Analysis of WAK Genes in Nine Cruciferous Species with a Focus on Brassica napus L.
Abstract
:1. Introduction
2. Results
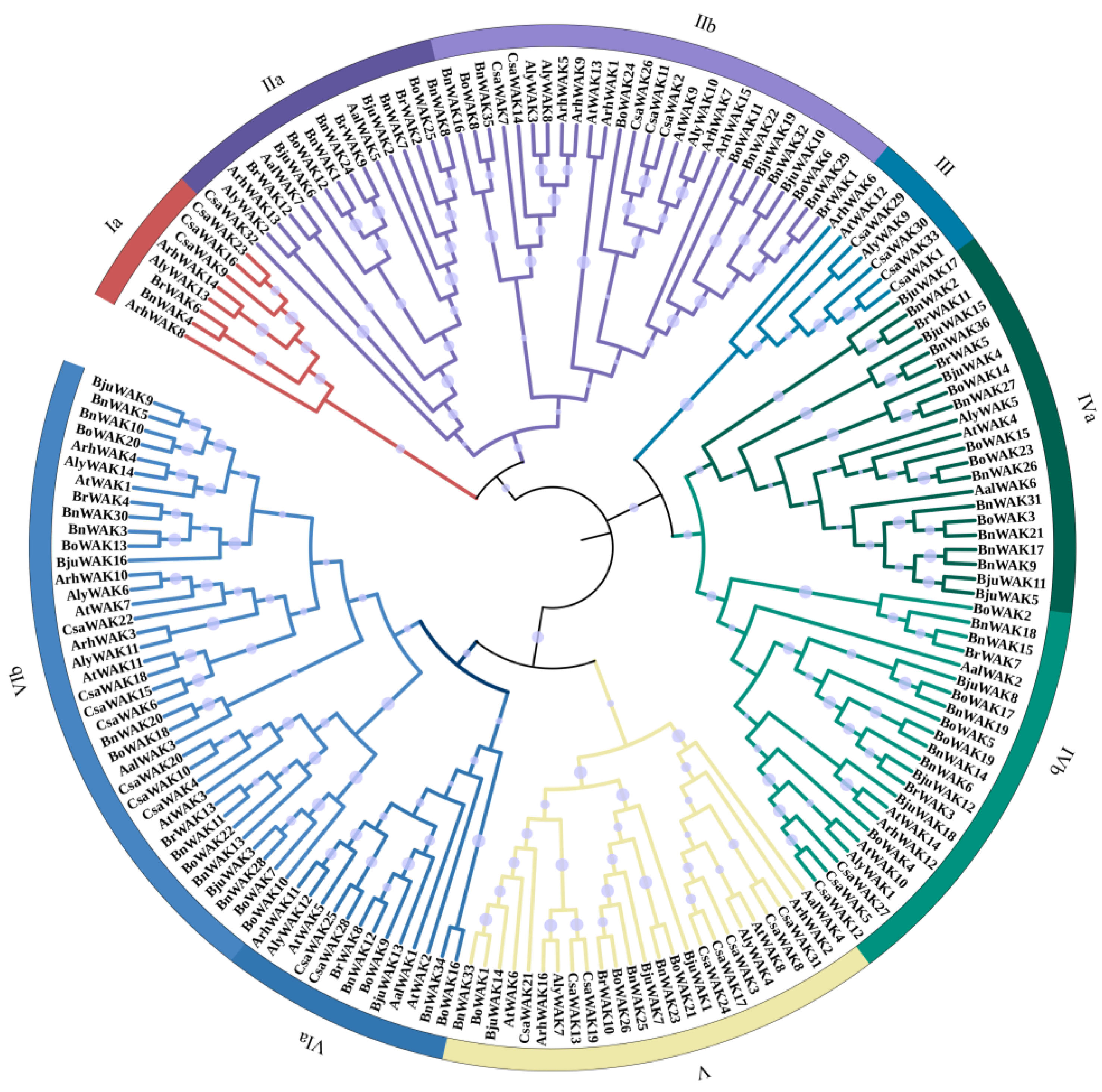
2.1. Analysis of the WAK Protein Phylogeny and Characterization
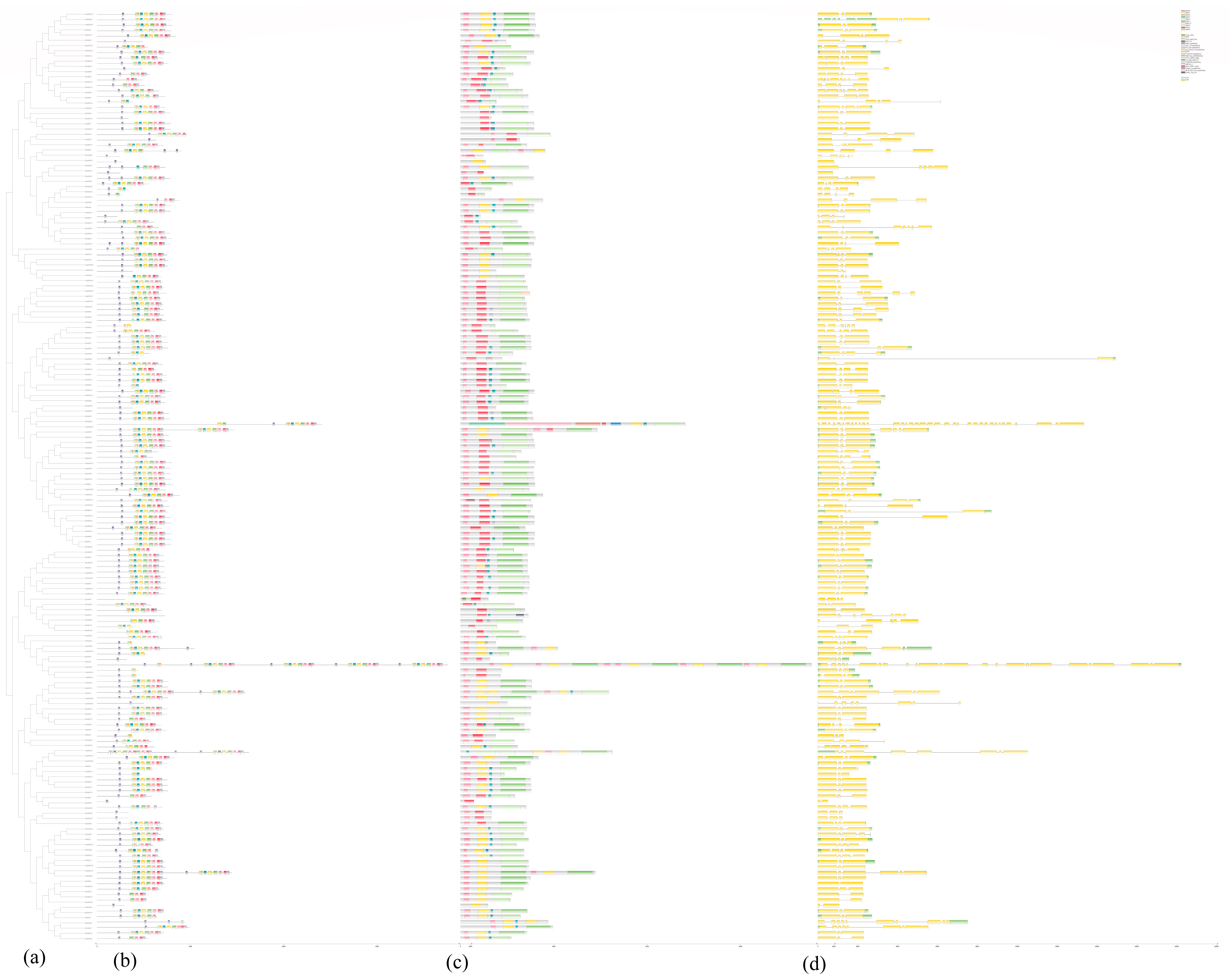
2.2. Gene Organization and Conserved Motif of the WAK Gene Family in B. napus
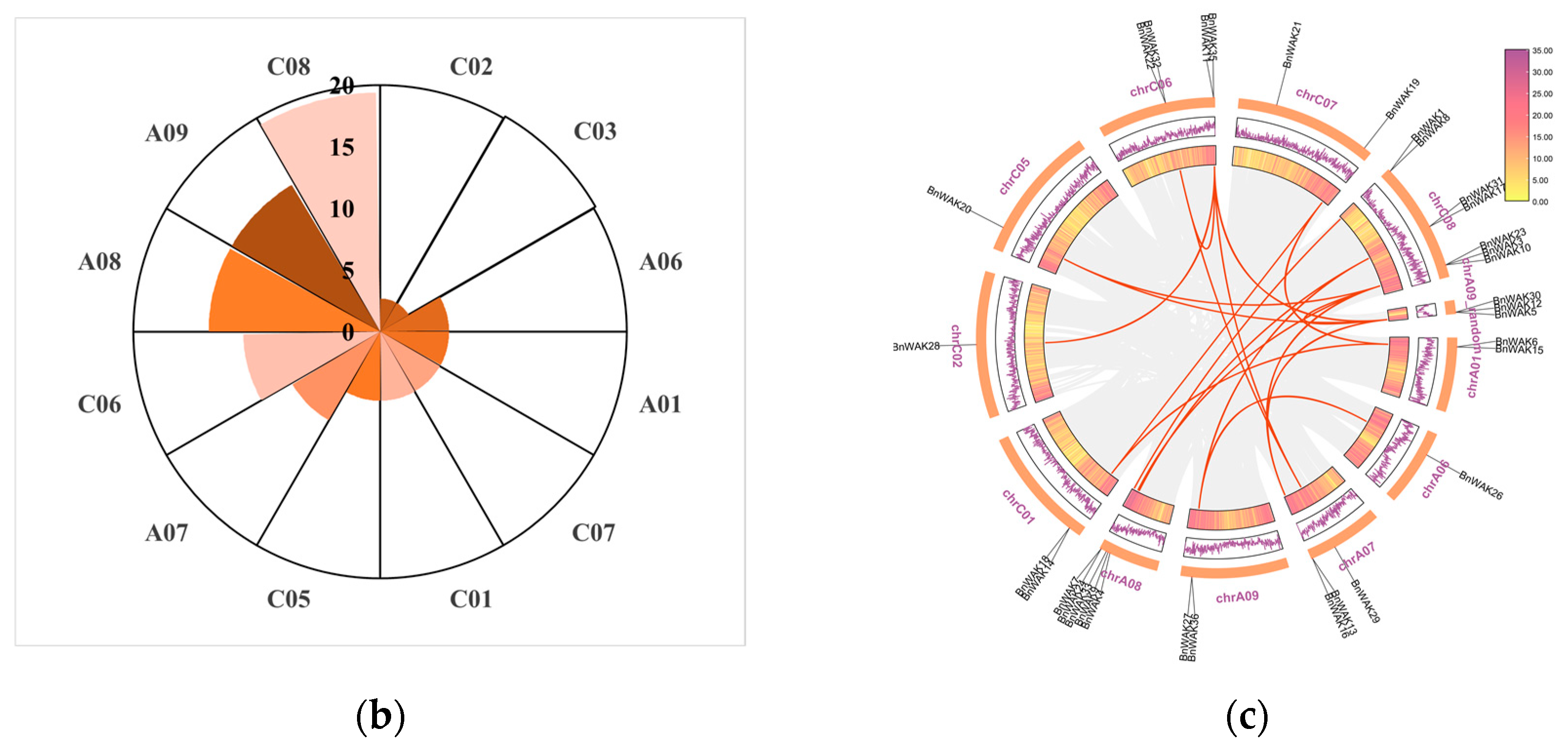
2.3. Chromosomal Localization and Collinearity Analysis of BnWAKs
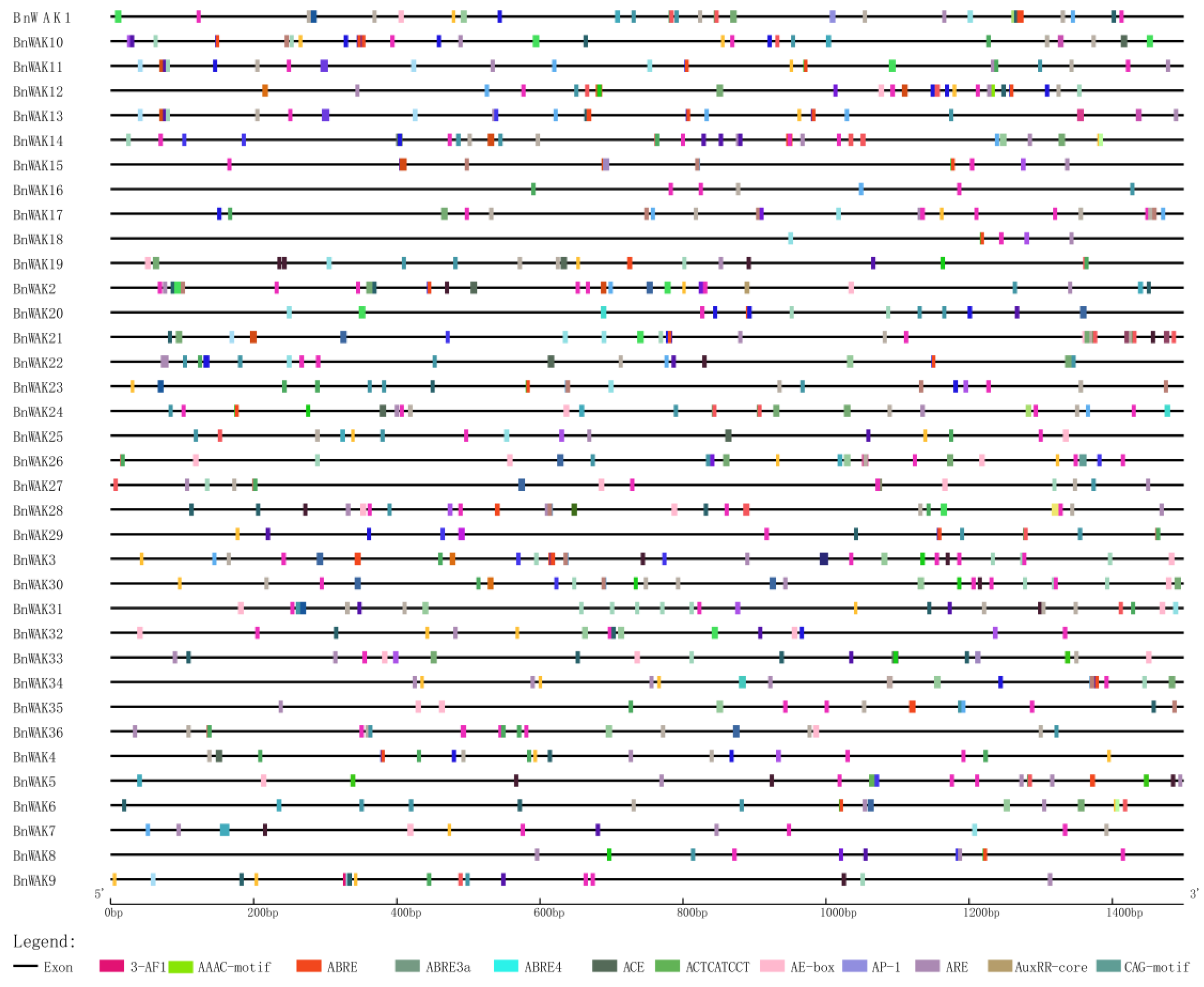
2.4. Cis-Acting Element Analysis of WAK Promoters in B. napus
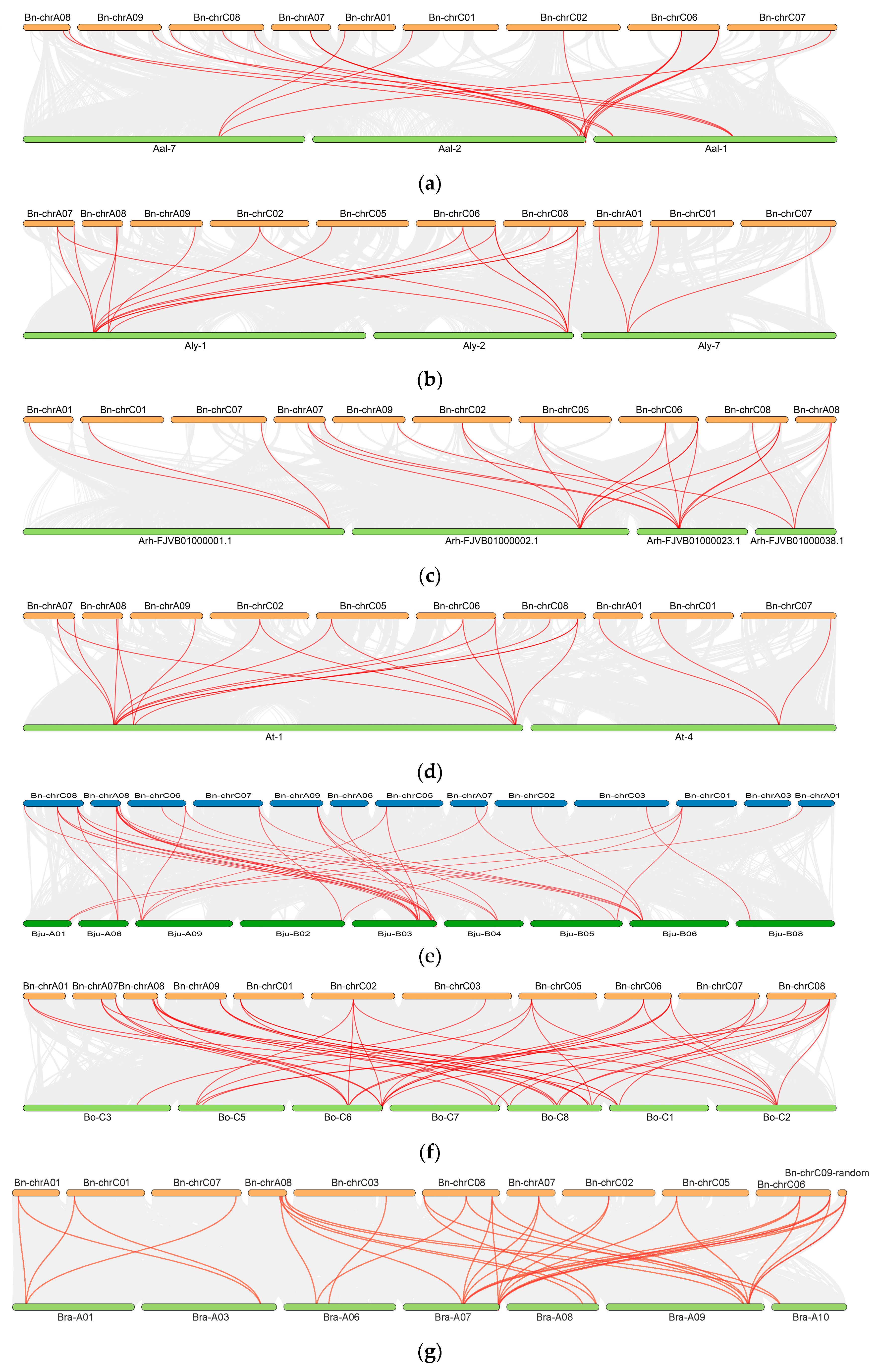
2.5. Interspecies Syntenic Analysis in Cruciferous Species
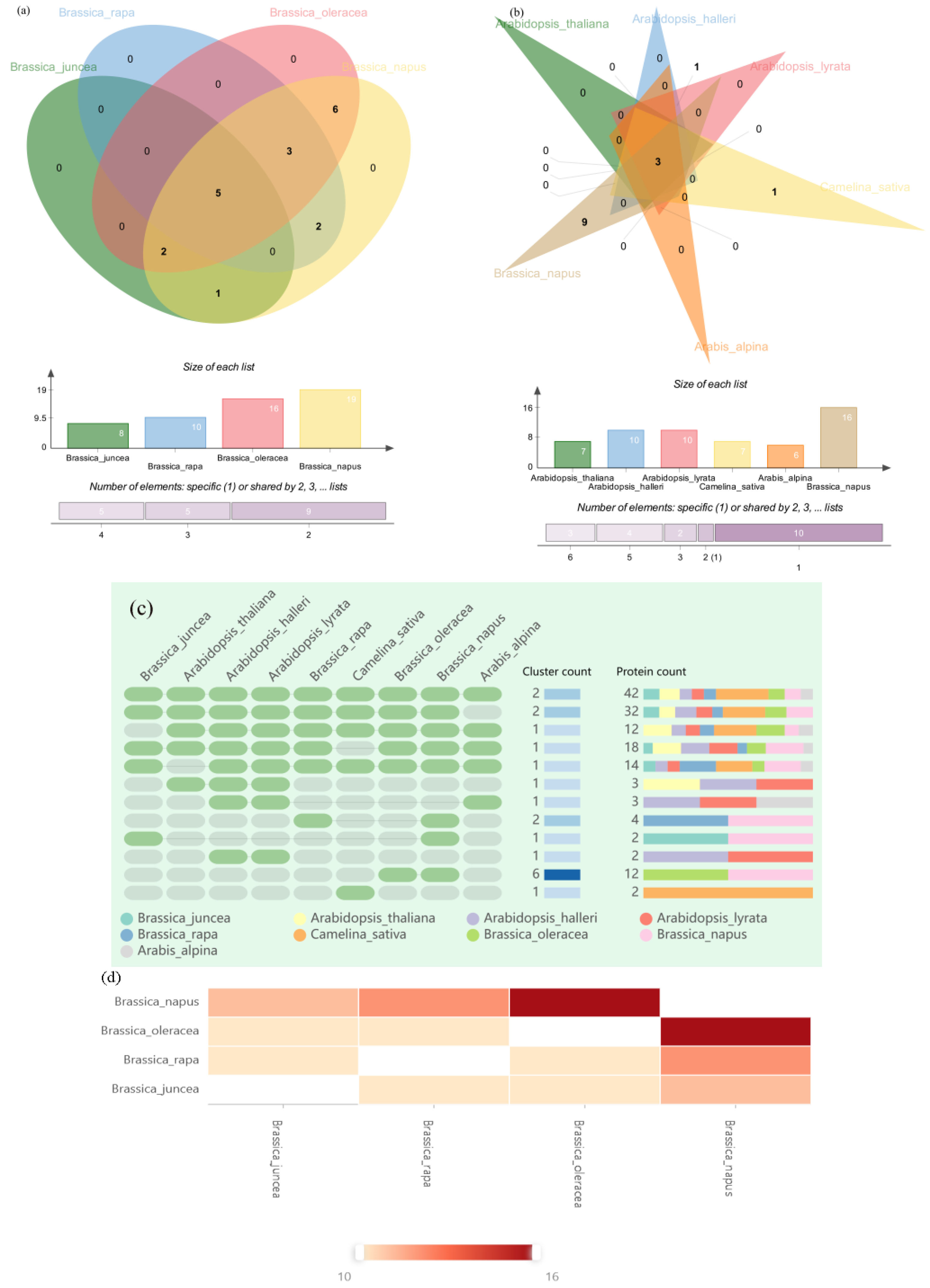
2.6. Orthologous Gene Clustering of WAK Gene Family in Five Cruciferous Species
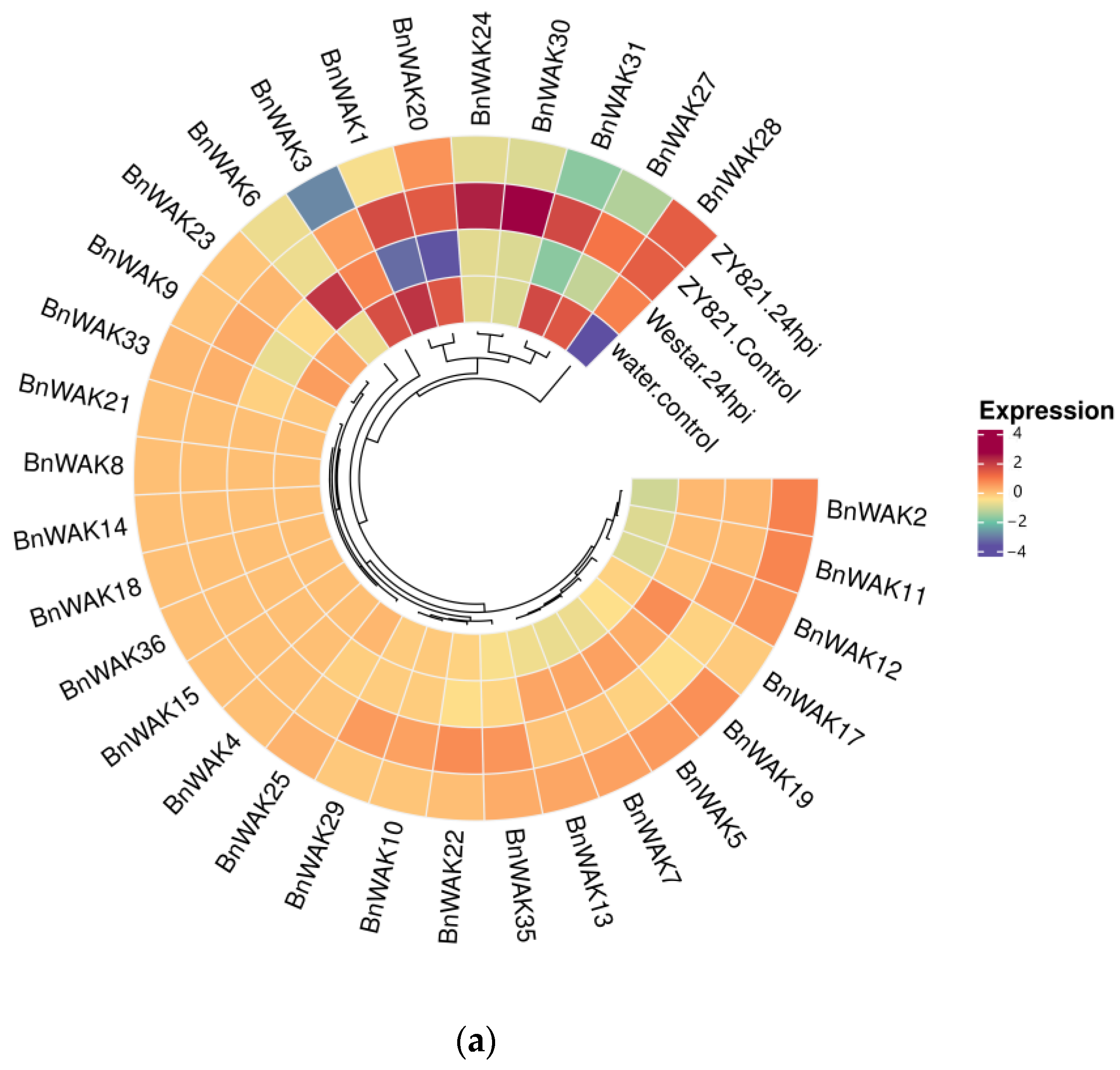
2.7. Expression Pattern of BnWAK Gene in Response to Different Stresses
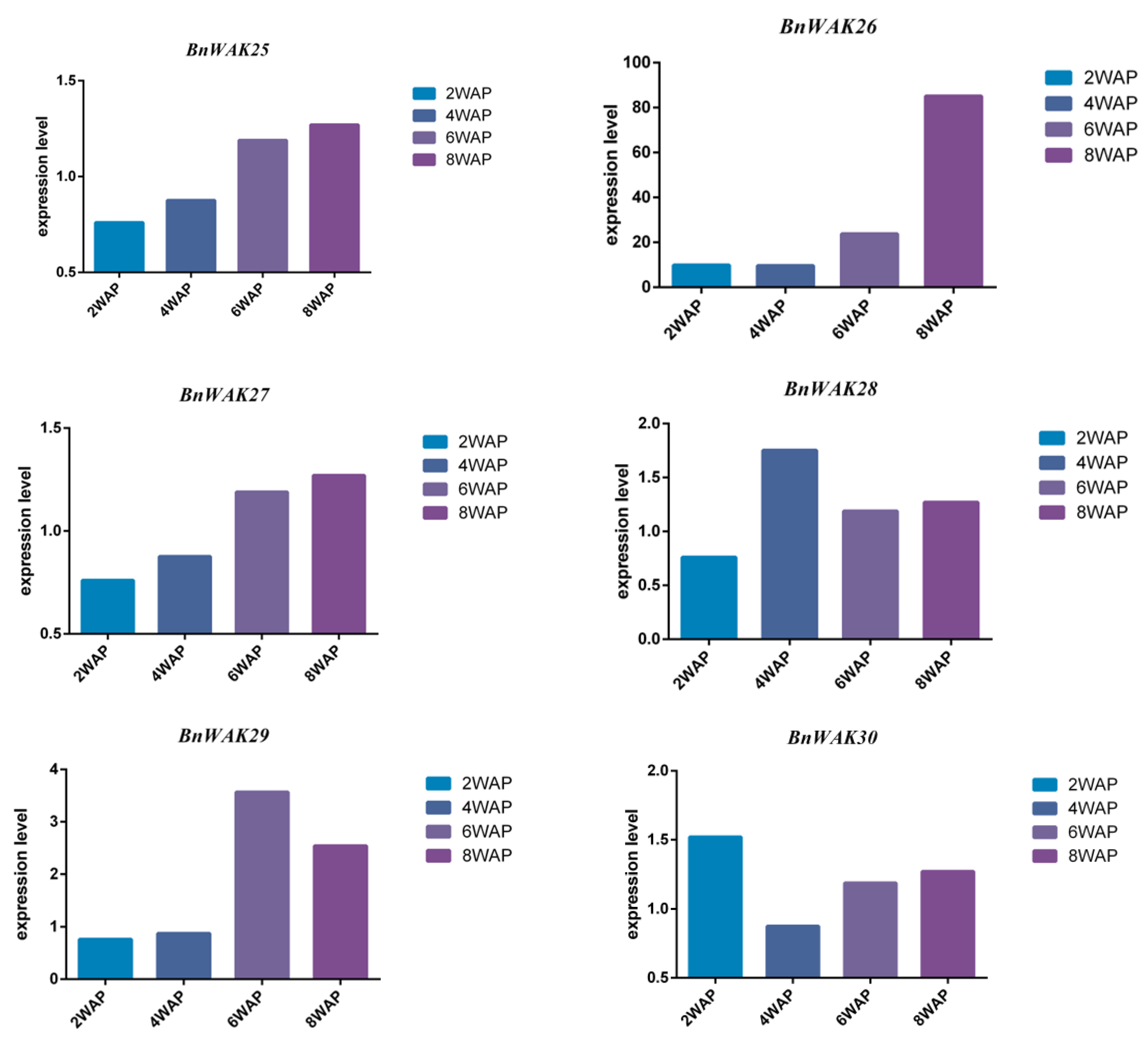
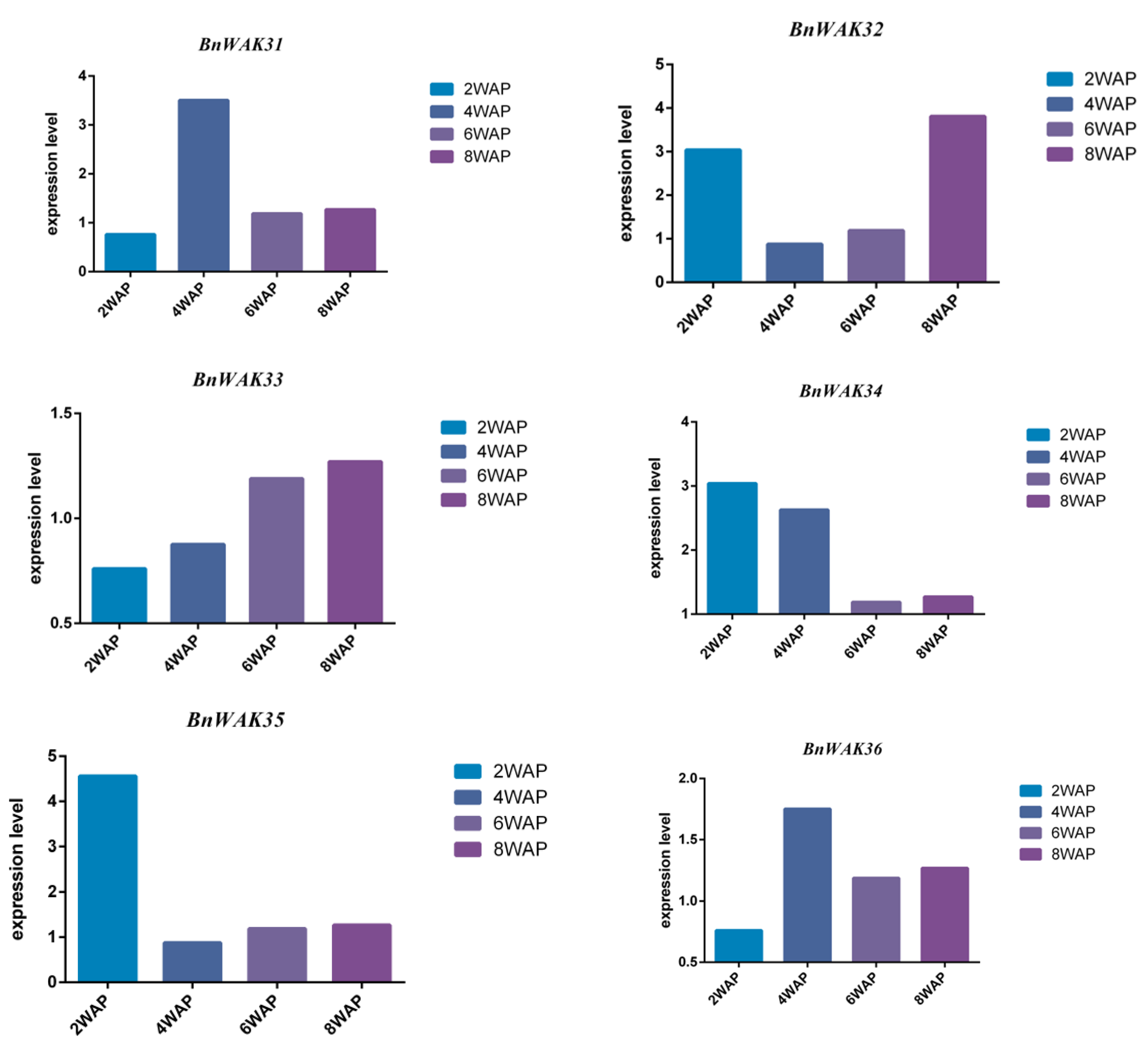
2.8. Expression Pattern of BnWAK Gene in Oil Accumulation
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Plant Materials
4.2. Identification and Analysis of WAK Family Members in Nine Cruciferous Species
4.3. Alignment of Multiple Sequences and Construction of Phylogenetic Trees
4.4. Genomic Information and Analysis of Physiological and Biochemical Properties
4.5. Analysis of WAK Family Protein Sequences for Gene Structure, Motifs and Gene Promoter Cis-Acting Elements
4.6. Collinearity Analysis and Homologous Gene Analysis
4.7. Expression and Stress Response Expression Profiling of BnWAK
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.H.; Cheeseman, I.; He, D.; Kohorn, B.D. A cluster of five cell wall-associated receptor kinase genes, Wak1-5, are expressed in specific organs of Arabidopsis. Plant Mol. Biol. 1999, 39, 1189–1196. [Google Scholar] [CrossRef]
- Decreux, A.; Messiaen, J. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 2005, 46, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Hammond-Kosack, K.E.; Kanyuka, K. WAKsing plant immunity, waning diseases. J. Exp. Bot. 2022, 73, 22–37. [Google Scholar] [CrossRef]
- Kohorn, B.D. WAKs; cell wall associated kinases. Curr. Opin. Cell Biol. 2001, 13, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Decreux, A.; Thomas, A.; Spies, B.; Brasseur, R.; Van Cutsem, P.; Messiaen, J. In vitro characterization of the homogalacturonan-binding domain of the wall-associated kinase WAK1 using site-directed mutagenesis. Phytochemistry 2006, 67, 1068–1079. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Liu, H.; Ou, M.; Ye, H.; Zhao, P. Identification and Characterization of Wall-Associated Kinase (WAK) and WAK-like (WAKL) Gene Family in Juglans regia and Its Wild Related Species Juglans mandshurica. Genes 2022, 13, 134. [Google Scholar] [CrossRef]
- Kohorn, B.D. Cell wall-associated kinases and pectin perception. J. Exp. Bot. 2016, 67, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kohorn, B.D.; Kohorn, S.L.; Todorova, T.; Baptiste, G.; Stansky, K.; McCullough, M. A dominant allele of Arabidopsis pectin-binding wall-associated kinase induces a stress response suppressed by MPK6 but not MPK3 mutations. Mol. Plant 2012, 5, 841–851. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; Lorenzo, G.D. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Kanyuka, K.; Rudd, J.J. Cell surface immune receptors: The guardians of the plant’s extracellular spaces. Curr. Opin. Plant Biol. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Zheng, X.; Tao, L.; Yang, Y.; Gao, L.; Xiong, J. Aeration Increases Cadmium (Cd) Retention by Enhancing Iron Plaque Formation and Regulating Pectin Synthesis in the Roots of Rice (Oryza sativa) Seedlings. Rice 2019, 12, 28. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Chao, Q.; Zhang, N.; Ye, J.; Tan, G.; Li, B.; Xing, Y.; Zhang, B.; Liu, H.; Fengler, K.A.; et al. A maize wall-associated kinase confers quantitative resistance to head smut. Nat. Genet. 2015, 47, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Praz, C.; Li, B.; Singla, J.; Robert, C.; Kessel, B.; Scheuermann, D.; Lüthi, L.; Ouzunova, M.; Erb, M.; et al. Fungal resistance mediated by maize wall-associated kinase ZmWAK-RLK1 correlates with reduced benzoxazinoid content. New Phytol. 2019, 221, 976–987. [Google Scholar] [CrossRef]
- Hu, K.; Cao, J.; Zhang, J.; Xia, F.; Ke, Y.; Zhang, H.; Xie, W.; Liu, H.; Cui, Y.; Cao, Y.; et al. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, L.; Jamieson, P.; Zhang, L.; Zhao, Z.; Babilonia, K.; Shao, W.; Wu, L.; Mustafa, R.; Amin, I.; et al. The Cotton Wall-Associated Kinase GhWAK7A Mediates Responses to Fungal Wilt Pathogens by Complexing with the Chitin Sensory Receptors. Plant Cell 2020, 32, 3978–4001. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, C.; Zhou, J.; Yuan, Y.; Feng, Z.; Shi, Y.; Zhao, L.; Zhang, Y.; Wei, F.; Zhu, H. A cotton WAKL protein interacted with a DnaJ protein and was involved in defense against Verticillium dahliae. Int. J. Biol. Macromol. 2021, 167, 633–643. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Yang, Y.; Fu, G.; Tao, L.; Xiong, J. Effects and oxygen-regulated mechanisms of water management on cadmium (Cd) accumulation in rice (Oryza sativa). Sci. Total Environ. 2022, 846, 157484. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, B.; Gao, C.; Zhang, H.; Wen, F.; Tao, L.; Fu, G.; Xiong, J. The Evolution and Functional Roles of miR408 and Its Targets in Plants. Int. J. Mol. Sci. 2022, 23, 530. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Tao, L.; Cao, Z.; Tang, W.; Zhang, J.; Yu, X.; Fu, G.; Zhang, X.; Lu, Y. Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: A review. Sci. Total Environ. 2020, 708, 135186. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chang, J.; Chen, R.; Li, H.; Lu, H.; Tao, L.; Xiong, J. Comparison on cellular mechanisms of iron and cadmium accumulation in rice: Prospects for cultivating Fe-rich but Cd-free rice. Rice 2016, 9, 39. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, X.; Wang, M.; Li, C.; Li, C.; Zhao, R.; Zhu, S.; He, Q.; Chen, J. Identification and profiling of upland cotton microRNAs at fiber initiation stage under exogenous IAA application. BMC Genom. 2019, 20, 421. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Zhang, F.; Yu, J.; Li, C.; Zhao, T.; Zhang, Y.; Xie, Q.; Su, B.; Mei, L.; et al. Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. Cell Mol. Biol. 2019, 100, 784–800. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, X.; Li, H.; Ussi, A.; Gao, Y.; Xiong, J. Roles of lncRNAs in Rice: Advances and Challenges. Rice Sci. 2020, 27, 3. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Chen, R.; Fu, G.; Chen, T.; Tao, L. Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa). Environ. Exp. Bot. 2016, 122, 141–149. [Google Scholar] [CrossRef]
- Zhang, N.; Jing, P. Anthocyanins in Brassicaceae: Composition, stability, bioavailability, and potential health benefits. Crit. Rev. Food Sci. Nutr. 2022, 62, 2205–2220. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; German, D.A.; Al-Shehbaz, I.A.; Yue, J.; Sun, H. Phylogeny of Euclidieae (Brassicaceae) based on plastome and nuclear ribosomal DNA data. Mol. Phylogenet. Evol. 2020, 153, 106940. [Google Scholar] [CrossRef]
- Cardoza, V.; Stewart, C.N., Jr. Canola (Brassica napus L.). Methods Mol. Biol. 2006, 343, 257–266. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, H.; Lu, S.; Yu, L.; Zhang, G.; Zhang, Y.; Yang, Q.Y.; Zhou, Y.; Wang, X.; Ma, W.; et al. Genome- and transcriptome-wide association studies provide insights into the genetic basis of natural variation of seed oil content in Brassica napus. Mol. Plant 2021, 14, 470–487. [Google Scholar] [CrossRef]
- Tan, Z.; Xie, Z.; Dai, L.; Zhang, Y.; Zhao, H.; Tang, S.; Wan, L.; Yao, X.; Guo, L.; Hong, D. Genome- and transcriptome-wide association studies reveal the genetic basis and the breeding history of seed glucosinolate content in Brassica napus. Plant Biotechnol. J. 2022, 20, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Cui, Y.; Ding, Y.; Mei, J.; Dong, H.; Zhang, W.; Wu, S.; Liang, Y.; Zhang, C.; Li, J.; et al. Time-Series Analyses of Transcriptomes and Proteomes Reveal Molecular Networks Underlying Oil Accumulation in Canola. Front. Plant Sci. 2017, 7, 2007. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Hussain, M.A.; Wei, S.; He, H.; Zaman, Q.U.; Xuekun, Z.; Hasanuzzaman, M. Omics: The way forward to enhance abiotic stress tolerance in Brassica napus L. GM Crops Food 2021, 12, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.F.; Strelkov, S.E.; Feng, J.; Gossen, B.D.; Howard, R.J. Plasmodiophora brassicae: A review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol. Plant Pathol. 2012, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Girard, I.J.; Tong, C.; Becker, M.G.; Mao, X.; Huang, J.; de Kievit, T.; Fernando, W.; Liu, S.; Belmonte, M.F. RNA sequencing of Brassica napus reveals cellular redox control of Sclerotinia infection. J. Exp. Bot. 2017, 68, 5079–5091. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Z.; Xing, M.; Yang, Y.; Wu, X.; Liu, H.; Liang, W. Heat Stress Suppresses Brassica napus Seed Oil Accumulation by Inhibition of Photosynthesis and BnWRI1 Pathway. Plant Cell Physiol. 2019, 60, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Fankhauser, C.; Coupland, G.; Blázquez, M.A. Signalling for developmental plasticity. Trends Plant Sci. 2004, 9, 309–314. [Google Scholar] [CrossRef]
- de Oliveira, L.F.V.; Christoff, A.P.; de Lima, J.C.; de Ross, B.C.F.; Sachetto-Martins, G.; Margis-Pinheiro, M.; Margis, R. The Wall-associated Kinase gene family in rice genomes. Plant Sci. 2014, 229, 181–192. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Tian, Y.; Zhang, S.; Li, D.; Dong, W.; Zhang, C.; Zhang, Z. Characterization of wall-associated kinase/wall-associated kinase-like (WAK/WAKL) family in rose (Rosa chinensis) reveals the role of RcWAK4 in Botrytis resistance. BMC Plant Biol. 2021, 21, 526. [Google Scholar] [CrossRef]
- Koh, J.; Barbulescu, D.M.; Norton, S.; Redden, B.; Salisbury, P.A.; Kaur, S.; Cogan, N.; Slater, A.T. A multiplex PCR for rapid identification of Brassica species in the triangle of U. Plant Methods 2017, 13, 49. [Google Scholar] [CrossRef]
- Han, Y.; Hou, Z.; He, Q.; Zhang, X.; Yan, K.; Han, R.; Liang, Z. Genome-Wide Characterization and Expression Analysis of bZIP Gene Family Under Abiotic Stress in Glycyrrhiza uralensis. Front. Genet. 2021, 12, 754237. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhang, M.; Liang, Z.S.; He, Q.L. Characterization and Comparative Analysis of Chloroplast Genomes in Five Uncaria Species Endemic to China. Int. J. Mol. Sci. 2022, 23, 11617. [Google Scholar] [CrossRef]
- Niu, T.; Wang, X.; Abbas, M.; Shen, J.; Liu, R.; Wang, Z.; Liu, A. Expansion of constans-like genes in sunflower confers putative neofunctionalization in the adaptation to abiotic stresses. Ind. Crops Prod. 2022, 176, 114400. [Google Scholar] [CrossRef]
- Yue, Z.L.; Liu, N.; Deng, Z.P.; Zhang, Y.; Wu, Z.M.; Zhao, J.L.; Sun, Y.; Wang, Z.Y.; Zhang, S.W. The receptor kinase OsWAK11 monitors cell wall pectin changes to fine-tune brassinosteroid signaling and regulate cell elongation in rice. Curr. Biol. CB 2022, 32, 2454–2466.e7. [Google Scholar] [CrossRef]
- Delteil, A.; Gobbato, E.; Cayrol, B.; Estevan, J.; Michel-Romiti, C.; Dievart, A.; Kroj, T.; Morel, J.B. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016, 16, 17. [Google Scholar] [CrossRef]
- Chen, P.; Giarola, V.; Bartels, D. The Craterostigma plantagineum protein kinase CpWAK1 interacts with pectin and integrates different environmental signals in the cell wall. Planta 2021, 253, 92. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.A.; Kohorn, B.D. Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 2001, 13, 303–318. [Google Scholar] [CrossRef]
- Huang, K.L.; Tian, J.; Wang, H.; Fu, Y.F.; Li, Y.; Zheng, Y.; Li, X.B. Fatty acid export protein BnFAX6 functions in lipid synthesis and axillary bud growth in Brassica napus. Plant Physiol. 2021, 186, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Pan, J.; Ullah, N.; Duan, Y.; Hao, R.; Li, J.; Huang, Q.; Xu, L. 5-Aminolevulinic acid mitigates the chromium-induced changes in Helianthus annuus L. as revealed by plant defense system enhancement. Plant Physiol. Biochem. PPB 2023, 198, 107701. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, J.; Chen, H.; Jin, W.; Liang, Z. Characterization of NAC family genes in Salvia miltiorrhiza and NAC2 potentially involved in the biosynthesis of tanshinones. Phytochemistry 2021, 191, 112932. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Chen, X.; Mao, Y.; Chai, W.; Yan, K.; Liang, Z.; Xia, P. Genome-wide identification and expression analysis of MYB gene family under nitrogen stress in Panax notoginseng. Protoplasma 2023, 260, 189–205. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chang, Q.; Xia, P.; Liang, Z.; Yan, K. The complete chloroplast genome of Clematis henryi var. ernate (Ranunculaceae). Mitochondrial DNA. Part B Resour. 2021, 6, 1319–1320. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Feng, T.; Jiang, Y.; Jia, Q.; Han, R.; Wang, D.; Zhang, X.; Liang, Z. Transcriptome Analysis of Different Sections of Rhizome in Polygonatum sibiricum Red. and Mining Putative Genes Participate in Polysaccharide Biosynthesis. Biochem. Genet. 2022, 60, 1547–1566. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Xia, P.; Hu, W.; Zheng, Y.; Wang, Y.; Yan, K.; Liang, Z. Structural and interactions analysis of a transcription factor PnMYB2 in Panax notoginseng. J. Plant Physiol. 2022, 275, 153756. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, M.; Guan, W.; Zhang, F.; Dai, W.; Yuan, L.; Gao, G.; Xu, K.; Chen, B.; Li, L.; et al. Genome-Wide Identification and Functional Characterization of Stress Related Glyoxalase Genes in Brassica napus L. Int. J. Mol. Sci. 2023, 24, 2130. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, Q.; Su, X.; Cheng, Y.; Wu, H.; Huang, Z.; Xu, A.; Dong, J.; Yu, C. Microscopic and Transcriptomic Comparison of Powdery Mildew Resistance in the Progenies of Brassica carinata × B. napus. Int. J. Mol. Sci. 2022, 23, 9961. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, H.; Zhang, X.; Liang, Z.; He, Q. Systematic Identification and Validation of Suitable Reference Genes for the Normalization of Gene Expression in Prunella vulgaris under Different Organs and Spike Development Stages. Genes 2022, 13, 1947. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Pei, T.; Bai, Z.; Jia, Y.; Ma, P.; Liang, Z. SmMYB36, a Novel R2R3-MYB Transcription Factor, Enhances Tanshinone Accumulation and Decreases Phenolic Acid Content in Salvia miltiorrhiza Hairy Roots. Sci. Rep. 2017, 7, 5104. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using 677 Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Duan, Y.; Liu, H.; Xu, M.; Zhang, Z.; Xu, L. Analysis of WAK Genes in Nine Cruciferous Species with a Focus on Brassica napus L. Int. J. Mol. Sci. 2023, 24, 13601. https://doi.org/10.3390/ijms241713601
Xu Z, Duan Y, Liu H, Xu M, Zhang Z, Xu L. Analysis of WAK Genes in Nine Cruciferous Species with a Focus on Brassica napus L. International Journal of Molecular Sciences. 2023; 24(17):13601. https://doi.org/10.3390/ijms241713601
Chicago/Turabian StyleXu, Zishu, Yi Duan, Hui Liu, Mingchao Xu, Zhi Zhang, and Ling Xu. 2023. "Analysis of WAK Genes in Nine Cruciferous Species with a Focus on Brassica napus L." International Journal of Molecular Sciences 24, no. 17: 13601. https://doi.org/10.3390/ijms241713601





