrahydrobiopterin (BH4) biosynthesis pathway affects cuticle pigmentation in Henosepilachna vigintioctopunctata. J. Insect. Physiol. 2023, 144, 104457. [Google Scholar] [CrossRef]
- Hasse, S.; Gibbons, N.C.; Rokos, H.; Marles, L.K.; Schallreuter, K.U. Perturbed 6-tetrahydrobiopterin recycling via decreased dihydropteridine reductase in vitiligo: More evidence for H2O2 stress. J. Investig. Dermatol. 2004, 122, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Gibbons, N.C.; Rokos, H.; Peters, E.M.; Wood, J.M.; Schallreuter, K.U. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J. Investig. Dermatol. 2007, 127, 411–420. [Google Scholar] [CrossRef]
- Eskandani, M.; Golchai, J.; Pirooznia, N.; Hasannia, S. Oxidative stress level and tyrosinase activity in vitiligo patients. Indian J. Dermatol. 2010, 55, 15–19. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Salem, M.A.; Holtz, S.; Panske, A. Basic evidence for epidermal H2O2/ONOO(-)-mediated oxidation/nitration in segmental vitiligo is supported by repigmentation of skin and eyelashes after reduction of epidermal H2O2 with topical NB-UVB-activated pseudocatalase PC-KUS. FASEB J. 2013, 27, 3113–3122. [Google Scholar] [CrossRef]
- Passi, S.; Grandinetti, M.; Maggio, F.; Stancato, A.; De Luca, C. Epidermal oxidative stress in vitiligo. Pigment. Cell Res. 1998, 11, 81–85. [Google Scholar] [CrossRef]
- Schallreuter, K.U. Successful treatment of oxidative stress in vitiligo. Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 132–138. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Gaze, D.C.; Tobin, D.J.; Marshall, H.S.; Panske, A.; Panzig, E.; Hibberts, N.A. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Investig. Symp. Proc. 1999, 4, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; van Geel, N. Vitiligo: An Update on Pathophysiology and Treatment Options. Am. J. Clin. Dermatol. 2017, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Rokos, H.; Beazley, W.D.; Schallreuter, K.U. Oxidative stress in vitiligo: Photo-oxidation of pterins produces H(2)O(2) and pterin-6-carboxylic acid. Biochem. Biophys. Res. Commun. 2002, 292, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S. The structure of the phenylalanine-hydroxylation cofactor. Proc. Natl. Acad. Sci. USA 1963, 50, 1085–1093. [Google Scholar] [CrossRef]
- Davis, M.D.; Kaufman, S. Evidence for the formation of the 4a-carbinolamine during the tyrosine- dependent oxidation of tetrahydrobiopterin by rat liver phenylalanine hydroxylase. J. Biol. Chem. 1989, 264, 8585–8596. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Krüger, C.; Würfel, B.A.; Panske, A.; Wood, J.M. From basic research to the bedside: Efficacy of topical treatment with pseudocatalase PC-KUS in 71 children with vitiligo. Int. J. Dermatol. 2008, 47, 743–753. [Google Scholar] [CrossRef]
- Telegina, T.A.; Vechtomova, Y.L.; Kritsky, M.S.; Nizamutdinov, A.S.; Madirov, E.I.; Makarova, D.A.; Buglak, A.A. Photooxidation of tetrahydrobiopterin as the basis of vitiligo phototherapy. Opt. Spectrosc. 2022, 130, 602–607. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Lyudnikova, T.A.; Vechtomova, Y.L.; Kritsky, M.S. Photooxidation of tetrahydrobiopterin under UV irradiation: Possible pathways and mechanisms. Photochem. Photobiol. 2014, 90, 1017–1026. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Kritsky, M.S. A quantitative structure-property relationship (QSPR) study of singlet oxygen generation by pteridines. Photochem. Photobiol. Sci. 2016, 15, 801–811. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Vorotelyak, E.A.; Kononov, A.I. Theoretical study of photoreactions between oxidized pterins and molecular oxygen. J. Photochem. Photobiol. A 2019, 372, 254–259. [Google Scholar] [CrossRef]
- Gawkrodger, D.J. Pseudocatalase and narrowband ultraviolet B for vitiligo: Clearing the picture. Br. J. Dermatol. 2009, 161, 721–722. [Google Scholar] [CrossRef]
- Telegina, T.A.; Lyudnikova, T.A.; Buglak, A.A.; Vechtomova, Y.L.; Biryukov, M.V.; Demin, V.V.; Kritsky, M.S. Transformation of 6-tetrahydrobiopterin in aqueous solutions under UV-irradiation. J. Photochem. Photobiol. A 2018, 354, 155–162. [Google Scholar] [CrossRef]
- Kirsch, M.; Korth, H.G.; Stenert, V.; Sustmann, R.; De Groot, H. The autoxidation of tetrahydrobiopterin revisited. J. Biol. Chem. 2003, 278, 24481–24490. [Google Scholar] [CrossRef] [PubMed]
- Buglak, A.A.; Telegina, T.A. A theoretical study of 5,6,7,8-tetrahydro-6-hydroxymethylpterin: Insight into intrinsic photoreceptor properties of 6-substituted tetrahydropterins. Photochem. Photobiol. Sci. 2019, 18, 516–523. [Google Scholar] [CrossRef]
- Dántola, L.M.; Gojanovich, A.D.; Thomas, A.H. Inactivation of tyrosinase photoinduced by pterin. Biochem. Biophys. Res. Commun. 2012, 424, 568–572. [Google Scholar] [CrossRef]
- Gogonea, V.; Shy, J.M., 2nd; Biswas, P.K. Electronic structure, ionization potential, and electron affinity of the enzyme cofactor (6R)-5,6,7,8-tetrahydrobiopterin in the gas phase, solution, and protein environments. J. Phys. Chem. B 2006, 110, 22861–22871. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Vechtomova, Y.L.; Kritsky, M.S. Autoxidation and photooxidation of tetrahydrobiopterin: A theoretical study. Free Radic. Res. 2021, 55, 499–509. [Google Scholar] [CrossRef]
- Goebbert, D.J.; Sanov, A.J. Photodetachment, photofragmentation and fragment autodetachment of [O2n(H2O)m]- clusters: Core-anion structures and fragment energy partitioning. J. Chem. Phys. 2009, 131, 104308. [Google Scholar] [CrossRef]
- Vignoni, M.; Cabrerizo, F.M.; Lorente, C.; Claparols, C.; Oliveros, E.; Thomas, A.H. Photochemistry of dihydrobiopterin in aqueous solution. Org. Biomol. Chem. 2010, 8, 800–810. [Google Scholar] [CrossRef]
- Telegina, T.A.; Vechtomova, Y.L.; Kritsky, M.S.; Madirov, E.I.; Nizamutdinov, A.S.; Obuhov, Y.N.; Buglak, A.A. Tetrahydrobiopterin photooxidation: A key process in vitiligo phototherapy. Appl. Biochem. Microbiol. 2021, 57, 571–578. [Google Scholar] [CrossRef]
- Fontas, E.; Montaudie, H.; Passeron, T. Oral gliadin-protected superoxidedismutase in addition to phototherapy for treating nonsegmental vitiligo: A 24-week prospective randomized placebo controlled study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Rodrigues, M. An update and review of narrowband ultraviolet B phototherapy for vitiligo. Dermat. Rev. 2022, 3, 326–335. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gwinner, W.; Grone, H.J. Role of reactive oxygen species in glomerulonephritis. Nephrol. Dial. Transplant. 2000, 15, 1127–1132. [Google Scholar] [CrossRef]
- Sozarukova, M.M.; Proskurnina, E.V.; Vladimirov, Y.A. Serum albumin as a source of and a target for free radicals in pathology. Bull. RSMU 2016, 1, 56–61. [Google Scholar] [CrossRef]
- Thomas, A.H.; Lorente, C.; Roitman, K.; Morales, M.M.; Dántola, M.L. Photosensitization of bovine serum albumin by pterin: A mechanistic study. J. Photochem. Photobiol. B 2013, 120, 52–58. [Google Scholar] [CrossRef]
- Dantola, M.; Reid, L.; Castaño, C.; Lorente, C.; Oliveros, E.; Thomas, A. Photosensitization of peptides and proteins by pterin derivatives. Pteridines 2017, 28, 105–114. [Google Scholar] [CrossRef]
- Oettl, K.; Reibnegger, G.; Schmut, O. The redox state of human serum albumin in eye diseases with and without complications. Acta Ophthalmol. 2011, 89, e174–e179. [Google Scholar] [CrossRef]
- Bourdon, E.; Loreau, N.; Blache, D. Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J. 1999, 13, 233–244. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.H.; Serrano, M.P.; Rahal, V.; Vicendo, P.; Claparols, C.; Oliveros, E.; Lorente, C. Tryptophan oxidation photosensitized by pterin. Free Radic. Biol. Med. 2013, 63, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]



| Reaction | ∆fGº, kcal mol−1 | ||
|---|---|---|---|
| 3Ptr* + H4Bip → Ptr•− + H4Bip•+ | (3) | chain initiation | −21.4 |
| Ptr•− + O2 → Ptr + O2•− | (16) | chain initiation | −30.4 |
| H4Bip + HOO• → H3Bip• + H2O2 | (7) | chain prolongation | −18.0 |
| H3Bip• + O2 → H2Bip + HOO• | (8) | chain prolongation | −1.5 |
| 2H3Bip• → H2Bip + H4Bip | (12) | chain termination | −32.4 |
| 2HOO• → H2O2 + O2 | (13) | chain termination | −48.9 |
| Sample | FOP | Irradiation Time, min |
|---|---|---|
| HSA | 0.24 ± 0.010 | 16 |
| H4Bip + HSA | 0.39 ± 0.017 | 16 |
| H4Bip + HSA | 0.64 ± 0.028 | 0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telegina, T.A.; Vechtomova, Y.L.; Borzova, V.A.; Buglak, A.A. Tetrahydrobiopterin as a Trigger for Vitiligo: Phototransformation during UV Irradiation. Int. J. Mol. Sci. 2023, 24, 13586. https://doi.org/10.3390/ijms241713586
Telegina TA, Vechtomova YL, Borzova VA, Buglak AA. Tetrahydrobiopterin as a Trigger for Vitiligo: Phototransformation during UV Irradiation. International Journal of Molecular Sciences. 2023; 24(17):13586. https://doi.org/10.3390/ijms241713586
Chicago/Turabian StyleTelegina, Taisiya A., Yuliya L. Vechtomova, Vera A. Borzova, and Andrey A. Buglak. 2023. "Tetrahydrobiopterin as a Trigger for Vitiligo: Phototransformation during UV Irradiation" International Journal of Molecular Sciences 24, no. 17: 13586. https://doi.org/10.3390/ijms241713586
APA StyleTelegina, T. A., Vechtomova, Y. L., Borzova, V. A., & Buglak, A. A. (2023). Tetrahydrobiopterin as a Trigger for Vitiligo: Phototransformation during UV Irradiation. International Journal of Molecular Sciences, 24(17), 13586. https://doi.org/10.3390/ijms241713586







