Isothiocyanates as Tubulin Polymerization Inhibitors—Synthesis and Structure–Activity Relationship Studies
Abstract
:1. Introduction
2. Results
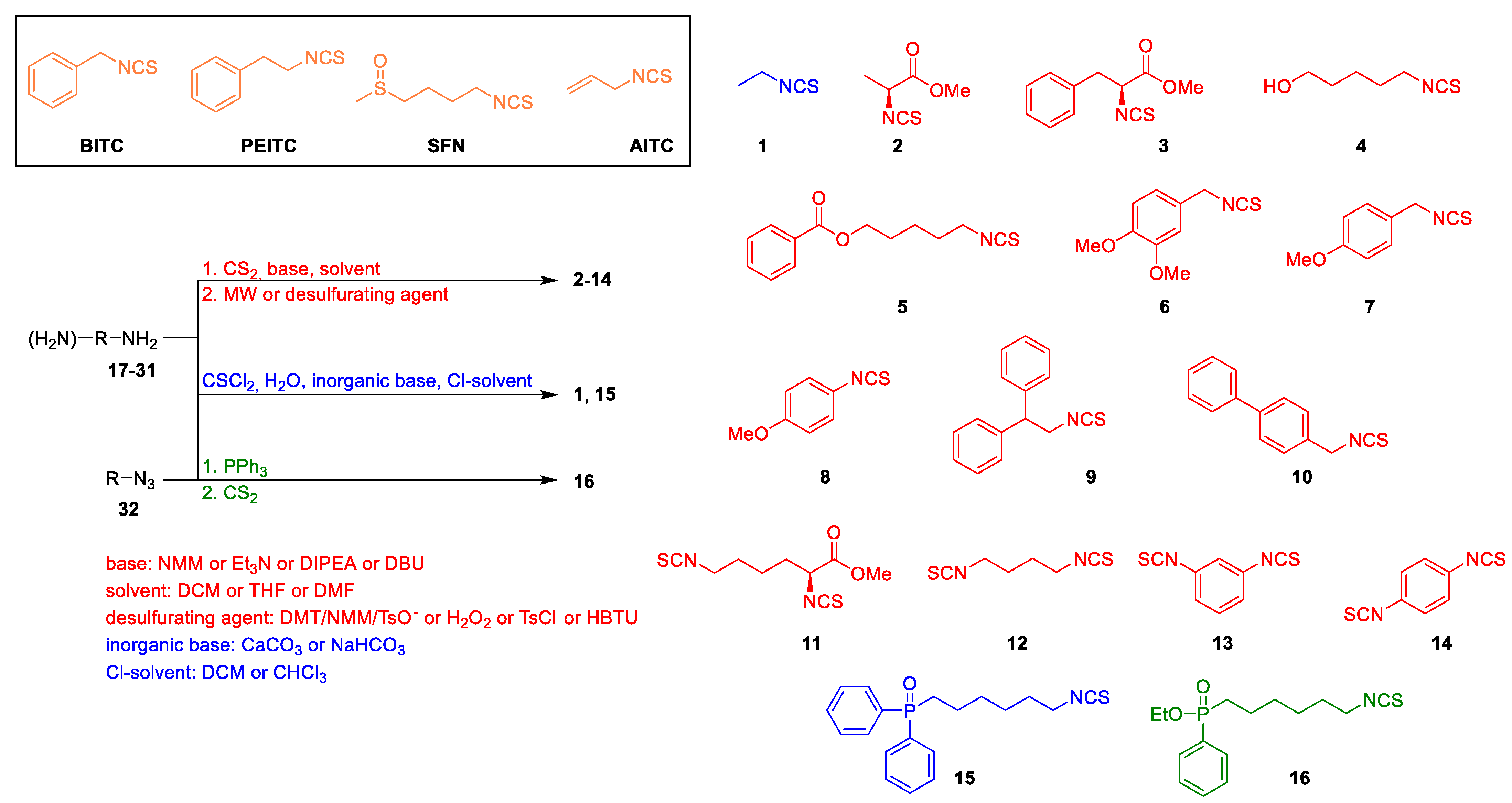
2.1. Chemistry
2.2. In Vitro Antiproliferative Activity of ITCs
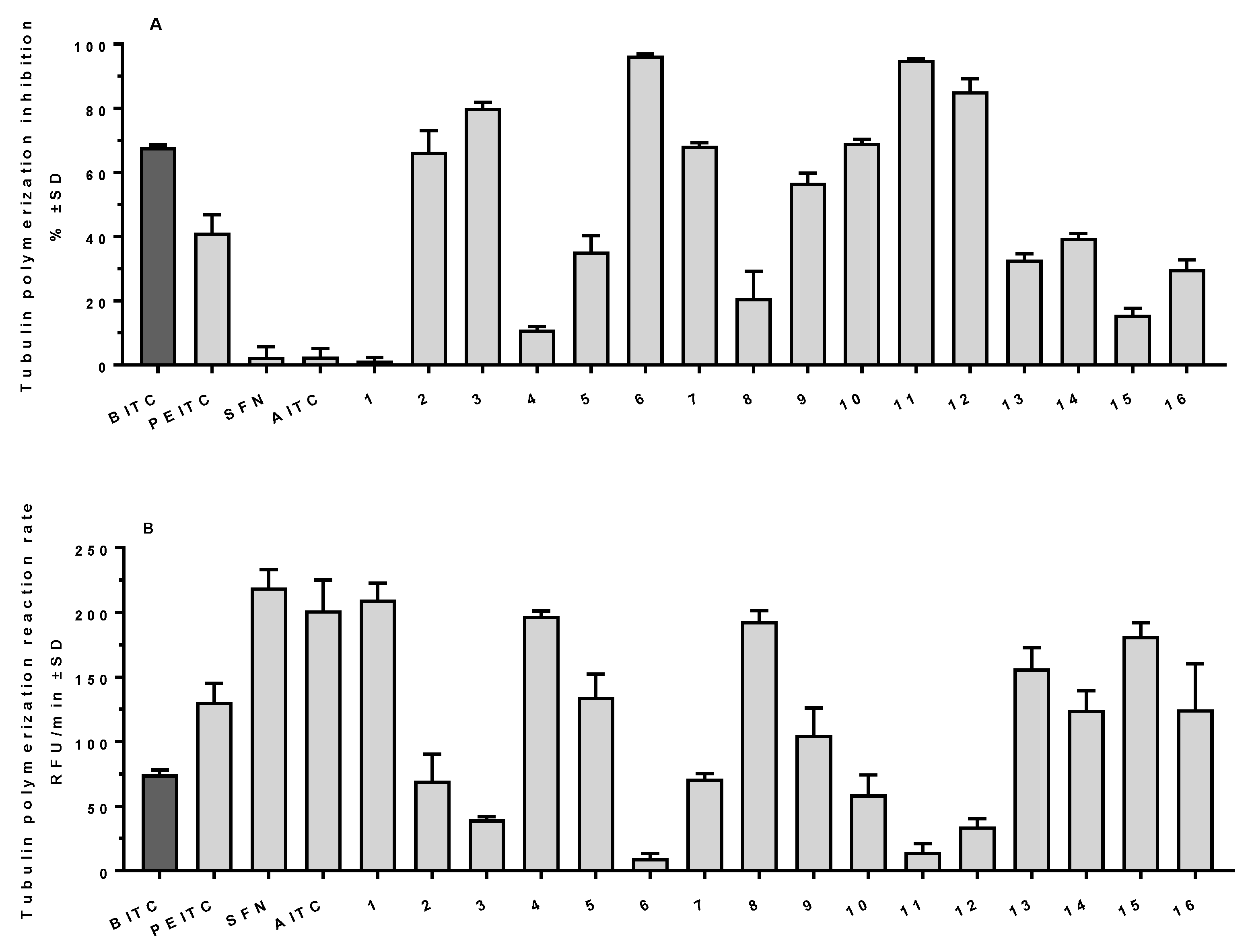
2.3. ITCs as Tubulin Polymerization Inhibitors
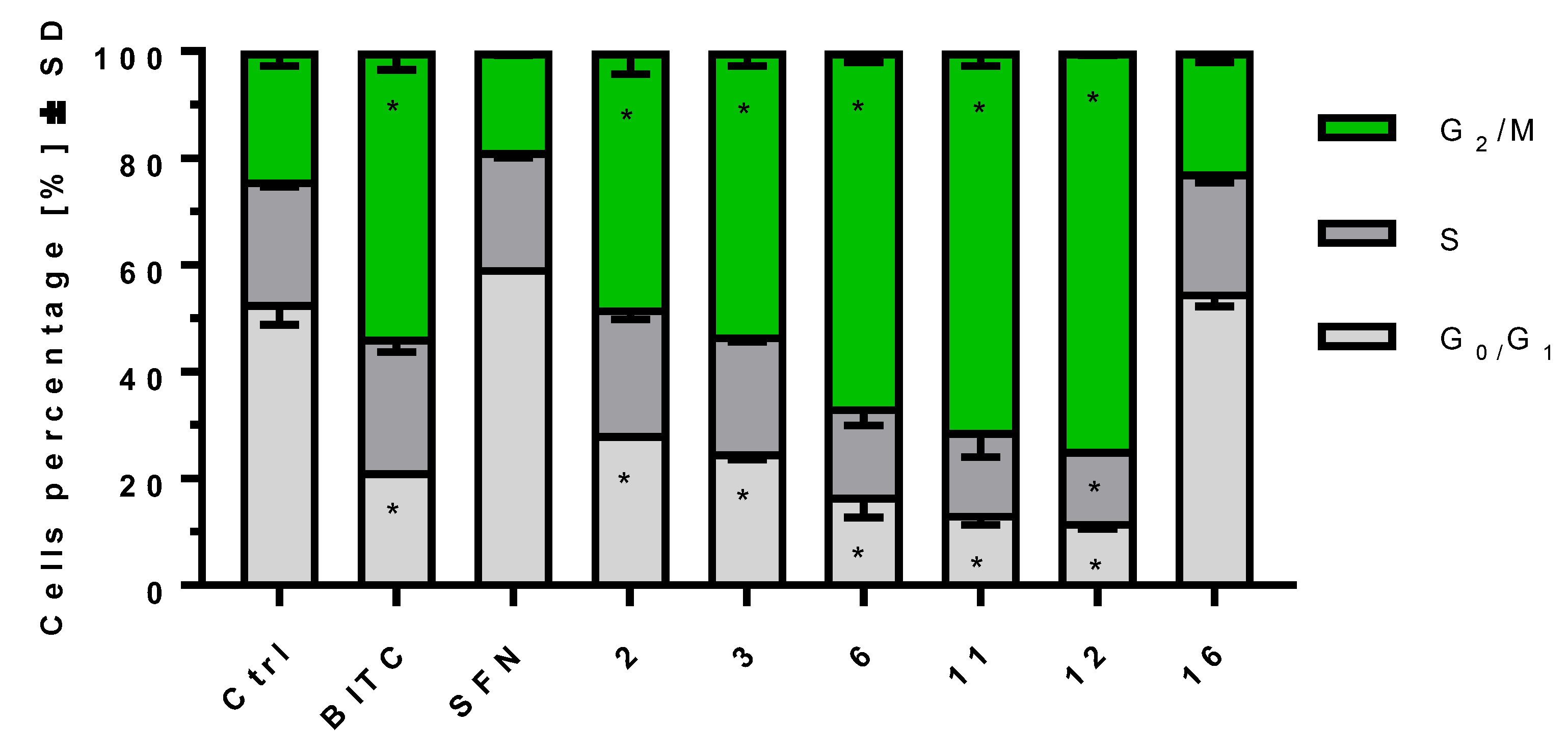
2.4. ITCs Influence on Cell Cycle Progression on MV-4-11 Cells
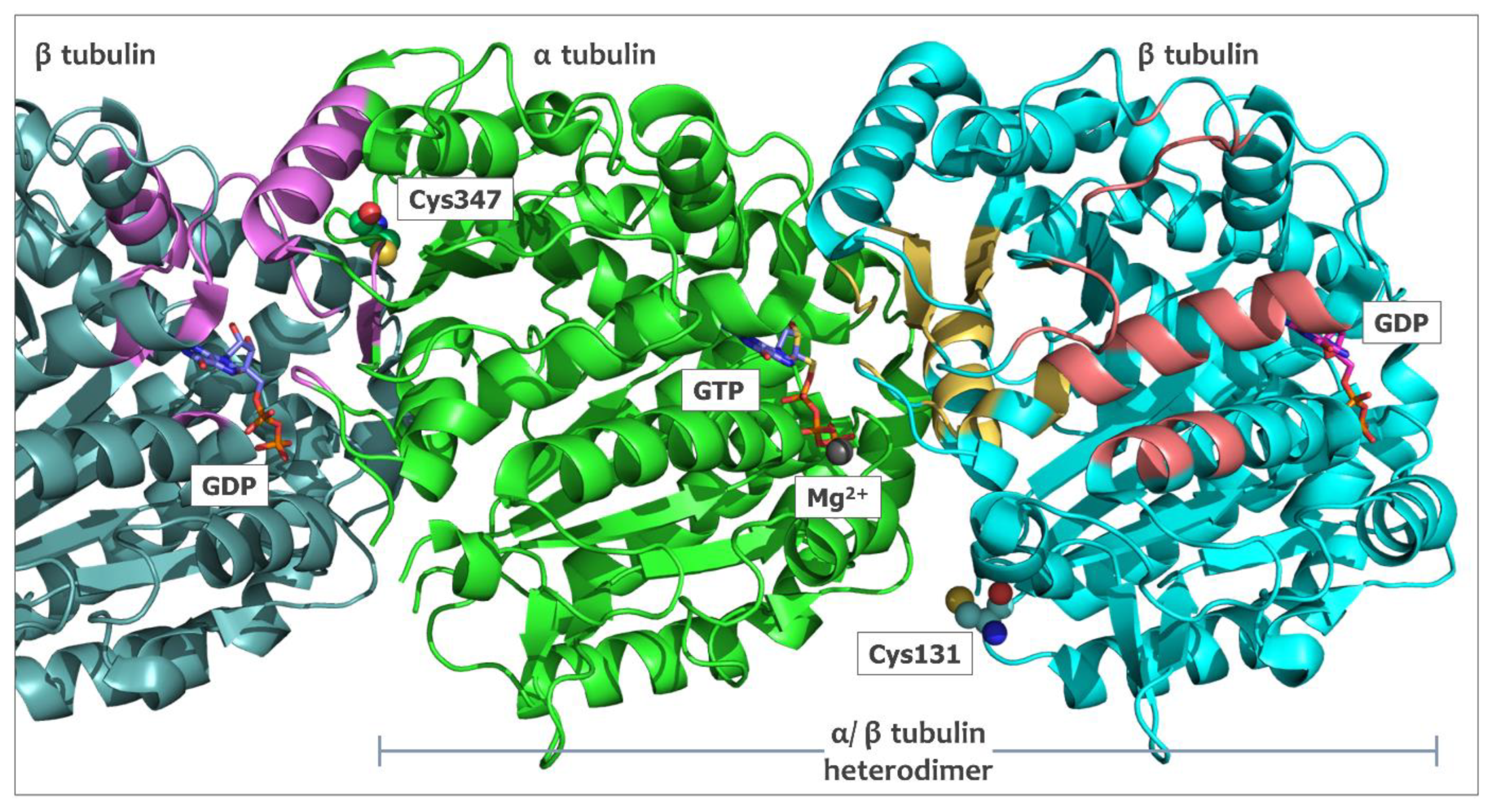
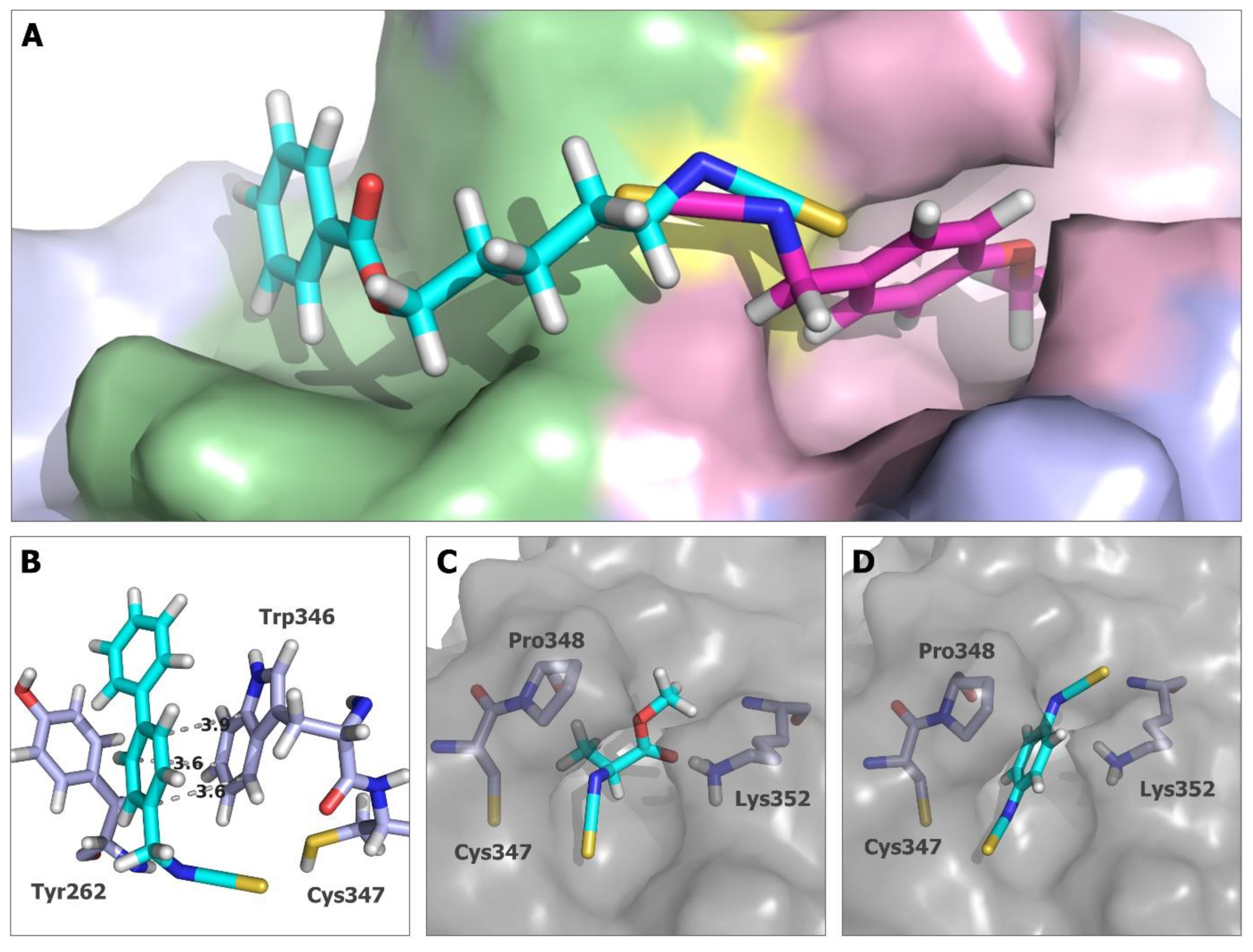
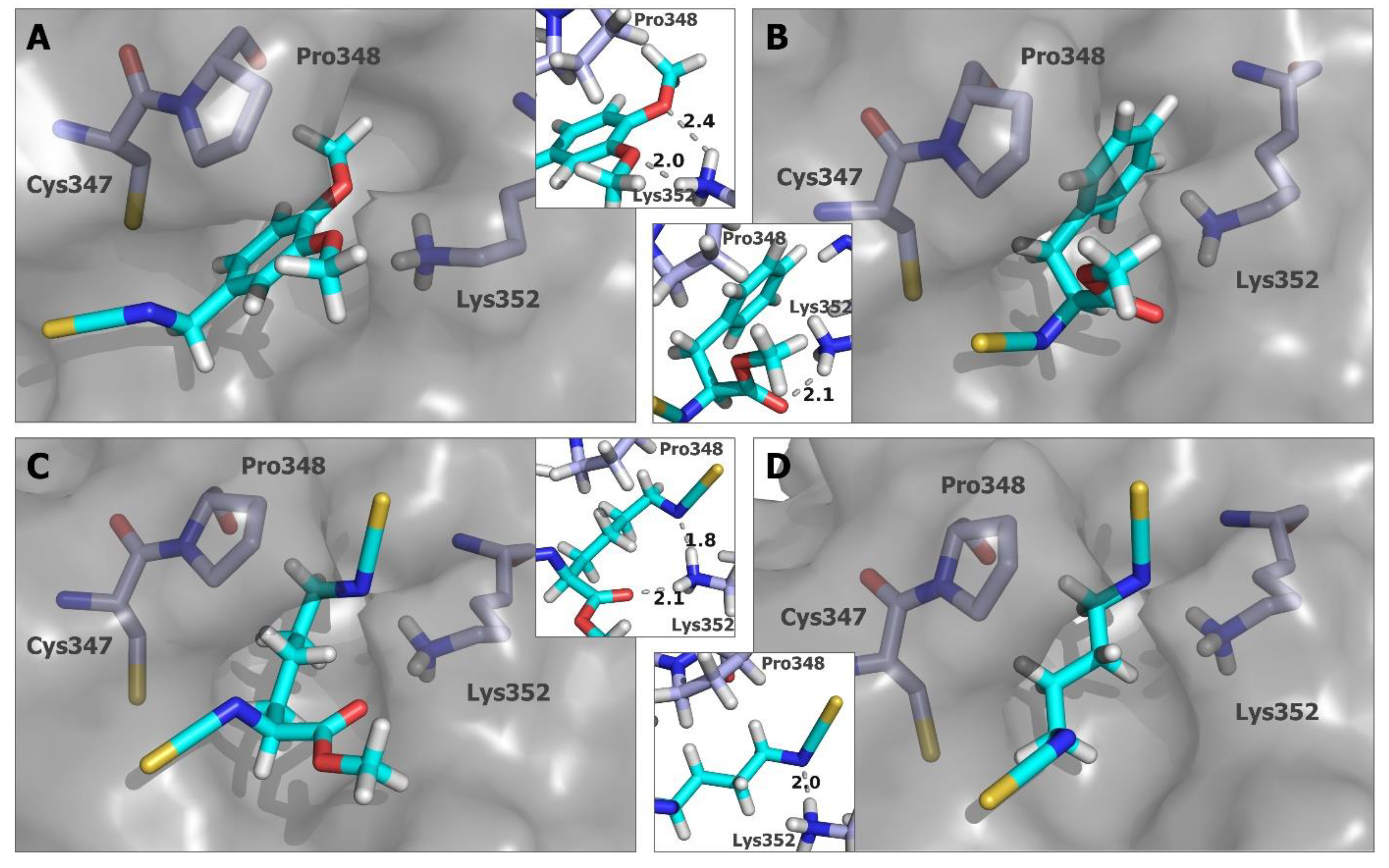
2.5. In Silico Studies for Isothiocyanates–Tubulin Interactions
3. Discussion
4. Materials and Methods
4.1. Biological Studies
4.2. In Cellulo Antiproliferative Studies
4.3. Cell-Free Tubulin Polymerization Assay
4.4. Cell Cycle Analyses
4.5. In Silico Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altmann, K.-H.; Gertsch, J. Anticancer Drugs from Nature—Natural Products as a Unique Source of New Microtubule-Stabilizing Agents. Nat. Prod. Rep. 2007, 24, 327–357. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-Binding Agents: A Dynamic Field of Cancer Therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- Jordan, M. Mechanism of Action of Antitumor Drugs That Interact with Microtubules and Tubulin. Curr. Med. Chem.-Anti-Cancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic Catastrophe: A Mechanism for Avoiding Genomic Instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Chot, C.-G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Singh, S.V.; Singh, K. Cancer Chemoprevention with Dietary Isothiocyanates Mature for Clinical Translational Research. Carcinogenesis 2012, 33, 1833–1842. [Google Scholar] [CrossRef]
- Zhang, Y. The Molecular Basis That Unifies the Metabolism, Cellular Uptake and Chemopreventive Activities of Dietary Isothiocyanates. Carcinogenesis 2012, 33, 2–9. [Google Scholar] [CrossRef]
- Mi, L.; Hood, B.L.; Stewart, N.A.; Xiao, Z.; Govind, S.; Wang, X.; Conrads, T.P.; Veenstra, T.D.; Chung, F.-L. Identification of Potential Protein Targets of Isothiocyanates by Proteomics. Chem. Res. Toxicol. 2011, 24, 1735–1743. [Google Scholar] [CrossRef]
- Mi, L.; Xiao, Z.; Hood, B.L.; Dakshanamurthy, S.; Wang, X.; Govind, S.; Conrads, T.P.; Veenstra, T.D.; Chung, F.-L. Covalent Binding to Tubulin by Isothiocyanates: A Mechanism of Cell Growth Arrest and Apoptosis. J. Biol. Chem. 2008, 283, 22136–22146. [Google Scholar] [CrossRef]
- Xiao, Z.; Mi, L.; Chung, F.-L.; Veenstra, T.D. Proteomic Analysis of Covalent Modifications of Tubulins by Isothiocyanates. J. Nutr. 2012, 142, 1377S–1381S. [Google Scholar] [CrossRef]
- Mi, L.; Gan, N.; Cheema, A.; Dakshanamurthy, S.; Wang, X.; Yang, D.C.H.; Chung, F.L. Cancer Preventive Isothiocyanates Induce Selective Degradation of Cellular α-and β-Tubulins by Proteasomes. J. Biol. Chem. 2009, 284, 17039–17051. [Google Scholar] [CrossRef]
- Geng, F.; Tang, L.; Li, Y.; Yang, L.; Choi, K.-S.; Kazim, A.L.; Zhang, Y. Allyl Isothiocyanate Arrests Cancer Cells in Mitosis, and Mitotic Arrest in Turn Leads to Apoptosis via Bcl-2 Protein Phosphorylation. J. Biol. Chem. 2011, 286, 32259–32267. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Kręgiel, D.; Kolesińska, B. Synthesis of Isothiocyanates Using DMT/NMM/TsO− as a New Desulfurization Reagent. Molecules 2021, 26, 2740. [Google Scholar] [CrossRef]
- Li, G.; Tajima, H.; Ohtani, T. An Improved Procedure for the Preparation of Isothiocyanates from Primary Amines by Using Hydrogen Peroxide as the Dehydrosulfurization Reagent. J. Org. Chem. 1997, 62, 4539–4540. [Google Scholar] [CrossRef]
- Wong, R.; Dolman, S.J. Isothiocyanates from Tosyl Chloride Mediated Decomposition of in Situ Generated Dithiocarbamic Acid Salts. J. Org. Chem. 2007, 72, 3969–3971. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Gajda, A.; Gajda, T. Direct, Microwave-Assisted Synthesis of Isothiocyanates. Eur. J. Org. Chem. 2019, 2019, 2528–2532. [Google Scholar] [CrossRef]
- Grzywa, R.; Winiarski, Ł.; Psurski, M.; Rudnicka, A.; Wietrzyk, J.; Gajda, T.; Oleksyszyn, J. Synthesis and Biological Activity of Diisothiocyanate-Derived Mercapturic Acids. Bioorg. Med. Chem. Lett. 2016, 26, 667–671. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Psurski, M.; Świtalska, M.; Gajda, A.; Goszczyński, T.M.; Oleksyszyn, J.; Wietrzyk, J.; Gajda, T. Design, Synthesis, and Evaluation of ω-(Isothiocyanato)Alkylphosphinates and Phosphine Oxides as Antiproliferative Agents. ChemMedChem 2018, 13, 105–115. [Google Scholar] [CrossRef]
- Nam, K.D.; Han, M.; Yoon, J.; Kim, E.-A.; Cho, S.-W.; Hahn, H.-G. 2-Amino-1,3-Thiazoles Suppressed Lipopolysaccharide-Induced IL-β and TNF-α. Bull. Korean Chem. Soc. 2013, 34, 271–274. [Google Scholar] [CrossRef]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies to Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Yan, W.; Li, W.; Qiu, Q.; Ye, H.; Chen, L. Covalent Modification of Cys-239 in β-Tubulin by Small Molecules as a Strategy to Promote Tubulin Heterodimer Degradation. J. Biol. Chem. 2019, 294, 8161–8170. [Google Scholar] [CrossRef]
- LaFrance, B.J.; Roostalu, J.; Henkin, G.; Greber, B.J.; Zhang, R.; Normanno, D.; McCollum, C.O.; Surrey, T.; Nogales, E. Structural Transitions in the GTP Cap Visualized by Cryo-Electron Microscopy of Catalytically Inactive Microtubules. Proc. Natl. Acad. Sci. USA 2022, 119, e2114994119. [Google Scholar] [CrossRef] [PubMed]
- Burkert, U.; Allinger, N.L. Molecular Mechanics; American Chemical Society: Washington, DC, USA, 1982. [Google Scholar]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical Scoring Functions for Advanced Protein−Ligand Docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Korb, O.; Stützle, T.; Exner, T.E. PLANTS: Application of Ant Colony Optimization to Structure-Based Drug Design. In Ant Colony Optimization and Swarm Intelligence; Dorigo, M., Gambardella, L.M., Birattari, M., Martinoli, A., Poli, R., Stützle, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 247–259. [Google Scholar]
- Korb, O.; Stützle, T.; Exner, T.E. An Ant Colony Optimization Approach to Flexible Protein–Ligand Docking. Swarm Intell. 2007, 1, 115–134. [Google Scholar] [CrossRef]
- Hecht, S.S. Chemoprevention of Cancer by Isothiocyanates, Modifiers of Carcinogen Metabolism. J. Nutr. 1999, 129, 768S–774S. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Q.; Xu, K. Are Isothiocyanates Potential Anti-Cancer Drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef]
- Gupta, P.; Kim, B.; Kim, S.-H.; Srivastava, S.K. Molecular Targets of Isothiocyanates in Cancer: Recent Advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef]
- Usui, T.; Watanabe, H.; Nakayama, H.; Tada, Y.; Kanoh, N.; Kondoh, M.; Asao, T.; Takio, K.; Watanabe, H.; Nishikawa, K.; et al. The Anticancer Natural Product Pironetin Selectively Targets Lys352 of α-Tubulin. Chem. Biol. 2004, 11, 799–806. [Google Scholar] [CrossRef]
- Chi, S.; Xie, W.; Zhang, J.; Xu, S. Theoretical Insight into the Structural Mechanism for the Binding of Vinblastine with Tubulin. J. Biomol. Struct. Dyn. 2015, 33, 2234–2254. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Psurski, M.; Janczewski, Ł.; Świtalska, M.; Gajda, A.; Goszczyński, T.M.; Oleksyszyn, J.; Wietrzyk, J.; Gajda, T. Novel Phosphonate Analogs of Sulforaphane: Synthesis, in Vitro and in Vivo Anticancer Activity. Eur. J. Med. Chem. 2017, 132, 63–80. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Krishan, A. Rapid Flow Cytofluorometric Analysis of Mammalian Cell Cycle by Propidium Iodide Staining. J. Cell Biol. 1975, 66, 188–193. [Google Scholar] [CrossRef]
- ten Brink, T.; Exner, T.E. PKa Based Protonation States and Microspecies for Protein–Ligand Docking. J. Comput.-Aided Mol. Des. 2010, 24, 935–942. [Google Scholar] [CrossRef]
- Li, D.; Shu, Y.; Li, P.; Zhang, W.; Ni, H.; Cao, Y. Synthesis and structure-activity relationships of aliphatic isothiocyanate analogs as antibiotic agents. Med. Chem. Res. 2013, 22, 3119–3125. [Google Scholar] [CrossRef]
- Park, S.; Hayes, B.L.; Marankan, F.; Mulhearn, D.C.; Wanna, L.; Mesecar, A.D.; Santarsiero, B.D.; Johnson, M.E.; Venton, D.L. Regioselective covalent modyfication of hemoglobin in search of antisicking agents. J. Med. Chem. 2003, 46, 936–953. [Google Scholar] [CrossRef]
- De Nicola, G.R.; Montaut, S.; Rollin, P.; Nyegue, M.; Menut, C.; Iori, R.; Tatibouët, A. Stability of benzylic-type isothiocyanates in hydrodistillation-mimicking conditions. J. Agric. Food Chem. 2013, 61, 137–142. [Google Scholar] [CrossRef]
- Kiaku, C.; Walsh, J.M.; Leech, M.C.; Poole, D.L.; Mason, J.; Goodall, I.C.A.; Devo, P.; Lam, K. Eletrochemical isothiocyanation of primary amines. Org. Lett. 2023, 25, 1147–1150. [Google Scholar] [CrossRef]
- Tournaire-Arellano, C.; Younes-El Hage, S.; Valès, P.; Caujolle, R.; Sanon, A.; Bories, C.; Loiseau, P.M. Synthesis and biological evaluation of ureido and thioureido derivatives of 2-amino-2-deoxy-d-glucose and related aminoalcohols as N-acetyl-β-d-hexosaminidase inhibitors. Carbohydr. Res. 1998, 314, 47–63. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Deng, J.-C.; Ke, Y.-P.; Zhong, X.-L.; Xu, L.; Tang, R.-Y.; Zheng, W. Isothiocyanation of amines using the Langlois reagent. Chem. Commun. 2017, 53, 6073–6076. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Gajda, A.; Frankowski, S.; Goszczyński, T.M.; Gajda, T. T3P®—A Benign Desulfurating Reagent in the Synthesis of Isothiocyanates. Synthesis 2018, 50, 1141–1151. [Google Scholar]


| IC50 ± SD [µM] * | ||||
|---|---|---|---|---|
| Compd | LoVo | A2780 | MV-4-11 | U-937 |
| BITC | 4.09 ± 0.96 | 3.46 ± 0.58 | 1.44 ± 0.64 | 8.47 ± 0.22 |
| PEITC | 8.22 ± 1.64 | 6.32 ± 1.16 | 2.67 ± 0.78 | 9.13 ± 3.12 |
| SFN | 7.99 ± 2.30 | 4.40 ± 1.50 | 3.51 ± 0.09 | 9.90 ± 3.74 |
| AITC | 22.58 ± 6.77 | 11.41 ± 2.50 | 7.48 ± 2.31 | 10.50 ± 0.70 |
| 1 | [14.61] ** ± 5.65 | [13.55] ** ± 2.12 | [8.03] ** ± 4.32 | [5.37] ** ± 2.36 |
| 2 | 8.68 ± 1.82 | 4.27 ± 1.12 | 6.04 ± 1.80 | 8.03 ± 0.44 |
| 3 | 7.88 ± 0.60 | 6.46 ± 0.28 | 2.38 ± 0.68 | 8.16 ± 0.32 |
| 4 | 4.01 ± 1.01 | 3.39 ± 0.56 | 1.87 ± 0.27 | 5.50 ± 0.20 |
| 5 | 1.84 ± 0.96 | 3.26 ± 0.31 | 0.59 ± 0.07 | 3.28 ± 1.39 |
| 6 | 1.92 ± 0.38 | 1.38 ± 0.20 | 0.87 ± 0.45 | 2.02 ± 0.60 |
| 7 | 1.70 ± 0.26 | 1.24 ± 0.09 | 0.81 ± 0.20 | 1.53 ± 0.12 |
| 8 | [14.31] ** ± 1.75 | [14.25] ** ± 4.32 | [18.43] ** ± 4.52 | [15.35] ** ± 8.43 |
| 9 | 1.86 ± 0.16 | 1.53 ± 0.44 | 1.11 ± 0.36 | 3.22 ± 1.10 |
| 10 | 3.97 ± 0.41 | 2.57 ± 0.81 | 1.28 ± 0.17 | 4.15 ± 1.94 |
| 11 | 1.64 ± 0.53 | 1.00 ± 0.22 | 1.66 ± 0.22 | 2.95 ± 1.07 |
| 12 | 0.68 ± 0.09 | 0.32 ± 0.07 | 0.22 ± 0.05 | 0.72 ± 0.06 |
| 13 | [24.21] ** ± 8.64 | [33.65] ** ± 2.12 | [28.03] ** ± 3.72 | [25.35] ** ± 5.46 |
| 14 | 8.00 ± 0.53 | 7.73 ± 1.66 | 4.36 ± 1.19 | 15.85 ± 2.71 |
| 15 | 13.21 ± 1.12 | 11.20 ± 2.36 | 8.69 ± 1.18 | 11.21 ± 3.66 |
| 16 | 11.87 ± 2.21 | 9.97 ± 2.69 | 8.47 ± 3.21 | 10.22 ± 4.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzywa, R.; Psurski, M.; Gajda, A.; Gajda, T.; Janczewski, Ł. Isothiocyanates as Tubulin Polymerization Inhibitors—Synthesis and Structure–Activity Relationship Studies. Int. J. Mol. Sci. 2023, 24, 13674. https://doi.org/10.3390/ijms241813674
Grzywa R, Psurski M, Gajda A, Gajda T, Janczewski Ł. Isothiocyanates as Tubulin Polymerization Inhibitors—Synthesis and Structure–Activity Relationship Studies. International Journal of Molecular Sciences. 2023; 24(18):13674. https://doi.org/10.3390/ijms241813674
Chicago/Turabian StyleGrzywa, Renata, Mateusz Psurski, Anna Gajda, Tadeusz Gajda, and Łukasz Janczewski. 2023. "Isothiocyanates as Tubulin Polymerization Inhibitors—Synthesis and Structure–Activity Relationship Studies" International Journal of Molecular Sciences 24, no. 18: 13674. https://doi.org/10.3390/ijms241813674
APA StyleGrzywa, R., Psurski, M., Gajda, A., Gajda, T., & Janczewski, Ł. (2023). Isothiocyanates as Tubulin Polymerization Inhibitors—Synthesis and Structure–Activity Relationship Studies. International Journal of Molecular Sciences, 24(18), 13674. https://doi.org/10.3390/ijms241813674






