Binding of βL-Crystallin with Models of Animal and Human Eye Lens-Lipid Membrane
Abstract
1. Introduction
2. Results
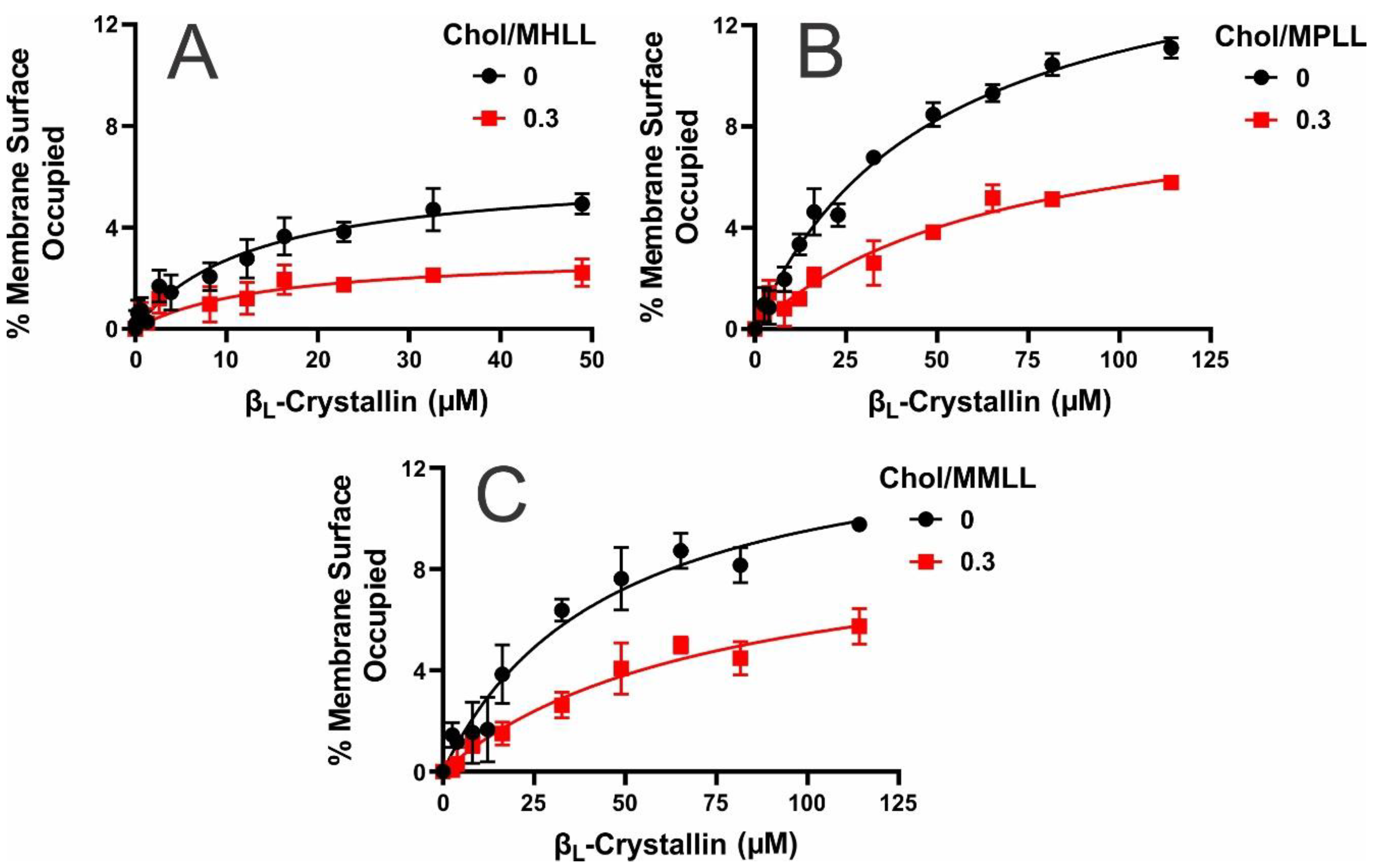
2.1. MSO by βL-Crystallin in Models of Human, Porcine, and Mouse Lens-Lipid Membrane
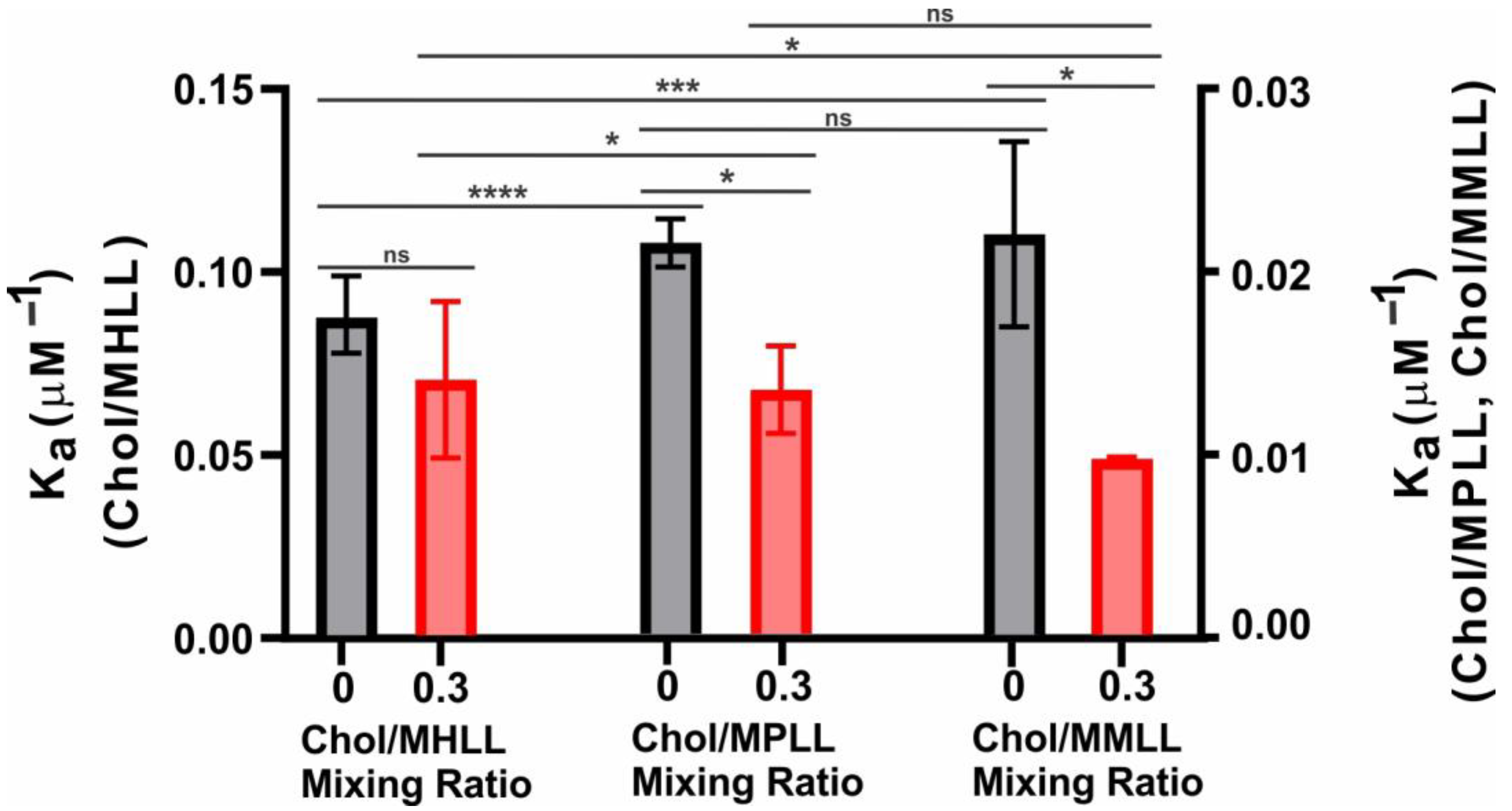
2.2. Ka of βL-Crystallin to the Models’ Lens-Lipid Membranes and the Effects of Chol
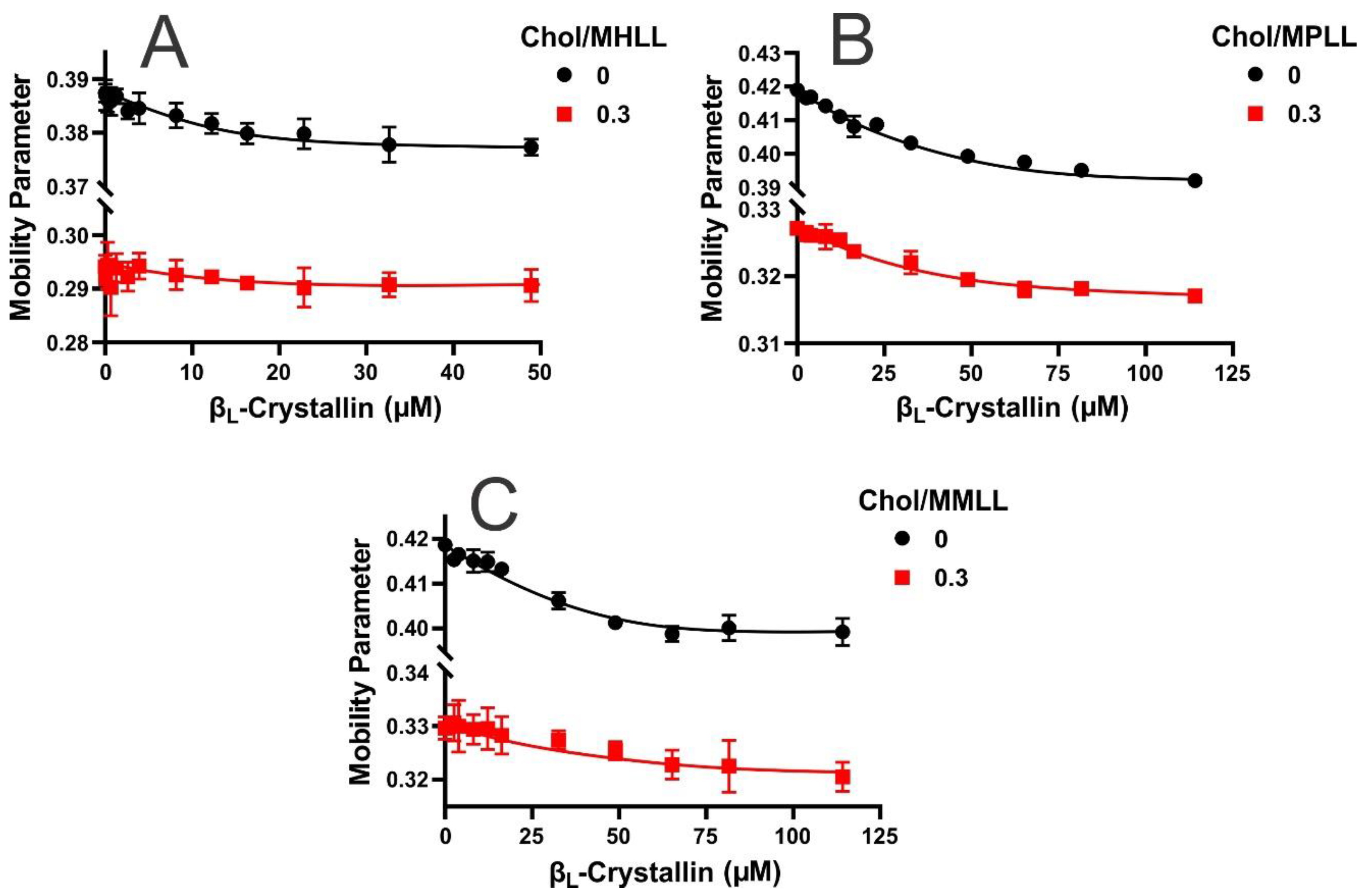
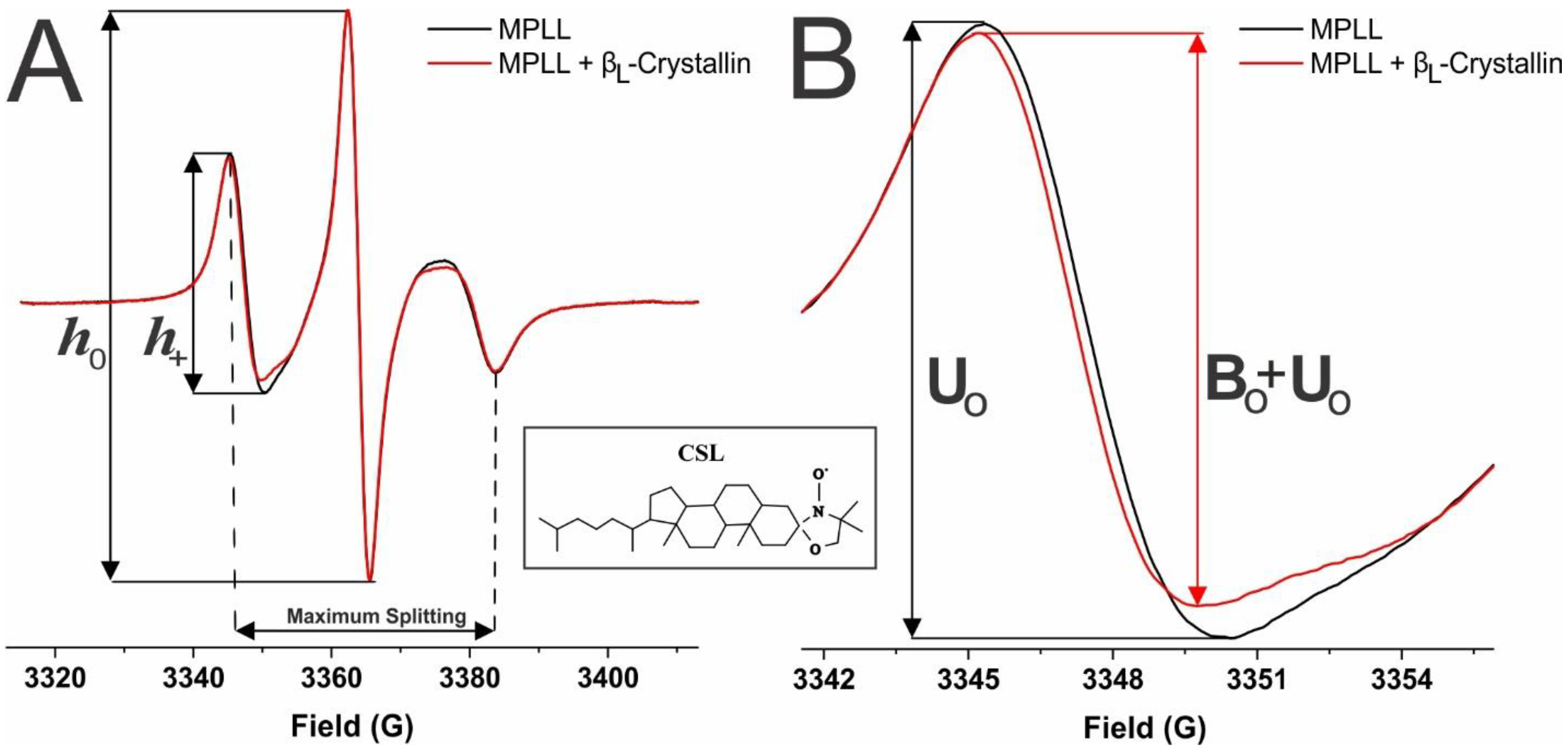
2.3. Mobility near the Surface of the Model Lens-Lipid Membranes with Chol and βL-Crystallin Binding
2.4. Order Below the Surface of Model Lens-Lipid Membranes with the Addition of Chol and βL-Crystallin Binding
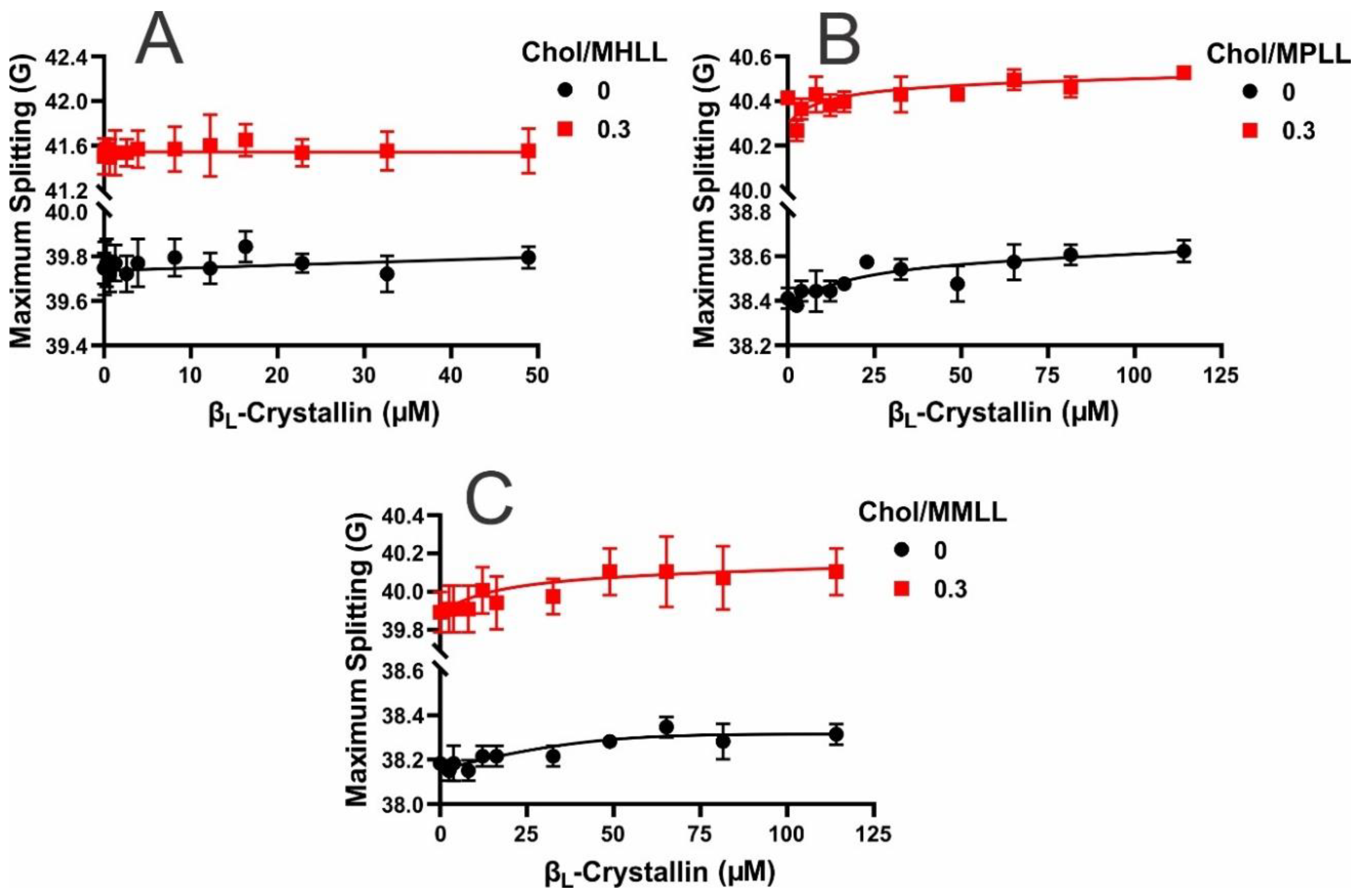
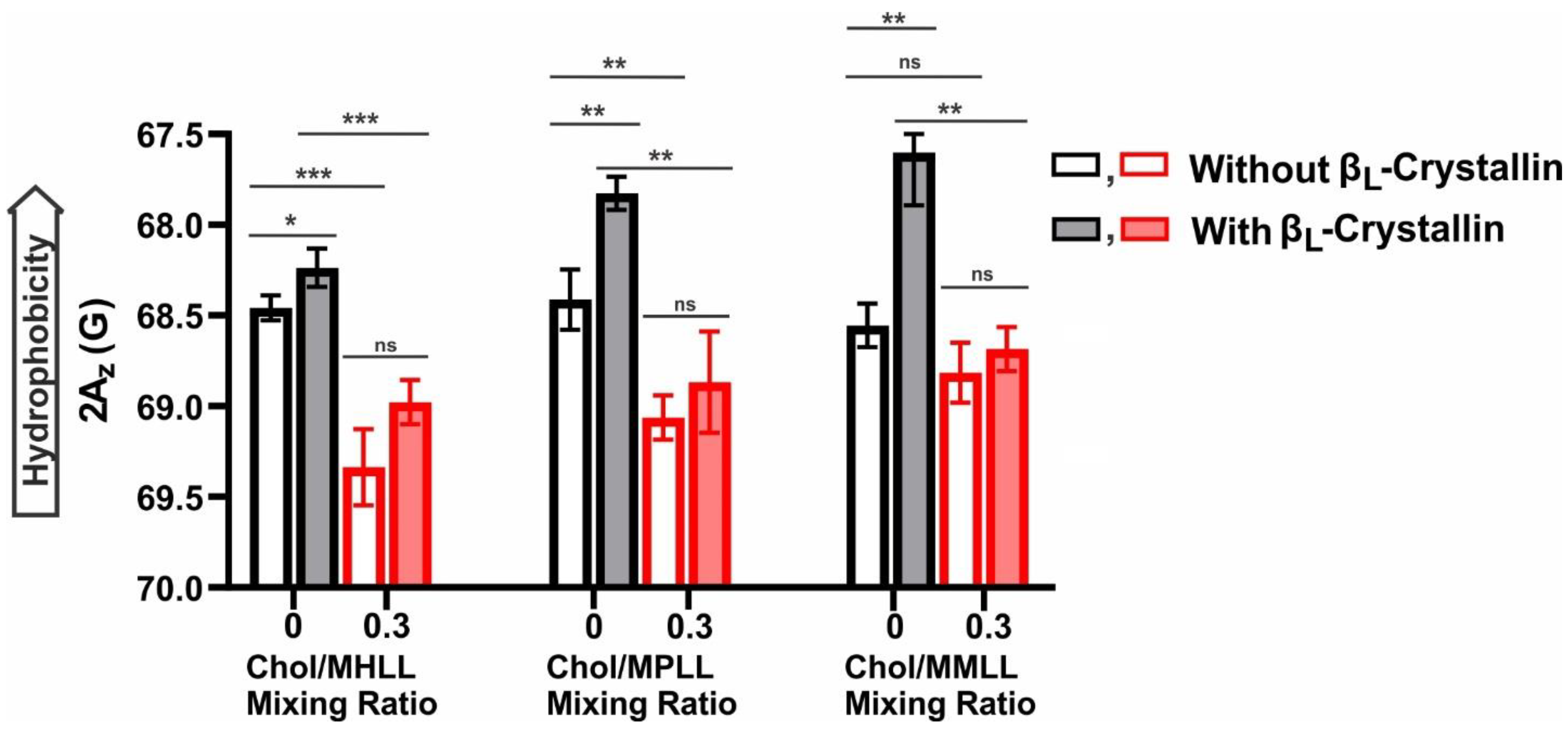
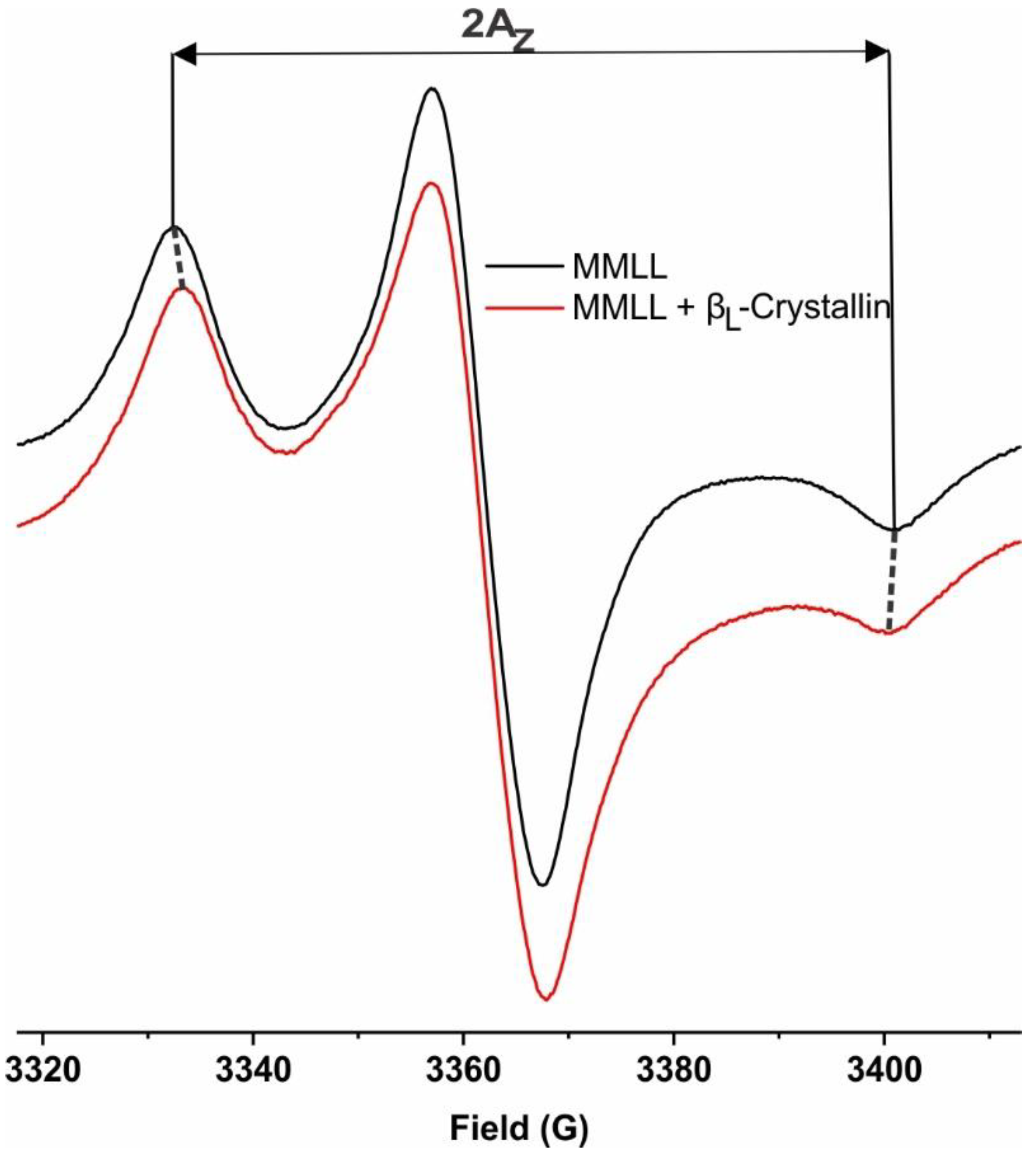
2.5. Hydrophobicity near the Surface of Model Lens-Lipid Membranes with the Addition of βL-Crystallin and Chol
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Purification of Native Bovine βL-Crystallin
4.3. Preparation of Model Human, Porcine, and Mouse Lens-Lipid Membranes
4.4. Electron Paramagnetic Resonance (EPR) Spin-Labeling Method for Investigation of βL-Crystallin Binding to Models of Human and Animal Eye Lens-Lipid Membranes
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPR | Electron paramagnetic resonance |
| MHLL | Model of human lens-lipid |
| MPLL | Model of porcine lens-lipid |
| MMLL | Model of mouse lens-lipid |
| MSO | Percentage of membrane surface occupied |
| MMSO | Maximum percentage of membrane surface occupied |
| CSL | Cholesterol analog cholestane spin-label |
| PL | Phospholipid |
| SM | Sphingomyelin |
| Chol | Cholesterol |
References
- Truscott, R.J.W.; Comte-Walters, S.; Ablonczy, Z.; Schwacke, J.H.; Berry, Y.; Korlimbinis, A.; Friedrich, M.G.; Schey, K.L. Tight Binding of Proteins to Membranes from Older Human Cells. Age 2011, 33, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J. Alpha-Crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Riazuddin, S.A.; McGreal, R.; Liu, W.; Cvekl, A.; Shiels, A. Lens Biology and Biochemistry. Prog. Mol. Biol. Transl. Sci. 2015, 134, 169–201. [Google Scholar] [CrossRef]
- Horwitz, J.; Bova, M.P.; Ding, L.-L.; Haley, D.A.; Stewart, P.L. Lens α-Crystallin: Function and Structure. Eye 1999, 13, 403–408. [Google Scholar] [CrossRef]
- Bloemendal, H.; De Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and Vision: Structure, Stability and Function of Lens Crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Augusteyn, R.C. On the Interaction of Alpha-Crystallin with Membranes. Curr. Eye Res. 1994, 13, 225–230. [Google Scholar] [CrossRef]
- Rocha, M.A.; Sprague-Piercy, M.A.; Kwok, A.O.; Roskamp, K.W.; Martin, R.W. Chemical Properties Determine Solubility and Stability in Βγ-Crystallins of the Eye Lens. ChemBioChem 2021, 22, 1329–1346. [Google Scholar] [CrossRef]
- Maulucci, G.; Papi, M.; Arcovito, G.; De Spirito, M. The Thermal Structural Transition of α-Crystallin Inhibits the Heat Induced Self-Aggregation. PLoS ONE 2011, 6, e18906. [Google Scholar] [CrossRef]
- Zhu, X.; Gaus, K.; Lu, Y.; Magenau, A.; Truscott, R.; Mitchell, T. α- and β-Crystallins Modulate the Head Group Order of Human Lens Membranes during Aging. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5162–5167. [Google Scholar] [CrossRef]
- Bloemendal, H.; Berbers, G.A.; De Jong, W.W.; Ramaekers, F.C.; Vermorken, A.J.; Dunia, I.; Benedetti, E.L. Interaction of Crystallins with the Cytoskeletal-Plasma Membrane Complex of the Bovine Lens. In Ciba Foundation Symposium 106—Human Cataract Formation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1984; pp. 177–190. [Google Scholar] [CrossRef]
- Datiles, M.B., III; Ansari, R.R.; Yoshida, J.; Brown, H.; Zambrano, A.I.; Tian, J.; Vitale, S.; Zigler, J.S., Jr.; Ferris, F.L., III; West, K.S.; et al. Longitudinal Study of Age-Related Cataract Using Dynamic Light Scattering: Loss of α-Crystallin Leads to Nuclear Cataract Development. Ophthalmology 2016, 12, 248–254. [Google Scholar] [CrossRef]
- Timsina, R.; Mainali, L. Association of Alpha-Crystallin with Fiber Cell Plasma Membrane of the Eye Lens Accompanied by Light Scattering and Cataract Formation. Membranes 2021, 11, 447. [Google Scholar] [CrossRef]
- Cenedella, R.J.; Fleschner, C.R. Selective Association of Crystallins with Lens “native” Membrane during Dynamic Cataractogenesis. Curr. Eye Res. 1992, 11, 801–815. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Truscott, R.J.W. Large-Scale Binding of α-Crystallin to Cell Membranes of Aged Normal Human Lenses: A Phenomenon That Can Be Induced by Mild Thermal Stress. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5145–5152. [Google Scholar] [CrossRef]
- Chandrasekher, G.; Cenedella, R.J. Protein Associated with Human Lens ‘Native’ Membrane during Aging and Cataract Formation. Exp. Eye Res. 1995, 60, 707–717. [Google Scholar] [CrossRef]
- Moffat, B.A.; Landman, K.A.; Truscott, R.J.W.; Sweeney, M.H.J.; Pope, J.M. Age-Related Changes in the Kinetics of Water Transport in Normal Human Lenses. Exp. Eye Res. 1999, 69, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, O.P.; Srivastava, K.; Silney, C. Levels of Crystallin Fragments and Identification of Their Origin in Water Soluble High Molecular Weight (HMW) Proteins of Human Lenses. Curr. Eye Res. 1996, 15, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A.; Petrash, J.M. Characterization of Alpha-Crystallin-Plasma Membrane Binding. J. Biol. Chem. 2000, 275, 6664–6672. [Google Scholar] [CrossRef]
- Srivastava, K.; Chaves, J.M.; Srivastava, O.P.; Kirk, M. Multi-Crystallin Complexes Exist in the Water-Soluble High Molecular Weight Protein Fractions of Aging Normal and Cataractous Human Lenses. Exp. Eye Res. 2008, 87, 356–366. [Google Scholar] [CrossRef]
- Harrington, V.; Srivastava, O.P.; Kirk, M. Proteomic Analysis of Water Insoluble Proteins from Normal and Cataractous Human Lenses. Mol. Vis. 2007, 13, 1680–1694. [Google Scholar]
- Cobb, B.A.; Petrash, J.M. Alpha-Crystallin Chaperone-like Activity and Membrane Binding in Age-Related Cataracts. Biochemistry 2002, 41, 483–490. [Google Scholar] [CrossRef]
- Trossi-Torres, G.; Timsina, R.; Mainali, L. Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation. Membranes 2022, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Wellisch, S.; Haemmerle, D.; Mainali, L. Binding of Alpha-Crystallin to Cortical and Nuclear Lens Lipid Membranes Derived from a Single Lens. Int. J. Mol. Sci. 2022, 23, 11295. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Trossi-Torres, G.; Thieme, J.; O’Dell, M.; Khadka, N.K.; Mainali, L. Alpha-Crystallin Association with the Model of Human and Animal Eye Lens-Lipid Membranes Is Modulated by Surface Hydrophobicity of Membranes. Curr. Eye Res. 2022, 47, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Trossi-Torres, G.; O’Dell, M.; Khadka, N.K.; Mainali, L. Cholesterol and Cholesterol Bilayer Domains Inhibit Binding of Alpha-Crystallin to the Membranes Made of the Major Phospholipids of Eye Lens Fiber Cell Plasma Membranes. Exp. Eye Res. 2021, 206, 108544. [Google Scholar] [CrossRef] [PubMed]
- Khadka, N.K.; Timsina, R.; Mainali, L. An AFM Approach Applied in a Study of α-Crystallin Membrane Association: New Insights into Lens Hardening and Presbyopia Development. Membranes 2022, 12, 522. [Google Scholar] [CrossRef]
- Mainali, L.; O’Brien, W.J.; Timsina, R. Interaction of Alpha-Crystallin with Phospholipid Membranes. Curr. Eye Res. 2021, 46, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Khadka, N.K.; Maldonado, D.; Mainali, L. Interaction of Alpha-Crystallin with Four Major Phospholipids of Eye Lens Membranes. Exp. Eye Res. 2021, 202, 108337. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A.; Petrash, J.M. Factors Influencing Alpha-Crystallin Association with Phospholipid Vesicles. Mol. Vis. 2002, 8, 85–93. [Google Scholar]
- Borchman, D.; Tang, D. Binding Capacity of Alpha-Crystallin to Bovine Lens Lipids. Exp. Eye Res. 1996, 63, 407–410. [Google Scholar] [CrossRef]
- Tang, D.; Borchman, D.; Yappert, M.C.; Cenedella, R.J. Influence of Cholesterol on the Interaction of Alpha-Crystallin with Phospholipids. Exp. Eye Res. 1998, 66, 559–567. [Google Scholar] [CrossRef]
- Grami, V.; Marrero, Y.; Huang, L.; Tang, D.; Yappert, M.C.; Borchman, D. α-Crystallin Binding in Vitro to Lipids from Clear Human Lenses. Exp. Eye Res. 2005, 81, 138–146. [Google Scholar] [CrossRef]
- Tang, D.; Borchman, D.; Yappert, M.C. Alpha-Crystallin/Lens Lipid Interactions Using Resonance Energy Transfer. Ophthalmic Res. 1999, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-P.; McArthur, J.D.; Friedrich, M.G.; Truscott, R.J.W.; Aquilina, J.A. Understanding the α-Crystallin Cell Membrane Conjunction. Mol. Vis. 2011, 17, 2798–2807. [Google Scholar] [PubMed]
- Borchman, D.; Stimmelmayr, R.; George, J.C. Whales, Lifespan, Phospholipids, and Cataracts. J. Lipid Res. 2017, 58, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D. Lipid Conformational Order and the Etiology of Cataract and Dry Eye. J. Lipid Res. 2021, 62, 100039. [Google Scholar] [CrossRef]
- Borchman, D.; Yappert, M.C. Lipids and the Ocular Lens. J. Lipid Res. 2010, 51, 2473–2488. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Grami, V.; Marrero, Y.; Tang, D.; Yappert, M.C.; Rasi, V.; Borchman, D. Human Lens Phospholipid Changes with Age and Cataract. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1682–1689. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Changes in the Properties and Organization of Human Lens Lipid Membranes Occurring with Age. Curr. Eye Res. 2017, 42, 721–731. [Google Scholar] [CrossRef]
- Paterson, C.A.; Zeng, J.; Husseini, Z.; Borchman, D.; Delamere, N.A.; Garland, D.; Jimenez-Asensio, J. Calcium ATPase Activity and Membrane Structure in Clear and Cataractous Human Lenses. Curr. Eye Res. 1997, 16, 333–338. [Google Scholar] [CrossRef]
- Deeley, J.M.; Mitchell, T.W.; Wei, X.; Korth, J.; Nealon, J.R.; Blanksby, S.J.; Truscott, R.J.W. Human Lens Lipids Differ Markedly from Those of Commonly Used Experimental Animals. Biochim. Biophys. Acta 2008, 1781, 288–298. [Google Scholar] [CrossRef]
- Stimmelmayr, R.; Borchman, D. Lens Lipidomes Among Phocidae and Odobenidae. Aquat. Mamm. 2018, 43, 506–518. [Google Scholar] [CrossRef]
- Deeley, J.M.; Hankin, J.A.; Friedrich, M.G.; Murphy, R.C.; Truscott, R.J.W.; Mitchell, T.W.; Blanksby, S.J. Sphingolipid Distribution Changes with Age in the Human Lens. J. Lipid Res. 2010, 51, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.F.; Cenedella, R.J.; Mason, R.P. Evidence for Distinct Cholesterol Domains in Fiber Cell Membranes from Cataractous Human Lenses. J. Biol. Chem. 2001, 276, 13573–13578. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Subczynski, W.K. Why Is Very High Cholesterol Content Beneficial for the Eye Lens but Negative for Other Organs? Nutrients 2019, 11, 1083. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of Membranes Derived from the Total Lipids Extracted from the Human Lens Cortex and Nucleus. Biochim. Biophys. Acta 2013, 1828, 1432–1440. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Raguz, M.; Widomska, J.; Mainali, L.; Konovalov, A. Functions of Cholesterol and the Cholesterol Bilayer Domain Specific to the Fiber-Cell Plasma Membrane of the Eye Lens. J. Membr. Biol. 2012, 245, 51–68. [Google Scholar] [CrossRef]
- Stein, N.; Subczynski, W.K. Differences in the Properties of Porcine Cortical and Nuclear Fiber Cell Plasma Membranes Revealed by Saturation Recovery EPR Spin Labeling Measurements. Exp. Eye Res. 2021, 206, 108536. [Google Scholar] [CrossRef]
- Lampi, K.J.; Wilmarth, P.A.; Murray, M.R.; David, L.L. Lens β-Crystallins: The Role of Deamidation and Related Modifications in Aging and Cataract. Prog. Biophys. Mol. Biol. 2014, 115, 21–31. [Google Scholar] [CrossRef]
- Slingsby, C.; Driessen, H.P.; Mahadevan, D.; Bax, B.; Blundell, T.L. Evolutionary and Functional Relationships between the Basic and Acidic Beta-Crystallins. Exp. Eye Res. 1988, 46, 375–403. [Google Scholar] [CrossRef]
- Sweeney, M.H.; Truscott, R.J. An Impediment to Glutathione Diffusion in Older Normal Human Lenses: A Possible Precondition for Nuclear Cataract. Exp. Eye Res. 1998, 67, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Truscott, R.J.W. Membrane Association of Proteins in the Aging Human Lens: Profound Changes Take Place in the Fifth Decade of Life. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4786–4793. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.W. Age-Related Nuclear Cataract-Oxidation Is the Key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.G.; Carver, J.A.; Truscott, R.J.W. 1H-NMR Spectroscopy of Bovine Lens β-Crystallin. Eur. J. Biochem. 1993, 213, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Polnaszek, C.F.; Smith, I.C. Spin Labels in Membranes. Problems in Practice. Biochim. Biophys. Acta 1978, 515, 395–436. [Google Scholar] [CrossRef]
- Raguz, M.; Mainali, L.; Widomska, J.; Subczynski, W.K. Using Spin-Label Electron Paramagnetic Resonance (EPR) to Discriminate and Characterize the Cholesterol Bilayer Domain. Chem. Phys. Lipids 2011, 164, 819–829. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Phases and Domains in Sphingomyelin-Cholesterol Membranes: Structure and Properties Using EPR Spin-Labeling Methods. Eur. Biophys. J. 2012, 41, 147–159. [Google Scholar] [CrossRef][Green Version]
- Kusumi, A.; Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Hyde, J.S.; Merkle, H. Spin-Label Studies on Phosphatidylcholine-Cholesterol Membranes: Effects of Alkyl Chain Length and Unsaturation in the Fluid Phase. Biochim. Biophys. Acta 1986, 854, 307–317. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Yin, J.J.; Hyde, J.S.; Kusumi, A. Hydrophobic Barriers of Lipid Bilayer Membranes Formed by Reduction of Water Penetration by Alkyl Chain Unsaturation and Cholesterol. Biochemistry 1994, 33, 7670–7681. [Google Scholar] [CrossRef]
- Barba-Bon, A.; Nilam, M.; Hennig, A. Supramolecular Chemistry in the Biomembrane. ChemBioChem 2020, 21, 886–910. [Google Scholar] [CrossRef]
- Edidin, M. The State of Lipid Rafts: From Model Membranes to Cells. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 257–283. [Google Scholar] [CrossRef]
- London, E. Insights into Lipid Raft Structure and Formation from Experiments in Model Membranes. Curr. Opin. Struct. Biol. 2002, 12, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L.C. Model Systems, Lipid Rafts, and Cell Membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.F.F.; Vaz, W.L.C.; Thompson, T.E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: A free volume analysis. Biochemistry 1992, 31, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, A.; Subczynski, W.K. The Liquid-Ordered Phase in Sphingomyelincholesterol Membranes as Detected by the Discrimination by Oxygen Transport (DOT) Method. Cell. Mol. Biol. Lett. 2008, 13, 430–451. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Das, K.P. Differential Recognition of Natural and Nonnatural Substrate by Molecular Chaperone Alpha-Crystallin-A Subunit Exchange Study. Biopolymers 2007, 85, 189–197. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Mainali, L.; Pasenkiewicz-Gierula, M.; Subczynski, W.K. Formation of Cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Membranes Made of the Major Phospholipids of Human Eye Lens Fiber Cell Plasma Membranes. Curr. Eye Res. 2020, 45, 162–172. [Google Scholar] [CrossRef]
- Khadka, N.K.; Mortimer, M.-F.; Marosvari, M.; Timsina, R.; Mainali, L. Membrane Elasticity Modulated by Cholesterol in Model of Porcine Eye Lens-Lipid Membrane. Exp. Eye Res. 2022, 220, 109131. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Phase-Separation and Domain-Formation in Cholesterol-Sphingomyelin Mixture: Pulse-EPR Oxygen Probing. Biophys. J. 2011, 101, 837–846. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazen, P.; Trossi-Torres, G.; Khadka, N.K.; Timsina, R.; Mainali, L. Binding of βL-Crystallin with Models of Animal and Human Eye Lens-Lipid Membrane. Int. J. Mol. Sci. 2023, 24, 13600. https://doi.org/10.3390/ijms241713600
Hazen P, Trossi-Torres G, Khadka NK, Timsina R, Mainali L. Binding of βL-Crystallin with Models of Animal and Human Eye Lens-Lipid Membrane. International Journal of Molecular Sciences. 2023; 24(17):13600. https://doi.org/10.3390/ijms241713600
Chicago/Turabian StyleHazen, Preston, Geraline Trossi-Torres, Nawal K. Khadka, Raju Timsina, and Laxman Mainali. 2023. "Binding of βL-Crystallin with Models of Animal and Human Eye Lens-Lipid Membrane" International Journal of Molecular Sciences 24, no. 17: 13600. https://doi.org/10.3390/ijms241713600
APA StyleHazen, P., Trossi-Torres, G., Khadka, N. K., Timsina, R., & Mainali, L. (2023). Binding of βL-Crystallin with Models of Animal and Human Eye Lens-Lipid Membrane. International Journal of Molecular Sciences, 24(17), 13600. https://doi.org/10.3390/ijms241713600






