Embedding and Backscattered Scanning Electron Microscopy (EM-BSEM) Is Preferential over Immunophenotyping in Relation to Bioprosthetic Heart Valves
Abstract
:1. Introduction
2. Results
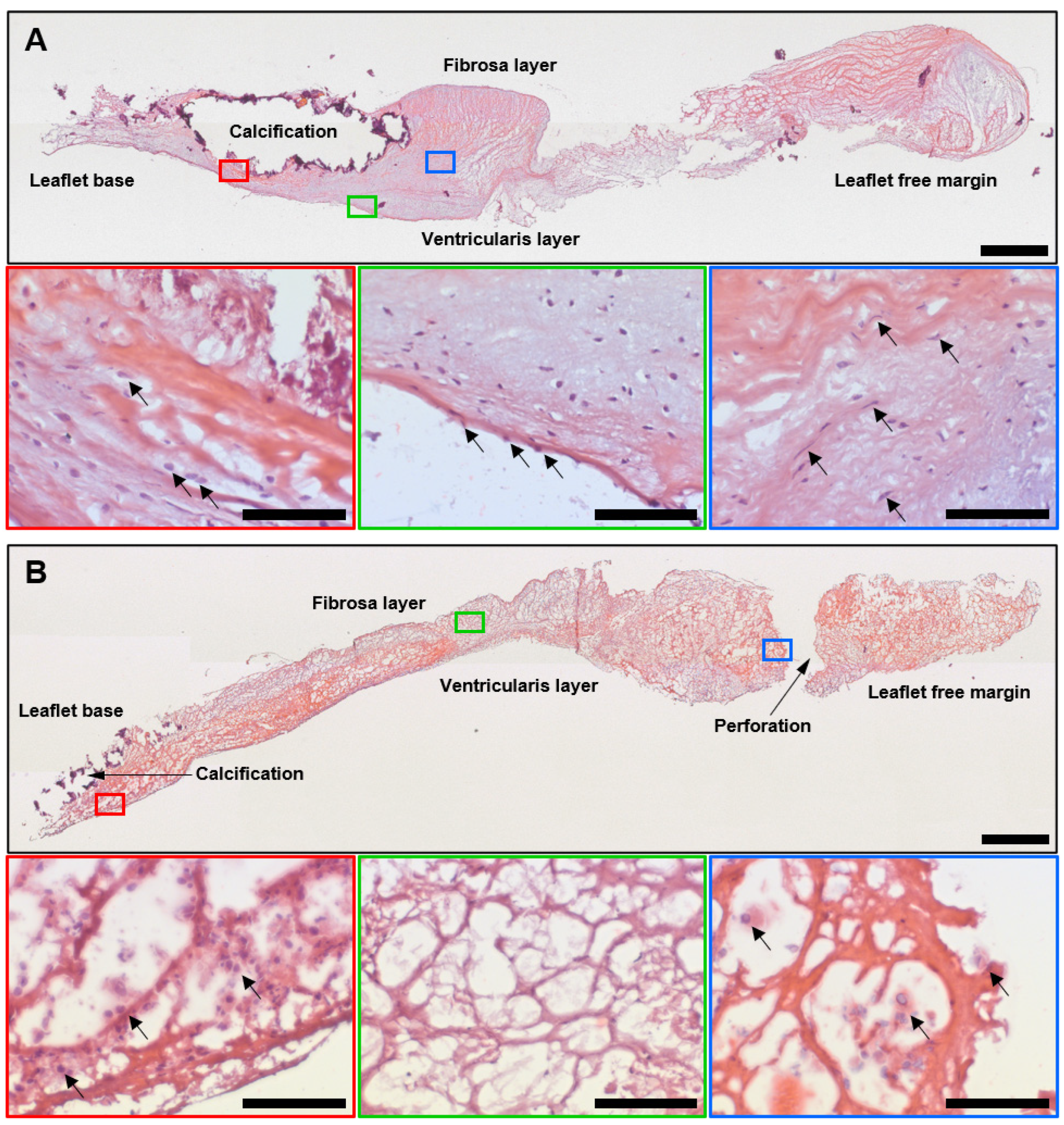
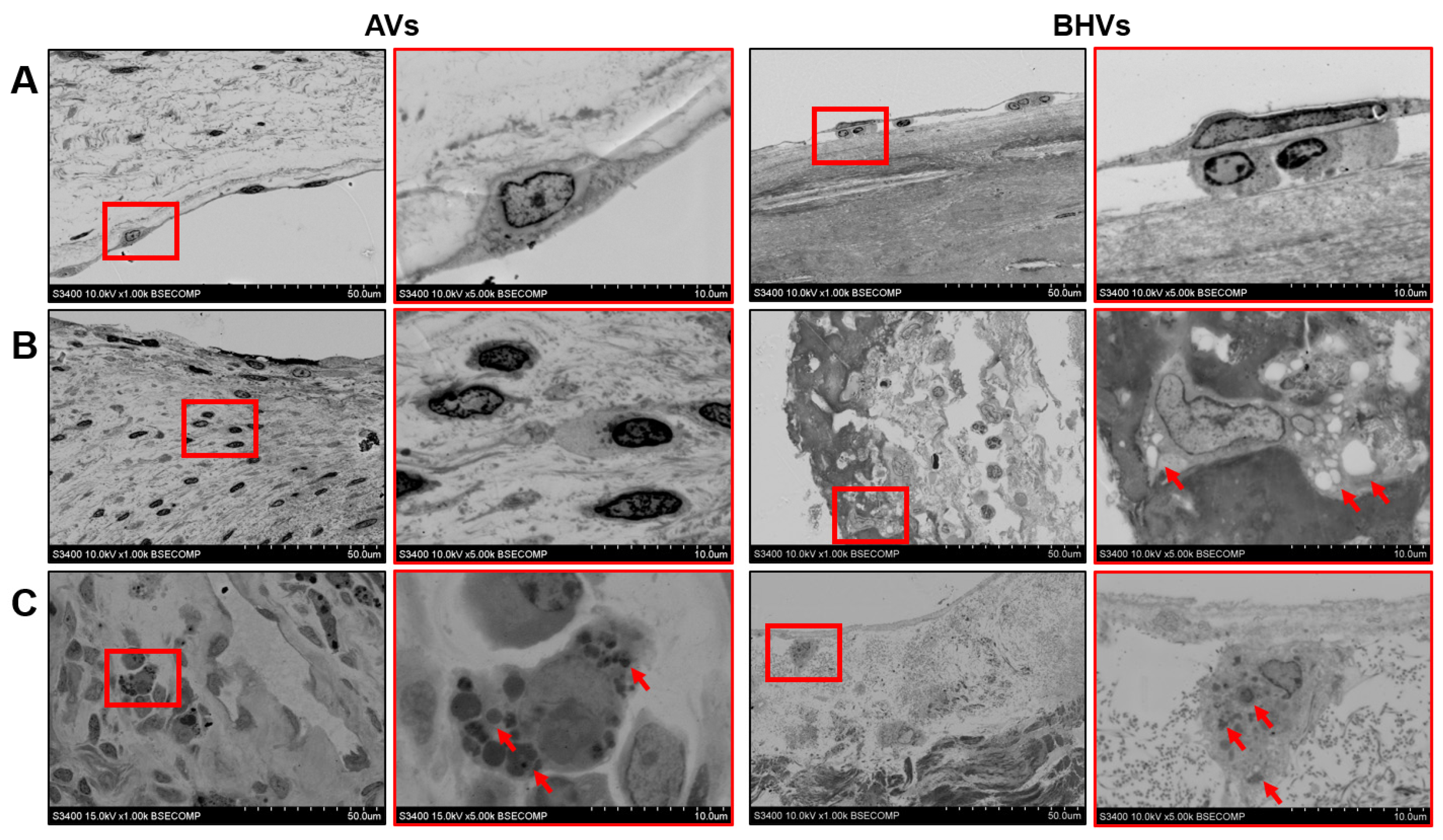
2.1. Histological, Immunohistochemical, and Electron Microscopy Examination of Calcified AVs and Failing BHVs Shows the Advantages of Ultrastructural Analysis by the EM-BSEM Approach
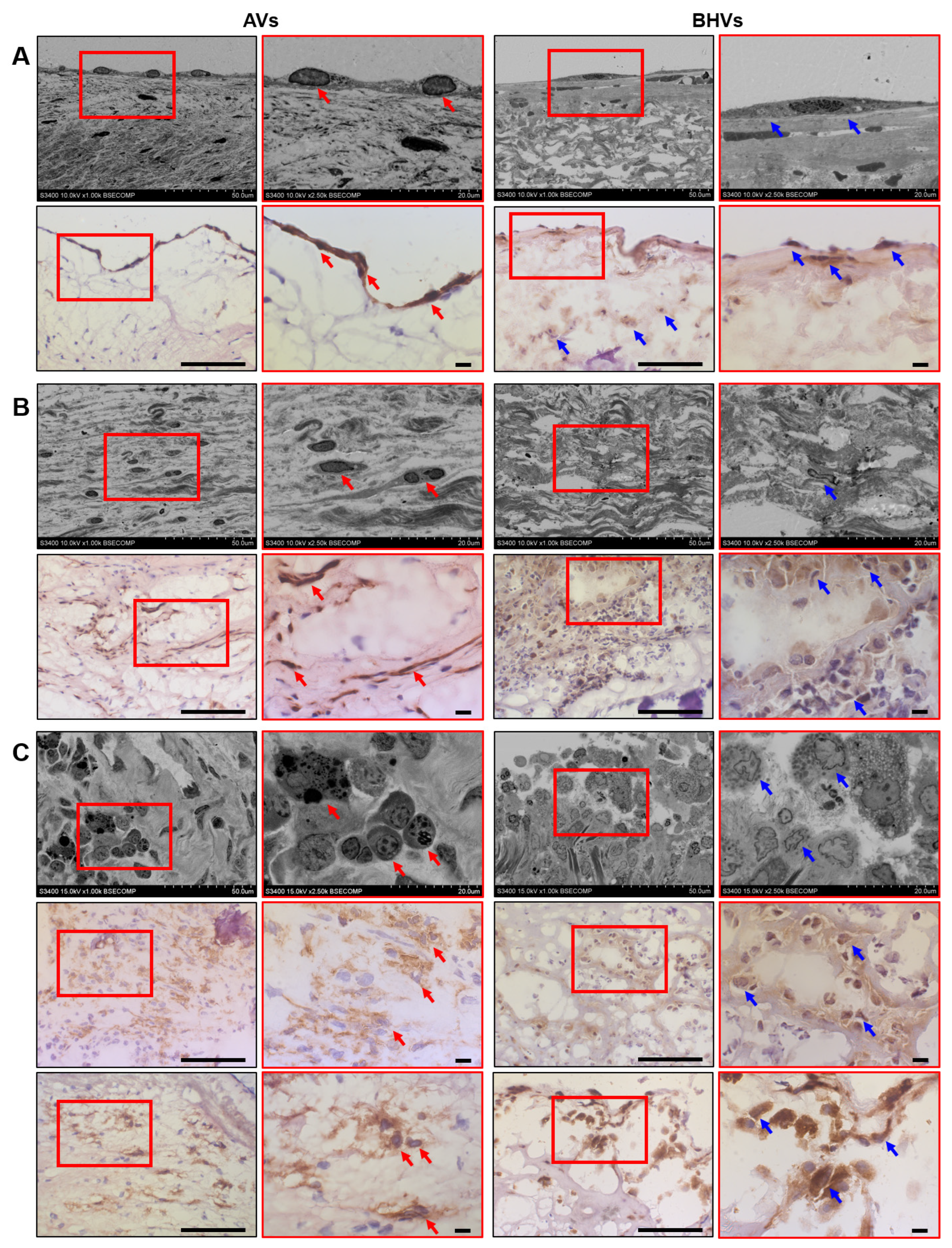
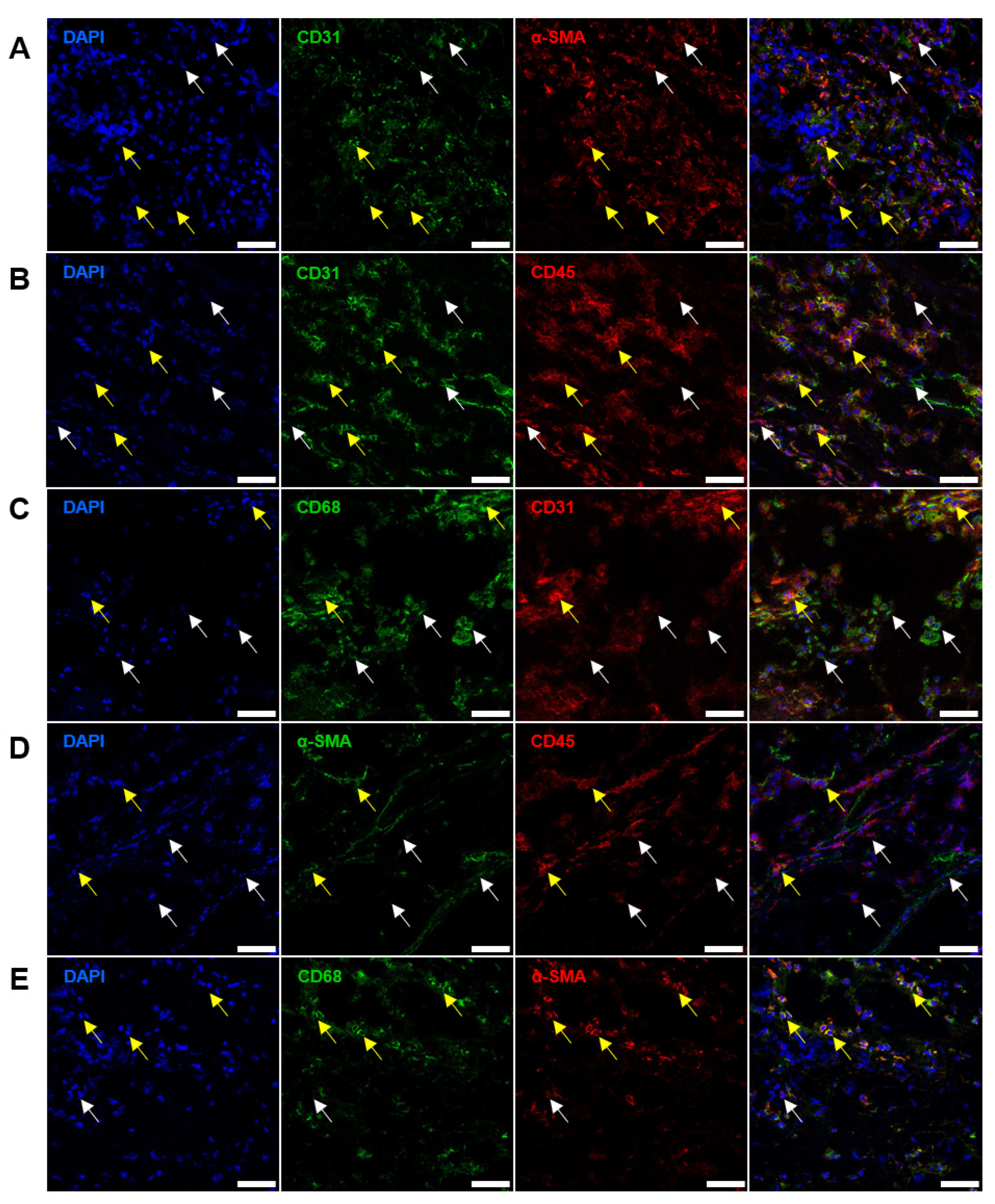
2.2. Immunostaining Well Defines the Phenotype of Cell Populations within the AVs but Fails to Tackle This Task in BHVs
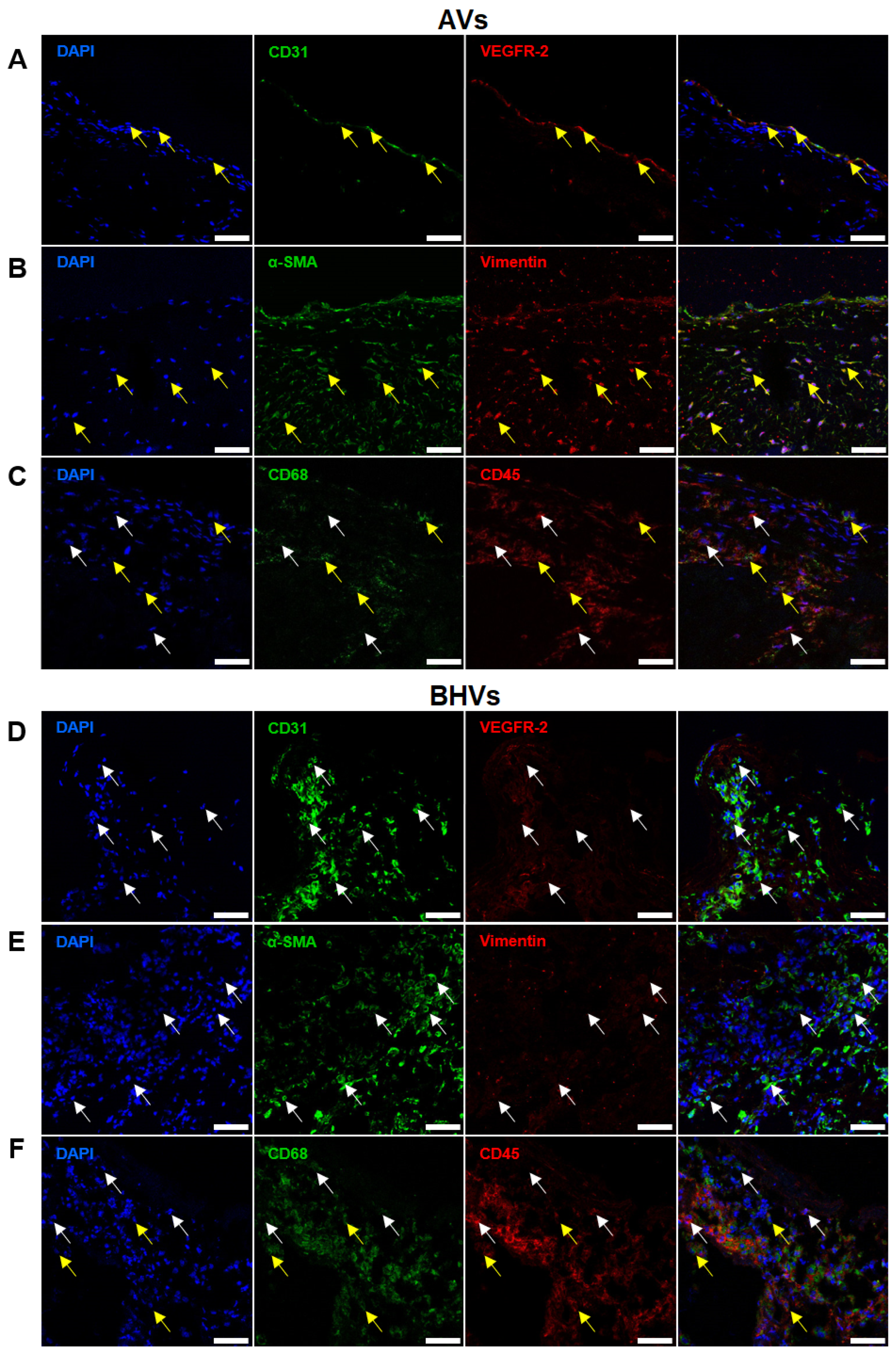
2.3. Quantitative Image Analysis Demonstrates That the Majority of Host Cells in the BHVs Have Mixed Immunophenotype
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Histological Examination and Immunostaining
4.3. Electron Microscopy
4.4. Quantitative Image Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Prosthetic Heart Valves: Selection of the Optimal Prosthesis and Long-Term Management. Circulation 2009, 119, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Brink, J.; Human, P.; Bezuidenhout, D. Prosthetic Heart Valves: Catering for the Few. Biomaterials 2008, 29, 385–406. [Google Scholar] [CrossRef]
- Head, S.J.; Çelik, M.; Kappetein, A.P. Mechanical versus Bioprosthetic Aortic Valve Replacement. Eur. Heart J. 2017, 38, 2183–2191. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized Definitions of Structural Deterioration and Valve Failure in Assessing Long-Term Durability of Transcatheter and Surgical Aortic Bioprosthetic Valves: A Consensus Statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar] [CrossRef]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.C.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Cote, N.; Pibarot, P.; Clavel, M.A. Incidence, Risk Factors, Clinical Impact, and Management of Bioprosthesis Structural Valve Degeneration. Curr. Opin. Cardiol. 2017, 32, 123–129. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Bottio, T.; Thiene, G.; Pettenazzo, E.; Ius, P.; Bortolotti, U.; Rizzoli, G.; Valfré, C.; Casarotto, D.; Valente, M. Hancock II Bioprosthesis: A Glance at the Microscope in Mid-Long-Term Explants. J. Thorac. Cardiovasc. Surg. 2003, 126, 99–105. [Google Scholar] [CrossRef]
- Butany, J.; Zhou, T.; Leong, S.W.; Cunningham, K.S.; Thangaroopan, M.; Jegatheeswaran, A.; Feindel, C.; David, T.E. Inflammation and Infection in Nine Surgically Explanted Medtronic Freestyle Stentless Aortic Valves. Cardiovasc. Pathol. 2007, 16, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kostyunin, A.E.; Glushkova, T.V.; Lobov, A.A.; Ovcharenko, E.A.; Zainullina, B.R.; Bogdanov, L.A.; Shishkova, D.K.; Markova, V.E.; Asanov, M.A.; Mukhamadiyarov, R.A.; et al. Proteolytic Degradation Is a Major Contributor to Bioprosthetic Heart Valve Failure. J. Am. Heart Assoc. 2023, 12, e028215. [Google Scholar] [CrossRef] [PubMed]
- Lepidi, H.; Casalta, J.P.; Fournier, P.E.; Habib, G.; Collart, F.; Raoult, D. Quantitative Histological Examination of Bioprosthetic Heart Valves. Clin. Infect. Dis. 2006, 42, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Pibarot, P.; Audet, A.; Janvier, R.; Dagenais, F.; Perron, J.; Couture, C.; Voisine, P.; Després, J.P.; Mathieu, P. Lipid-Mediated Inflammation and Degeneration of Bioprosthetic Heart Valves. Eur. J. Clin. Investig. 2009, 39, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wu, H.; Bai, Y.; Gong, D.; Xia, C.; Li, Q.; Lu, F.; Xu, Z. Evidence of Osteogenic Regulation in Calcific Porcine Aortic Valves. Heart Surg. Forum 2018, 21, E375–E381. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Law, K.B.; Li, A.Y.; Phillips, K.R.; David, T.E.; Butany, J. Characterizing the Inflammatory Reaction in Explanted Medtronic Freestyle Stentless Porcine Aortic Bioprosthesis over a 6-Year Period. Cardiovasc. Pathol. 2012, 21, 158–168. [Google Scholar] [CrossRef]
- Sakaue, T.; Nakaoka, H.; Shikata, F.; Aono, J.; Kurata, M.; Uetani, T.; Hamaguchi, M.; Kojima, A.; Uchita, S.; Yasugi, T.; et al. Biochemical and Histological Evidence of Deteriorated Bioprosthetic Valve Leaflets: The Accumulation of Fibrinogen and Plasminogen. Biol. Open 2018, 7, bio034009. [Google Scholar] [CrossRef]
- Sellers, S.L.; Turner, C.T.; Sathananthan, J.; Cartlidge, T.R.G.; Sin, F.; Bouchareb, R.; Mooney, J.; Nørgaard, B.L.; Bax, J.J.; Bernatchez, P.N.; et al. Transcatheter Aortic Heart Valves: Histological Analysis Providing Insight to Leaflet Thickening and Structural Valve Degeneration. JACC Cardiovasc. Imaging 2019, 12, 135–145. [Google Scholar] [CrossRef]
- Kostyunin, A.; Mukhamadiyarov, R.; Glushkova, T.; Bogdanov, L.; Shishkova, D.; Osyaev, N.; Ovcharenko, E.; Kutikhin, A. Ultrastructural Pathology of Atherosclerosis, Calcific Aortic Valve Disease, and Bioprosthetic Heart Valve Degeneration: Commonalities and Differences. Int. J. Mol. Sci. 2020, 21, 7434. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Ovcharenko, E.A.; Kutikhin, A.G. Development of Calcific Aortic Valve Disease: Do We Know Enough for New Clinical Trials? J. Mol. Cell. Cardiol. 2019, 132, 189–209. [Google Scholar] [CrossRef]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific Aortic Stenosis. Nat. Rev. Dis. Prim. 2016, 2, 16006. [Google Scholar] [CrossRef]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the Early Lesion of ‘Degenerative’ Valvular Aortic Stenosis. Histological and Immunohistochemical Studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Niepmann, S.T.; Willemsen, N.; Boucher, A.S.; Stei, M.; Goody, P.; Zietzer, A.; Bulic, M.; Billig, H.; Odainic, A.; Weisheit, C.K.; et al. Toll-like receptor-3 contributes to the development of aortic valve stenosis. Basic Res. Cardiol. 2023, 118, 6. [Google Scholar] [CrossRef]
- Cecoltan, S.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Mihaila, A.C.; Tucureanu, M.; Vlad, M.L.; Droc, I.; Gherghiceanu, M.; Simionescu, A.; et al. High Glucose Induced Changes in Human VEC Phenotype in a 3D Hydrogel Derived From Cell-Free Native Aortic Root. Front. Cardiovasc. Med. 2021, 8, 714573. [Google Scholar] [CrossRef]
- Newman, P.J. The biology of PECAM-1. J. Clin. Investig. 1997, 100, S25–S29. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.K.; Lee, H.J.; Choi, H.N.; Ahn, J.H.; Choi, J.Y.; Song, H.S.; Lee, K.H.; Yoon, Y.; Yi, L.S.; Kim, J.S.; et al. Characterization of CD45-/CD31+/CD105+ circulating cells in the peripheral blood of patients with gynecologic malignancies. Clin. Cancer Res. 2013, 19, 5340–5350. [Google Scholar] [CrossRef]
- Briand, M.; Pibarot, P.; Despres, J.P.; Voisine, P.; Dumesnil, J.G.; Dagenais, F.; Mathieu, P. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation 2006, 114, I512–I517. [Google Scholar] [CrossRef]
- Farivar, R.S.; Cohn, L.H. Hypercholesterolemia is a risk factor for bioprosthetic valve calcification and explantation. J. Thorac. Cardiovasc. Surg. 2003, 126, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Gelsomino, S.; Lucà, F.; De Cicco, G.; Billè, G.; Carella, R.; Villa, E.; Troise, G.; Viganò, M.; Banfi, C.; et al. Type 2 diabetes mellitus is associated with faster degeneration of bioprosthetic valve: Results from a propensity score-matched Italian multicenter study. Circulation 2012, 125, 604–614. [Google Scholar] [CrossRef]
- Mahjoub, H.; Mathieu, P.; Senechal, M.; Larose, E.; Dumesnil, J.; Despres, J.P.; Pibarot, P. ApoB/ApoA-I ratio is associated with increased risk of bioprosthetic valve degeneration. J. Am. Coll. Cardiol. 2013, 61, 752–761. [Google Scholar] [CrossRef]
- Mahmut, A.; Mahjoub, H.; Boulanger, M.C.; Fournier, D.; Després, J.P.; Pibarot, P.; Mathieu, P. Lp-PLA2 is associated with structural valve degeneration of bioprostheses. Eur. J. Clin. Investig. 2014, 44, 136–145. [Google Scholar] [CrossRef]
- Nollert, G.; Miksch, J.; Kreuzer, E.; Reichart, B. Risk factors for atherosclerosis and the degeneration of pericardial valves after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2003, 126, 965–968. [Google Scholar] [CrossRef]
- Nsaibia, M.J.; Mahmut, A.; Mahjoub, H.; Dahou, A.; Bouchareb, R.; Boulanger, M.C.; Després, J.P.; Bossé, Y.; Arsenault, B.J.; Larose, E.; et al. Association between plasma lipoprotein levels and bioprosthetic valve structural degeneration. Heart 2016, 102, 1915–1921. [Google Scholar] [CrossRef]
- Ochi, A.; Cheng, K.; Zhao, B.; Hardikar, A.A.; Negishi, K. Patient risk factors for bioprosthetic aortic valve degeneration: A systematic review and meta-analysis. Heart Lung Circ. 2020, 29, 668–678. [Google Scholar] [CrossRef]
- Wakami, T.; Koizumi, S.; Koyama, T. Impact of postoperative patient-prosthesis mismatch as a risk factor for early structural valve deterioration after aortic valve replacement with Trifecta bioprosthesis. J. Cardiothorac. Surg. 2022, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Kossar, A.P.; Abramov, A.; Frasca, A.; Sun, M.; Zyablitskaya, M.; Paik, D.; Kalfa, D.; Della Barbera, M.; Thiene, G.; et al. Age-related enhanced degeneration of bioprosthetic valves due to leaflet calcification, tissue crosslinking, and structural changes. Cardiovasc. Res. 2023, 119, 302–315. [Google Scholar] [CrossRef]
- Frasca, A.; Xue, Y.; Kossar, A.P.; Keeney, S.; Rock, C.; Zakharchenko, A.; Streeter, M.; Gorman, R.C.; Grau, J.B.; George, I.; et al. Glycation and Serum Albumin Infiltration Contribute to the Structural Degeneration of Bioprosthetic Heart Valves. JACC Basic Transl. Sci. 2020, 5, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Marie, P.; Farge, D.; Carpentier, S.; De Pollak, C.; Hott, M.; Chen, L.; Martinet, B.; Carpentier, A. Osteopontin is associated with bioprosthetic heart valve calcification in humans. Comptes Rendus Acad. Sci.-Ser. III 1997, 320, 49–57. [Google Scholar] [CrossRef]
- Naso, F.; Gandaglia, A.; Bottio, T.; Tarzia, V.; Nottle, M.B.; d’Apice, A.J.; Cowan, P.; Cozzi, E.; Galli, C.; Lagutina, I.; et al. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation 2013, 20, 252–261. [Google Scholar] [CrossRef]
- Reuven, E.M.; Leviatan Ben-Arye, S.; Marshanski, T.; Breimer, M.E.; Yu, H.; Fellah-Hebia, I.; Roussel, J.C.; Costa, C.; Galiñanes, M.; Mañez, R.; et al. Characterization of immunogenic Neu5Gc in bioprosthetic heart valves. Xenotransplantation 2016, 23, 381–392. [Google Scholar] [CrossRef]
- Shishkova, D.K.; Sinitskaya, A.V.; Sinitsky, M.Y.; Matveeva, V.G.; Velikanova, E.A.; Markova, V.E.; Kutikhin, A.G. Spontaneous endothelial-to-mesenchymal transition in human primary umbilical vein endothelial cells. Complex Issues Cardiovasc. Dis. 2022, 11, 97–114. [Google Scholar] [CrossRef]
- Mahler, G.J.; Farrar, E.J.; Butcher, J.T. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 121–130. [Google Scholar] [CrossRef]
- Dahal, S.; Huang, P.; Murray, B.T.; Mahler, G.J. Endothelial to mesenchymal transformation is induced by altered extracellular matrix in aortic valve endothelial cells. J. Biomed. Mater. Res. Part A 2017, 105, 2729–2741. [Google Scholar] [CrossRef]
- Nehl, D.; Goody, P.R.; Maus, K.; Pfeifer, A.; Aikawa, E.; Bakthiary, F.; Zimmer, S.; Nickenig, G.; Jansen, F.; Hosen, M.R. Human and porcine aortic valve endothelial and interstitial cell isolation and characterization. Front. Cardiovasc. Med. 2023, 10, 1151028. [Google Scholar] [CrossRef]
- Li, Y.; Lui, K.O.; Zhou, B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018, 15, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kovacic, J.C. Endothelial to Mesenchymal Transition in Health and Disease. Annu. Rev. Physiol. 2023, 85, 245–267. [Google Scholar] [CrossRef]
- Kostina, A.; Shishkova, A.; Ignatieva, E.; Irtyuga, O.; Bogdanova, M.; Levchuk, K.; Golovkin, A.; Zhiduleva, E.; Uspenskiy, V.; Moiseeva, O.; et al. Different Notch signaling in cells from calcified bicuspid and tricuspid aortic valves. J. Mol. Cell. Cardiol. 2018, 114, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xie, S.; Huang, Y.; Zhou, T.; Liu, M.; Zhu, P.; Wang, C.; Shi, J.; Li, F.; Sellke, F.W.; et al. Cell-Type Transcriptome Atlas of Human Aortic Valves Reveals Cell Heterogeneity and Endothelial to Mesenchymal Transition Involved in Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2910–2921. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, D.; Yuan, P.; Li, J.; Yun, Y.; Cui, Y.; Zhang, T.; Ma, J.; Sun, L.; Ma, H.; et al. Endothelial-to-Mesenchymal Transition in Calcific Aortic Valve Disease. Acta Cardiol. Sin. 2020, 36, 183–194. [Google Scholar] [CrossRef]
- Dayawansa, N.H.; Baratchi, S.; Peter, K. Uncoupling the Vicious Cycle of Mechanical Stress and Inflammation in Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2022, 9, 783543. [Google Scholar] [CrossRef]
- McKenney, J.K.; Weiss, S.W.; Folpe, A.L. CD31 expression in intratumoral macrophages: A potential diagnostic pitfall. Am. J. Surg. Pathol. 2001, 25, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; SivaSai, K.S.; Smith, M.A.; Trulock, E.P.; Lynch, J.P.; Patterson, G.A.; Mohanakumar, T. Increased expression of inflammatory cytokines and adhesion molecules by alveolar macrophages of human lung allograft recipients with acute rejection: Decline with resolution of rejection. J. Heart Lung Transplant. 2000, 19, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Wang, S.; Huang, X.R.; Yang, C.; Xiao, J.; Zhang, Y.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016, 7, e2495. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Jiang, H.; Pan, J.; Huang, X.-R.; Wang, Y.-C.; Huang, H.-F.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y.; Chen, J.-H. Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.O.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef]
- Bogdanova, M.; Zabirnyk, A.; Malashicheva, A.; Enayati, K.Z.; Karlsen, T.A.; Kaljusto, M.L.; Kvitting, J.-P.; Dissen, E.; Sullivan, G.J.; Kostareva, A.; et al. Interstitial Cells in Calcified Aortic Valves Have Reduced Differentiation Potential and Stem Cell-Like Properties. Sci. Rep. 2019, 9, 12934. [Google Scholar] [CrossRef] [PubMed]
- Semenova, D.; Zabirnyk, A.; Lobov, A.; Boyarskaya, N.; Kachanova, O.; Uspensky, V.; Zainullina, B.; Denisov, E.; Gerashchenko, T.; Kvitting, J.-P.; et al. Multi-Omics of In Vitro Aortic Valve Calcification. Front. Cardiovasc. Med. 2022, 9, 1043165. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, J.S.; Papadopoulos, J.; Wook Kim, S.; Maya, M.; Zhang, F.; He, J.; Fan, D.; Langley, R.; Fidler, I.J. Circulating Monocytes Expressing CD31: Implications for Acute and Chronic Angiogenesis. Am. J. Pathol. 2009, 174, 1972–1980. [Google Scholar] [CrossRef]
- Rohde, E.; Malischnik, C.; Thaler, D.; Maierhofer, T.; Linkesch, W.; Lanzer, G.; Guelly, C.; Strunk, D. Blood Monocytes Mimic Endothelial Progenitor Cells. Stem Cells 2006, 24, 357–367. [Google Scholar] [CrossRef]
- Schmeisser, A.; Garlichs, C.D.; Zhang, H.; Eskafi, S.; Graffy, C.; Ludwig, J.; Strasser, R.H.; Daniel, W.G. Monocytes Coexpress Endothelial and Macrophagocytic Lineage Markers and Form Cord-Like Structures in Matrigel under Angiogenic Conditions. Cardiovasc. Res. 2001, 49, 671–680. [Google Scholar] [CrossRef]
- Yan, D.; Wang, X.; Li, D.; Qu, Z.; Ruan, Q. Macrophages Overexpressing VEGF, Transdifferentiate into Endothelial-Like Cells In Vitro and In Vivo. Biotechnol. Lett. 2011, 33, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Krenning, G.; Dankers, P.Y.; Jovanovic, D.; van Luyn, M.J.; Harmsen, M.C. Efficient Differentiation of CD14+ Monocytic Cells into Endothelial Cells on Degradable Biomaterials. Biomaterials 2007, 28, 1470–1479. [Google Scholar] [CrossRef]
- Pilling, D.; Buckley, C.D.; Salmon, M.; Gomer, R.H. Inhibition of Fibrocyte Differentiation by Serum Amyloid P. J. Immunol. 2003, 171, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Scott, P.G.; Giuffre, J.; Shankowsky, H.A.; Ghahary, A.; Tredget, E.E. Peripheral Blood Fibrocytes from Burn Patients: Identification and Quantification of Fibrocytes in Adherent Cells Cultured from Peripheral Blood Mononuclear Cells. Lab. Investig. 2002, 82, 1183–1192. [Google Scholar] [CrossRef]
- Mukhamadiyarov, R.A.; Bogdanov, L.A.; Glushkova, T.V.; Shishkova, D.K.; Kostyunin, A.E.; Koshelev, V.A.; Shabaev, A.R.; Frolov, A.V.; Stasev, A.N.; Lyapin, A.A.; et al. Embedding and Backscattered Scanning Electron Microscopy: A Detailed Protocol for the Whole-Specimen, High-Resolution Analysis of Cardiovascular Tissues. Front. Cardiovasc. Med. 2021, 8, 739549. [Google Scholar] [CrossRef]
- Antonova, L.; Kutikhin, A.; Sevostianova, V.; Lobov, A.; Repkin, E.; Krivkina, E.; Velikanova, E.; Mironov, A.; Mukhamadiyarov, R.; Senokosova, E.; et al. Controlled and Synchronized Vascular Regeneration upon the Implantation of Iloprost- and Cationic Amphiphilic Drugs-Conjugated Tissue-Engineered Vascular Grafts into the Ovine Carotid Artery: A Proteomics-Empowered Study. Polymers 2022, 14, 5149. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Sevostianova, V.V.; Silnikov, V.N.; Krivkina, E.O.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Senokosova, E.A.; Khanova, M.Y.; Glushkova, T.V.; et al. Comparison of the Patency and Regenerative Potential of Biodegradable Vascular Prostheses of Different Polymer Compositions in an Ovine Model. Int. J. Mol. Sci. 2023, 24, 8540. [Google Scholar] [CrossRef]
- Kostyunin, A.; Glushkova, T.; Stasev, A.; Mukhamadiyarov, R.; Velikanova, E.; Bogdanov, L.; Sinitskaya, A.; Asanov, M.; Ovcharenko, E.; Barbarash, L.; et al. Early Postoperative Immunothrombosis of Bioprosthetic Mitral Valve and Left Atrium: A Case Report. Int. J. Mol. Sci. 2022, 23, 6736. [Google Scholar] [CrossRef]
- Bogdanov, L.; Shishkova, D.; Mukhamadiyarov, R.; Velikanova, E.; Tsepokina, A.; Terekhov, A.; Koshelev, V.; Kanonykina, A.; Shabaev, A.; Frolov, A.; et al. Excessive Adventitial and Perivascular Vascularisation Correlates with Vascular Inflammation and Intimal Hyperplasia. Int. J. Mol. Sci. 2022, 23, 12156. [Google Scholar] [CrossRef]



| Mouse Primary Antibody (Labelled by Donkey Antimouse Alexa Fluor 488-Labeled Secondary) | Rabbit Primary Antibody (Labelled by Donkey Antimouse Alexa Fluor 555-Labeled Secondary) | ||||
|---|---|---|---|---|---|
| Catalogue Number and Manufacturer | Dilution | Antigen | Catalogue Number and Manufacturer | Dilution | Antigen |
| MAB1393-I, Sigma-Aldrich | 1:100 | CD31 | ab39256, Abcam | 1:200 | VEGFR2 |
| ab7817, Abcam | 1:1000 | α-SMA | ab16700, Abcam | 1:1000 | VIM |
| ab955, Abcam | 1:200 | CD68 | ab10558, Abcam | 1:1500 | CD45 |
| MAB1393-I, Sigma-Aldrich | 1:100 | CD31 | ab5694, Abcam | 1:100 | α-SMA |
| MAB1393-I, Sigma-Aldrich | 1:100 | CD31 | ab10558, Abcam | 1:1500 | CD45 |
| ab955, Abcam | 1:200 | CD68 | ab182981, Abcam | 1:200 | CD31 |
| ab7817, Abcam | 1:1000 | α-SMA | ab10558, Abcam | 1:1500 | CD45 |
| ab955, Abcam | 1:200 | CD68 | ab5694, Abcam | 1:100 | α-SMA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostyunin, A.; Glushkova, T.; Velikanova, E.; Mukhamadiyarov, R.; Bogdanov, L.; Akentyeva, T.; Ovcharenko, E.; Evtushenko, A.; Shishkova, D.; Markova, Y.; et al. Embedding and Backscattered Scanning Electron Microscopy (EM-BSEM) Is Preferential over Immunophenotyping in Relation to Bioprosthetic Heart Valves. Int. J. Mol. Sci. 2023, 24, 13602. https://doi.org/10.3390/ijms241713602
Kostyunin A, Glushkova T, Velikanova E, Mukhamadiyarov R, Bogdanov L, Akentyeva T, Ovcharenko E, Evtushenko A, Shishkova D, Markova Y, et al. Embedding and Backscattered Scanning Electron Microscopy (EM-BSEM) Is Preferential over Immunophenotyping in Relation to Bioprosthetic Heart Valves. International Journal of Molecular Sciences. 2023; 24(17):13602. https://doi.org/10.3390/ijms241713602
Chicago/Turabian StyleKostyunin, Alexander, Tatiana Glushkova, Elena Velikanova, Rinat Mukhamadiyarov, Leo Bogdanov, Tatiana Akentyeva, Evgeny Ovcharenko, Alexey Evtushenko, Daria Shishkova, Yulia Markova, and et al. 2023. "Embedding and Backscattered Scanning Electron Microscopy (EM-BSEM) Is Preferential over Immunophenotyping in Relation to Bioprosthetic Heart Valves" International Journal of Molecular Sciences 24, no. 17: 13602. https://doi.org/10.3390/ijms241713602
APA StyleKostyunin, A., Glushkova, T., Velikanova, E., Mukhamadiyarov, R., Bogdanov, L., Akentyeva, T., Ovcharenko, E., Evtushenko, A., Shishkova, D., Markova, Y., & Kutikhin, A. (2023). Embedding and Backscattered Scanning Electron Microscopy (EM-BSEM) Is Preferential over Immunophenotyping in Relation to Bioprosthetic Heart Valves. International Journal of Molecular Sciences, 24(17), 13602. https://doi.org/10.3390/ijms241713602







