Development of Multiplex Polymerase Chain Reaction (PCR)-Based MSA Assay for Bladder Cancer Detection
Abstract
:1. Introduction
2. Results
2.1. Selection of 16 Markers
2.2. Development of Triplet Multiplex PCR
3. Discussion
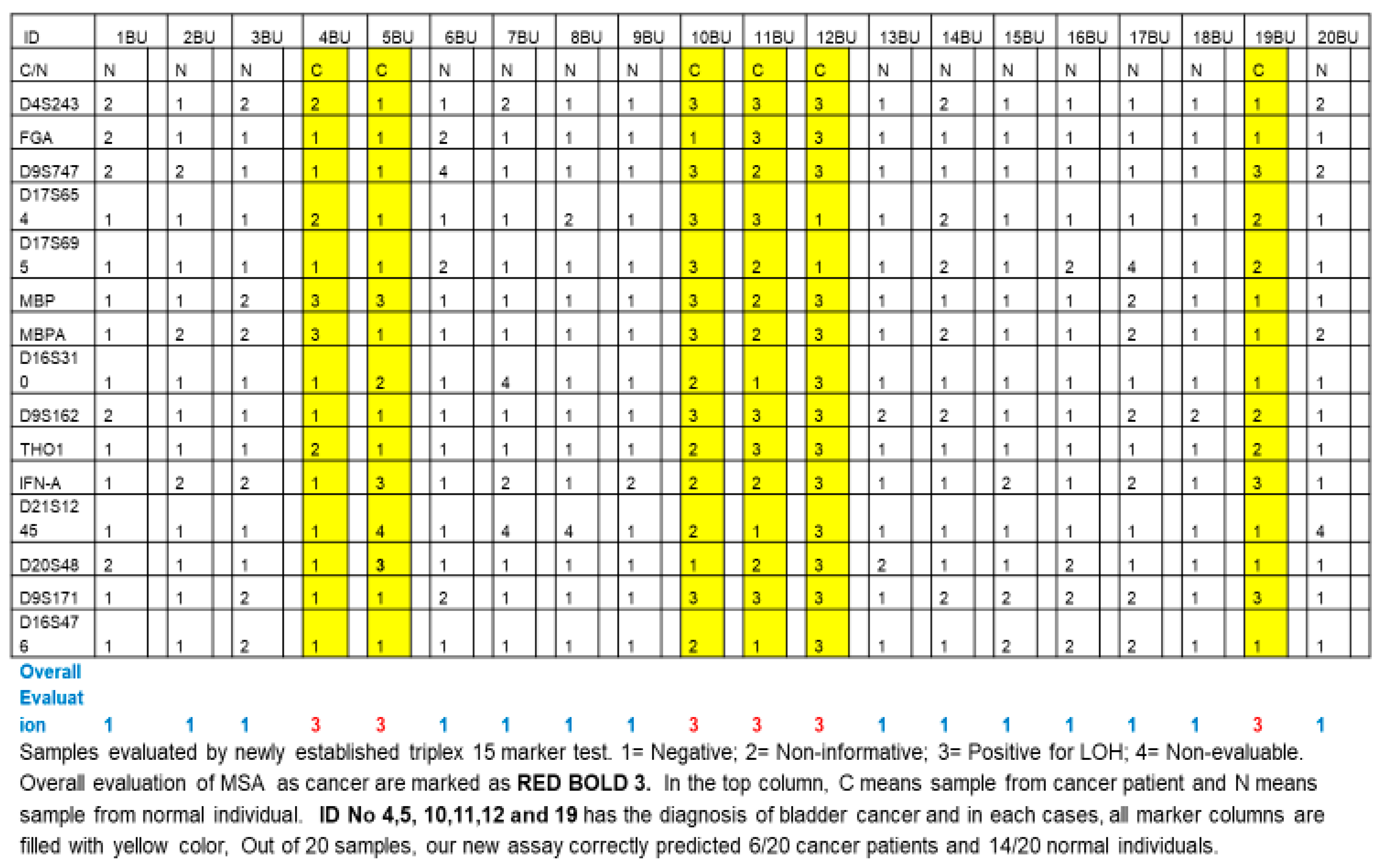
4. Material and Method
4.1. Matched Blood and Urine Genomic DNA Samples
4.2. EDRN QC Samples
4.3. DNA Extraction and Quantification
4.4. STR Targets and PCR Primers
4.5. Multiplex PCR: Triplet (Three Tube) Multiplex PCR
4.6. Capillary Electrophoresis
4.7. Calculation of Loss of Heterozygosity
4.8. Data Analysis
4.9. ABI 3100 DNA Analyzer Acceptance Criteria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Aaltonen, L.A.; Peltomäki, P.; Leach, F.S.; Sistonen, P.; Pylkkänen, L.; Mecklin, J.-P.; Järvinen, H.; Powell, S.M.; Jen, J.; Hamilton, S.R.; et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite instability in cancer of the proximal colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Yamamoto, H. Carcinogenesis and microsatellite instability: The interrelationship between genetics and epigenetics. Carcinogenesis 2008, 29, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H. Interrelationship between microsatellite instability and microRNA in gastrointestinal cancer. World J. Gastroenterol. 2012, 18, 2745–2755. [Google Scholar] [CrossRef]
- Yamamoto, H. An updated review of gastric cancer in the next-generation sequencing era: Insights from bench to bedside and vice versa. World J. Gastroenterol. 2014, 20, 3927–3937. [Google Scholar] [CrossRef]
- Yamamoto, H.; Imai, K. Microsatellite instability: An update. Arch. Toxicol. 2015, 89, 899–921. [Google Scholar] [CrossRef]
- Gelsomino, F.; Barbolini, M.; Spallanzani, A.; Pugliese, G.; Cascinu, S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat. Rev. 2016, 51, 19–26. [Google Scholar] [CrossRef]
- De la Chapelle, A.; Hampel, H. Clinical relevance of microsatellite instability in colorectal cancer. Clin. Oncol. 2010, 28, 3380–3387. [Google Scholar] [CrossRef]
- Bacher, J.W.; Flanagan, L.A.; Smalley, R.L.; Nassif, N.A.; Burgart, L.J.; Halberg, R.B.; Megid, W.M.A.; Thibodeau, S.N. Development of a fluorescent multiplex assay for detection of MSI-high tumors. Dis. Markers 2004, 20, 237–250. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Botteman, M.F.; Pashos, C.L.; Redaelli, A.; Laskin, B.; Hauser, R. The health economics of bladder cancer: A comprehensive review of the published literature. PharmacoEconomics 2003, 22, 1315–1330. [Google Scholar] [CrossRef]
- Lotan, Y.; Kamat, A.M.; Porter, M.P.; Robinson, V.L.; Shore, N.; Jewett, M.; Schelhammer, P.F.; White, R.d.; Quale, D.; Lee, C.T. Key concerns about the current state of bladder cancer: A position paper from the bladder cancer think tank, the bladder cancer advocacy network, and the society of urologic oncology. Cancer 2009, 115, 4096–4103. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Svatek, R.S.; Malats, N. Screening for bladder cancer: A perspective. World J. Urol. 2008, 26, 13–18. [Google Scholar] [CrossRef]
- Shariat, S.F.; Lotan, Y.; Vickers, A.; Karakiewicz, P.I.; Schmitz-Dräger, B.J.; Goebell, P.J.; Malats, N. Statistical consideration for clinical biomarker research in bladder cancer. Urol. Oncol. 2010, 28, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Roehrborn, C.G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: Results of a comprehensive literature review and meta-analyses. Urology 2003, 61, 109–118. [Google Scholar] [CrossRef]
- Bensalah, K.; Montorsi, F.; Shariat, S.F. Challenges of cancer biomarker profiling. Eur. Urol. 2007, 52, 1601–1609. [Google Scholar] [CrossRef]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Böhle, A.; Palou-Redorta, J.; Rouprêt, M. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 2011, 59, 997–1008. [Google Scholar] [CrossRef]
- Hall, M.C.; Chang, S.S.; Dalbagni, G.; Pruthi, R.S.; Seigne, J.D.; Skinner, E.C.; Wolf, J.S.; Schellhammer, P.F. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J. Urol. 2007, 178, 2314–2330. [Google Scholar] [CrossRef]
- Mitra, A.P.; Datar, R.H.; Cote, R.J. Molecular pathways in invasive bladder cancer: New insights into mechanisms, progression, and target identification. Clin. Oncol. 2006, 24, 5552–5564. [Google Scholar] [CrossRef]
- Mitra, A.P.; Cote, R.J. Molecular pathogenesis and diagnostics of bladder cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 251–285. [Google Scholar] [CrossRef]
- Moon, J.J.; Lu, A.; Moon, C. Role of genomic instability in human carcinogenesis. Exp. Biol. Med. 2019, 244, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Saran, K.K.; Gould, D.; Godec, C.J.; Verma, R.S. Genetics of bladder cancer. J. Mol. Med. 1996, 74, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Ørntoft, T.F.; Wolf, H. Molecular alterations in bladder cancer. Urol. Res. 1998, 26, 223–233. [Google Scholar] [CrossRef]
- Skacel, M.; Pettay, J.D.; Tsiftsakis, E.K.; Procop, G.W.; Biscotti, C.V.; Tubbs, R.R. Validation of a multicolor interphase fluorescence in situ hybridization assay for detection of transitional cell carcinoma on fresh and archival thin-layer, liquid-based cytology slides. Anal. Quant. Cytol. Histol. 2001, 23, 381–387. [Google Scholar] [PubMed]
- Lopez-Beltran, A.; Amin, M.B.; Oliveira, P.S.; Montironi, R.; Algaba, F.; McKenney, J.K.; de Torres, I.; Mazerolles, C.; Wang, M.; Cheng, L. Urothelial carcinoma of the bladder, lipid cell variant: Clinicopathologic findings and LOH analysis. Am. J. Surg. Pathol. 2010, 34, 371–376. [Google Scholar] [CrossRef]
- Ploussard, G.; Dubosq, F.; Soliman, H.; Verine, J.; Desgrandchamps, F.; De Thé, H.; Mongiat-Artus, P. Prognostic value of loss of heterozygosity at chromosome 9p in non–muscle-invasive bladder cancer. Urology 2010, 76, 513.e13–513.e18. [Google Scholar] [CrossRef]
- Cai, T.; Nesi, G.; Canto, M.D.; Mondaini, N.; Mauro, P.; Bartoletti, R. Prognostic role of loss of heterozygosity on chromosome 18 in patients with low-risk nonmuscle-invasive bladder cancer: Results from a prospective study. J. Surg. Res. 2010, 161, 89–94. [Google Scholar] [CrossRef]
- Sibley, K.; Cuthbert-Heavens, D.; Knowles, M.A. Loss of heterozygosity at 4p16.3 and mutation of FGFR3 in transitional cell carcinoma. Oncogene 2001, 20, 686–691. [Google Scholar] [CrossRef]
- Yoon, D.-S.; Li, L.; Zhang, R.-D.; Kram, A.; Ro, J.Y.; Johnston, D.; Grossman, H.B.; Scherer, S.; Czerniak, B. Genetic mapping and DNA sequence-based analysis of deleted regions on chromosome 16 involved in progression of bladder cancer from occult preneoplastic conditions to invasive disease. Oncogene 2001, 20, 5005–5014. [Google Scholar] [CrossRef]
- Docimo, S.G.; Chow, N.-H.; Steiner, G.; Silver, R.I.; Rodriguez, R.; Kinsman, S.; Sidransky, D.; Schoenberg, M. Detection of adenocarcinoma by urinary microsatellite analysis after augmentation cystoplasty. Urology 1999, 54, 561. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T. The diagnostic value of microsatellite LOH analysis and the prognostic relevance of angiogenic gene expression in urinary bladder cancer. Magy. Onkol. 2009, 53, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, R.; Cai, T.; Nesi, G.; Roberta Girardi, L.; Baroni, G.; Dal Canto, M. Loss of P16 expression and chromosome 9p21 LOH in predicting outcome of patients affected by superficial bladder cancer. J. Surg. Res. 2007, 143, 422–427. [Google Scholar] [CrossRef]
- Mao, L.; Schoenberg, M.P.; Scicchitano, M.; Erozan, Y.S.; Merlo, A.; Schwab, D.; Sidransky, D. Molecular detection of primary bladder cancer by microsatellite analysis. Science 1996, 271, 659–662. [Google Scholar] [CrossRef]
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435–445. [Google Scholar] [CrossRef]
- Seripa, D.; Parrella, P.; Gallucci, M.; Gravina, C.; Papa, S.; Fortunato, P.; Alcini, A.; Flammia, G.; Lazzari, M.; Fazio, V.M. Sensitive detection of transitional cell carcinoma of the bladder by microsatellite analysis of cells exfoliated in urine. Int. J. Cancer. 2001, 95, 364–369. [Google Scholar] [CrossRef]
- Frigerio, S.; Padberg, B.C.; Strebel, R.T.; Lenggenhager, D.M.; Messthaler, A.; Abdou, M.-T.; Moch, H.; Zimmermann, D.R. Improved detection of bladder carcinoma cells in voided urine by standardized microsatellite analysis. Int. J. Cancer. 2007, 121, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.O.; Lee, J.; Begum, S.; Yamashita, K.; Engles, J.M.; Schoenberg, M.; Westra, W.H.; Sidransky, D. High-throughput molecular analysis of urine sediment for the detection of bladder cancer by high-density single-nucleotide polymorphism array. Cancer Res. 2003, 63, 5723–5726. [Google Scholar]
- De Bekker-Grob, E.W.; van der Aa, M.N.; Zwarthoff, E.C.; Eijkemans, M.J.; van Rhijn, B.W.; van der Kwast, T.H.; Steyerberg, E.W. Non-muscle-invasive bladder cancer surveillance for which cystoscopy is partly replaced by microsatellite analysis of urine: A cost-effective alternative? BJU Int. 2009, 104, 41–47. [Google Scholar] [CrossRef]
- Van der Aa, M.N.M.; Zwarthoff, E.C.; Steyerberg, E.W.; Boogaard, M.W.; Nijsen, Y.; van der Keur, K.A.; van Exsel, A.J.A.; Kirkels, W.J.; Bangma, C.; van der Kwast, T.H. Microsatellite analysis of voided-urine samples for surveillance of low-grade non-muscle-invasive urothelial carcinoma: Feasibility and clinical utility in a prospective multicenter study (cost-effectiveness of follow-up of urinary bladder cancer trial [CEFUB]). Eur. Urol. 2009, 55, 659–668. [Google Scholar] [CrossRef]
- Wild, P.J.; Fuchs, T.; Stoehr, R.; Zimmermann, D.; Frigerio, S.; Padberg, B.; Steiner, I.; Zwarthoff, E.C.; Burger, M.; Denzinger, S.; et al. Detection of urothelial bladder cancer cells in voided urine can be improved by a combination of cytology and standardized microsatellite analysis. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.; Schoenberg, M.P.; Linn, J.F.; Mao, L.; Sidransky, D. Detection of bladder cancer recurrence by microsatellite analysis of urine. Nat. Med. 1997, 3, 621–624. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.G.; Lurkin, I.; Kirkels, W.J.; van der Kwast, T.H.; Zwarthoff, E.C. Microsatellite analysis? DNA test in urine competes with cystoscopy in follow-up of superficial bladder carcinoma: A Phase II trial. Cancer 2001, 92, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Amira, N.; Mourah, S.; Rozet, F.; Teillac, P.; Fiet, J.; Aubin, P.; Cortesse, A.; Desgrandchamps, F.; Le Duc, A.; Cussenot, O.; et al. Non-invasive molecular detection of bladder cancer recurrence. Int. J. Cancer. 2002, 101, 293–297. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Microsatellite Analysis of Urinary Sediment in Detecting Bladder Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00095589 (accessed on 6 September 2021).
- National Cancer Institute. Detection of Bladder CA by Microsatellite Analysis (MSA). Available online: https://edrn.nci.nih.gov/data-and-resources/protocols/108-detection-of-bladder-ca-by-microsatellite-analysis-msa/ (accessed on 6 September 2021).
- Linn, J.F.; Lango, M.; Halachmi, S.; Schoenberg, M.P.; Sidransky, D. Microsatellite analysis and telomerase activity in archived tissue and urine samples of bladder cancer patients. Int. J. Cancer. 1997, 74, 625–629. [Google Scholar] [CrossRef]
- Schneider, A.; Borgnat, S.; Lang, H.; Régine, O.; Lindner, V.; Kassem, M.; Saussine, C.; Oudet, P.; Jacqmin, D.; Gaub, M.P. Evaluation of microsatellite analysis in urine sediment for diagnosis of bladder cancer. Cancer Res. 2000, 60, 4617–4622. [Google Scholar] [PubMed]
- Sourvinos, G.; Kazanis, I.; Delakas, D.; Cranidis, A.; Spandidos, D.A. Genetic detection of bladder cancer by microsatellite analysis of p16, rb1 and p53 tumor suppressor genes. J. Urol. 2001, 165, 249–252. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, S.; Fan, Z.; Gao, Y.; Di, X.; Wang, D.; Xiao, Z.; Li, C.; An, Q.; Cheng, S. A comparison between microsatellite analysis and cytology of urine for the detection of bladder cancer. Cancer Lett. 2001, 172, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, Z.; Gao, Y.; Xiao, Z.; Li, C.; An, Q.; Cheng, S. Detecting bladder cancer in the Chinese by microsatellite analysis: Ethnic and etiologic considerations. J. Natl. Cancer Inst. 2001, 93, 45–50. [Google Scholar] [CrossRef]
- Mourah, S.; Cussenot, O.; Vimont, V.; Desgrandchamps, F.; Teillac, P.; Cochant-Priollet, B.; Le Duc, A.; Fiet, J.; Soliman, H. Assessment of microsatellite instability in urine in the detection of transitional-cell carcinoma of the bladder. Int. J. Cancer. 1998, 79, 629–633. [Google Scholar] [CrossRef]
- Steiner, G.; Reinschmidt, G.; Müller, S.C. Molecular genetic diagnosis of de novo and recurrent bladder cancer. Electrophoresis 1999, 20, 280–282. [Google Scholar] [CrossRef]
- Baron, A.; Mastroeni, F.; Moore, P.S.; Bonetti, F.; Orlandini, S.; Manfrin, E.; Schiavone, D.; Migliorini, F.; Lusuardi, L.; Mobilio, G.; et al. Detection of bladder cancer by semi-automated microsatellite analysis of urine sediment. Adv. Clin. Path. 2000, 4, 19–24. [Google Scholar] [PubMed]
- Christensen, M.; Wolf, H.; Orntoft, T.F. Microsatellite alterations in urinary sediments from patients with cystitis and bladder cancer. Int. J. Cancer. 2000, 85, 614–617. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.; Lurkin, I.; Chopin, D.K.; Kirkels, W.J.; Thiery, J.P.; van der Kwast, T.H.; Radvanyi, F.; Zwarthoff, E.C. Combined microsatellite and FGFR3 mutation analysis enables a highly sensitive detection of urothelial cell carcinoma in voided urine. Clin. Cancer Res. 2003, 9, 257–263. [Google Scholar]
- Zeger, S.L.; Liang, K.-Y. An overview of methods for the analysis of longitudinal data. Stat. Med. 1992, 11, 1825–1839. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.G.; van der Poel, H.G.; van der Kwast, T.H. Urine markers for bladder cancer surveillance: A systematic review. Eur. Urol. 2005, 47, 736–748. [Google Scholar] [CrossRef]
- Vanrhijn, B.; Smit, M.; Vangeenen, D.; Wijnmaalen, A.; Kirkels, W.; Vanderkwast, T.; Kuenenboumeester, V.; Zwarthoff, E. Surveillance with microsatellite analysis of urine in bladder cancer patients treated by radiotherapy. Eur. Urol. 2003, 43, 369–373. [Google Scholar] [CrossRef]
- Mian, C.; Maier, K.; Comploj, E.; Lodde, M.; Berner, L.; Lusuardi, L.; Palermo, S.; Vittadello, F.; Pycha, A. uCyt+/ImmunoCyt™ in the detection of recurrent urothelial carcinoma. Cancer 2006, 108, 60–65. [Google Scholar] [CrossRef]
- Lodde, M.; Mian, C.; Comploj, E.; Palermo, S.; Longhi, E.; Marberger, M.; Pycha, A. uCyt+ test: Alternative to cystoscopy for less-invasive follow-up of patients with low risk of urothelial carcinoma. Urology 2006, 67, 950–954. [Google Scholar] [CrossRef]
- Parekh, D.J.; Bochner, B.H.; Dalbagni, G. Superficial and muscle-invasive bladder cancer: Principles of management for outcomes assessments. Clin. Oncol. 2006, 24, 5519–5527. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Gupta, N.P. Comparison of NMP22 bladderchek test and urine cytology for the detection of recurrent bladder cancer. Jpn. J. Clin. Oncol. 2006, 36, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Kibar, Y.; Goktas, S.; Kilic, S.; Yaman, H.; Onguru, O.; Peker, A.F. Prognostic value of cytology, nuclear matrix protein 22 (NMP22) test, and urinary bladder cancer II (UBC II) test in early recurrent transitional cell carcinoma of the bladder. Ann. Clin. Lab. Sci. 2006, 36, 31–38. [Google Scholar] [PubMed]
- Mian, C. Multiprobe fluorescence in situ hybridisation: Prognostic perspectives in superficial bladder cancer. J. Clin. Pathol. 2006, 59, 984–987. [Google Scholar] [CrossRef]
- May, M.; Hakenberg, O.W.; Gunia, S.; Pohling, P.; Helke, C.; Lübbe, L.; Nowack, R.; Siegsmund, M.; Hoschke, B. Comparative diagnostic value of urine cytology, UBC-ELISA, and fluorescence in situ hybridization for detection of transitional cell carcinoma of urinary bladder in routine clinical practice. Urology 2007, 70, 449–453. [Google Scholar] [CrossRef]
- Bergman, J.; Reznichek, R.C.; Rajfer, J. Surveillance of patients with bladder carcinoma using fluorescent in-situ hybridization on bladder washings. BJU Int. 2008, 101, 26–29. [Google Scholar] [CrossRef]
- Raitanen, M.-P. The role of BTA stat Test in follow-up of patients with bladder cancer: Results from FinnBladder studies. World J. Urol. 2008, 26, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Moon, C.; Gordon, M.; Moon, D.; Reynolds, T. Microsatellite instability analysis (MSA) for bladder cancer: Past history and future directions. Int. J. Mol. Sci. 2021, 22, 12864. [Google Scholar] [CrossRef]
| Study | No. of Cancers Detected by MSA | Sensitivity (%) | Healthy Controls with Neg MSA Result | Specificity (%) |
|---|---|---|---|---|
| Mao et al. (1996) ♦ Science 271:659–662 [34] | 19/20 | 95 | 5 out of 5 | 100 |
| Steiner et al. (1997) ♦ Nat. Med. 3:621–624 [42] | 10/11 | 91 | 10 out of 10 | 100 |
| Linn et al. (1997) ♦ Int. J. Cancer 74:625–629 [47] | 13/15 | 87 | N/A | N/A |
| Schneider et al. (2000) ♦ Cancer Res. 60:4617–4622 [48] | 87/103 | 84 | N/A | N/A |
| Sourvinos et al. (2001) ♦ J. Urol. 165:249–252 [49] | 26/28 | 93 | 10 out of 10 | 100 |
| Zhang et al. (2001) ♦ Cancer Lett. 172:55–58 [50] | 73/81 | 90 | 19/19 | 100 |
| Seripa et al. (2001) ♦ Int. J. Cancer 95:364–369 [36] | 33/34 | 97 | 11 out of 11 | 100 |
| Zhang et al. (2001) ♦ J. Natl. Cancer Inst. 93:45–50 [51] | 22/23 | 96 | 17/17 | 100 |
| Amira et al. (2002) ♦ Int. J. Cancer 101:293–297 [44] | 44/47 | 94 | N/A | N/A |
| Overall | 327/362 | 90% | 72/72 | 100% |
| Locus | |
|---|---|
| D13S802 | Primer F: TGACACAGTGAGACTCTATCTCAAAAA |
| (AAAG)n | Primer R: CTTCAGACTGGCTTAGACTGAGG |
| D4S243 | Primer F: TCAGTCTCTCTTTCTCCTTGCA |
| (ATAG)n | Primer R: TAGGAGCCTGTGGTCCTGTT |
| FGA | Primer F: GACATCTTAACTGGCATTCATGG |
| (TTTC)n | Primer R: CTTCTCAGATCCTCTGACACTCG |
| D9S747 | Primer F: GCCATTATTGACTCTGGAAAAGAC |
| (GATA)n | Primer R: CAGGCTCTCAAAATATGAACAAAAT |
| D17S654 | Primer F: ACCTAGGCCATGTTCACAGC |
| (CA)n | Primer R: GAGCAGAATGAGAGGCCAAG |
| D9S162 | Primer F: GCAACCATTTATGTGGTTAGGG |
| (CA)n | Primer R: TCCCACAACAAATCTCCTCAC |
| D17S695 | Primer F: CTGGGCAACAAGAGCAAAAT |
| (AAAG)n | Primer R: TTTGTTGTTGTTCATTGACTTCAGTC |
| MBP | Primer F: GGACCTCGTGAATTACAATCACT |
| (ATGG)n | Primer R: ATCCATTTACCTACCTGTTCATCC |
| D21S1245 | Primer F: CCAGAAAATGACACATGAAGGA |
| (AAAG)n | Primer R: TTGTTGAGGATTTTTGCATCA |
| D16S310 | Primer F: GGGCAACAAGGAGAGACTCT |
| (ATAG)n | Primer R: AAAAAAGGACCTGCCTTTATCC |
| D20S48 | Primer F: ATGGTCTCCAGTCCCATCTG |
| (GT)n | Primer R: TTGACCTGGATGAGCATGTG |
| THO1 | Primer F: AGGCTCTAGCAGCAGCTCAT |
| (TCAT)n | Primer R: TGTACACAGGGCTTCCGAGT |
| D9S171 | Primer F: TCTGTCTGCTGCCTCCTACA |
| (CA)n | Primer R: GATCCTATTTTTCTTGGGGCTA |
| D16S476 | Primer F: GGCAACAAGAGCAAAACTCC |
| (AAAG)n | Primer R: GGTGCTCTCTGCCCTATCTG |
| IFN-A | Primer F: TGCGCGTTAAGTTAATTGGTT |
| (GT)n | Primer R: GTAAGGTGGAAACCCCCACT |
| Multiplex | Target | Chromosome | STR | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|---|---|---|
| MP1 | D4S243 | 4 | (ATAG)n | 6-FAM-TCAGTCTCTCTTTCTCCTTGCA | TAGGAGCCTGTGGTCCTGTT |
| FGA | 4 | (TTTC)n | VIC-GACATCTTAACTGGCATTCATGG | CTTCTCAGATCCTCTGACACTCG | |
| D9S747 | 9 | (GATA)n | VIC-GCCATTATTGACTCTGGAAAAGAC | CAGGCTCTCAAAATATGAACAAAAT | |
| D17S654 | 17 | (CA)n | NED-ACCTAGGCCATGTTCACAGC | GAGCAGAATGAGAGGCCAAG | |
| D17S695 | 16 | (AAAG)n | PET-CTGGGCAACAAGAGCAAAAT | TTTGTTGTTGTTCATTGACTTCAGTC | |
| MP2 | D9S162 | 9 | (CA)n | NED-GCAACCATTTATGTGGTTAGGG | TCCCACAACAAATCTCCTCAC |
| MBP | 18 | (ATGG)n | 6-FAM-GGACCTCGTGAATTACAATCACT | ATCCATTTACCTACCTGTTCATCC | |
| D16S310 | 16 | (ATAG)n | VIC-GGGCAACAAGGAGAGACTCT | AAAAAAGGACCTGCCTTTATCC | |
| THO1 | 11 | (TCAT)n | NED-AGGCTCTAGCAGCAGCTCAT | TGTACACAGGGCTTCCGAGT | |
| IFN-A | 9 | (GT)n | PET-TGCGCGTTAAGTTAATTGGTT | GTAAGGTGGAAACCCCCACT | |
| MP3 | D21S1245 | 21 | (AAAG)n | VIC-CCAGAAAATGACACATGAAGGA | TTGTTGAGGATTTTTGCATCA |
| D20S48 | 20 | (GT)n | NED-ATGGTCTCCAGTCCCATCTG | TTGACCTGGATGAGCATGTG | |
| D9S171 | 9 | (CA)n | NED-TCTGTCTGCTGCCTCCTACA | GATCCTATTTTTCTTGGGGCTA | |
| D16S476 | 16 | (AAAG)n | 6-FAM-GGCAACAAGAGCAAAACTCC | GGTGCTCTCTGCCCTATCTG |
| Locus: D4S243 | Repeat Type: (ATAG)n | Size Range: Color 165–192 bp | Channel: Blue | K562 -Allele Sizes: 169 bp |
|---|---|---|---|---|
| FGA | (TTTC)n | 299–361 bp | Green | 328 bp |
| D9S747 | (GATA)n | 179–201 bp | Green | 185 bp |
| Dl 7S654 | (CA)n | 194–218 bp | Yellow | 216 bp |
| D9S162 | (CA)n | 117–148 bp | Yellow | 143 bp |
| D17S695 | (AAAG)n | 170–220 bp | Red | 185, 200 bp |
| MBP & A | (ATGG)n | 200–242/119–151 | Blue | 207, 215, 119 bp |
| D21S1245 | (AAAG)n | 209–293 bp | Green | 236, 255 bp |
| D16S310 | (ATAG)n | 127–170 bp | Green | 155, 160 bp |
| D20S48 | (GT)n | 251–269 bp | Yellow | 261 bp |
| THOl | (TCAT)n | 174–209 bp | Yellow | 198 bp |
| D9Sl 71 | (CA)n | 109–129 bp | Yellow | 126 bp |
| D16S476 | (AAAG)n | 176–230 bp | Red | 187 209 bp |
| IFN-A | (GT)n | 132–152 bp | Red | no product |
| Marker Lower | Limit | Upper Limit |
|---|---|---|
| D4S243 | 0.68 | 1.33 |
| FGA | 0.60 | 1.43 |
| D9S747 | 0.63 | 1.42 |
| Dl 7S654 | 0.71 | 1.36 |
| DI 7S695 | 0.49 | 1.61 |
| MBP | 0.63 | 1.45 |
| MBPA | 0.71 | 1.37 |
| D16S310 | 0.6 | 1.34 |
| D9S162 | 0.51 | 1.53 |
| THOl | 0.46 | 1.53 |
| IFN-A | 0.68 | 1.43 |
| D21Sl245 | 0.58 | 1.42 |
| D20S48 | 0.62 | 1.46 |
| D9Sl 71 | 0.72 | 1.36 |
| D16S476 | 0.54 | 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, T.; Gordon, M.; Monar, G.V.F.; Moon, D.; Moon, C. Development of Multiplex Polymerase Chain Reaction (PCR)-Based MSA Assay for Bladder Cancer Detection. Int. J. Mol. Sci. 2023, 24, 13651. https://doi.org/10.3390/ijms241713651
Reynolds T, Gordon M, Monar GVF, Moon D, Moon C. Development of Multiplex Polymerase Chain Reaction (PCR)-Based MSA Assay for Bladder Cancer Detection. International Journal of Molecular Sciences. 2023; 24(17):13651. https://doi.org/10.3390/ijms241713651
Chicago/Turabian StyleReynolds, Thomas, Maxie Gordon, Gabriela Vanessa Flores Monar, David Moon, and Chulso Moon. 2023. "Development of Multiplex Polymerase Chain Reaction (PCR)-Based MSA Assay for Bladder Cancer Detection" International Journal of Molecular Sciences 24, no. 17: 13651. https://doi.org/10.3390/ijms241713651
APA StyleReynolds, T., Gordon, M., Monar, G. V. F., Moon, D., & Moon, C. (2023). Development of Multiplex Polymerase Chain Reaction (PCR)-Based MSA Assay for Bladder Cancer Detection. International Journal of Molecular Sciences, 24(17), 13651. https://doi.org/10.3390/ijms241713651






