Humoral and Cellular Immune Response Elicited by the BNT162b2 COVID-19 Vaccine Booster in Elderly
Abstract
:1. Introduction
2. Results
2.1. Study Participants
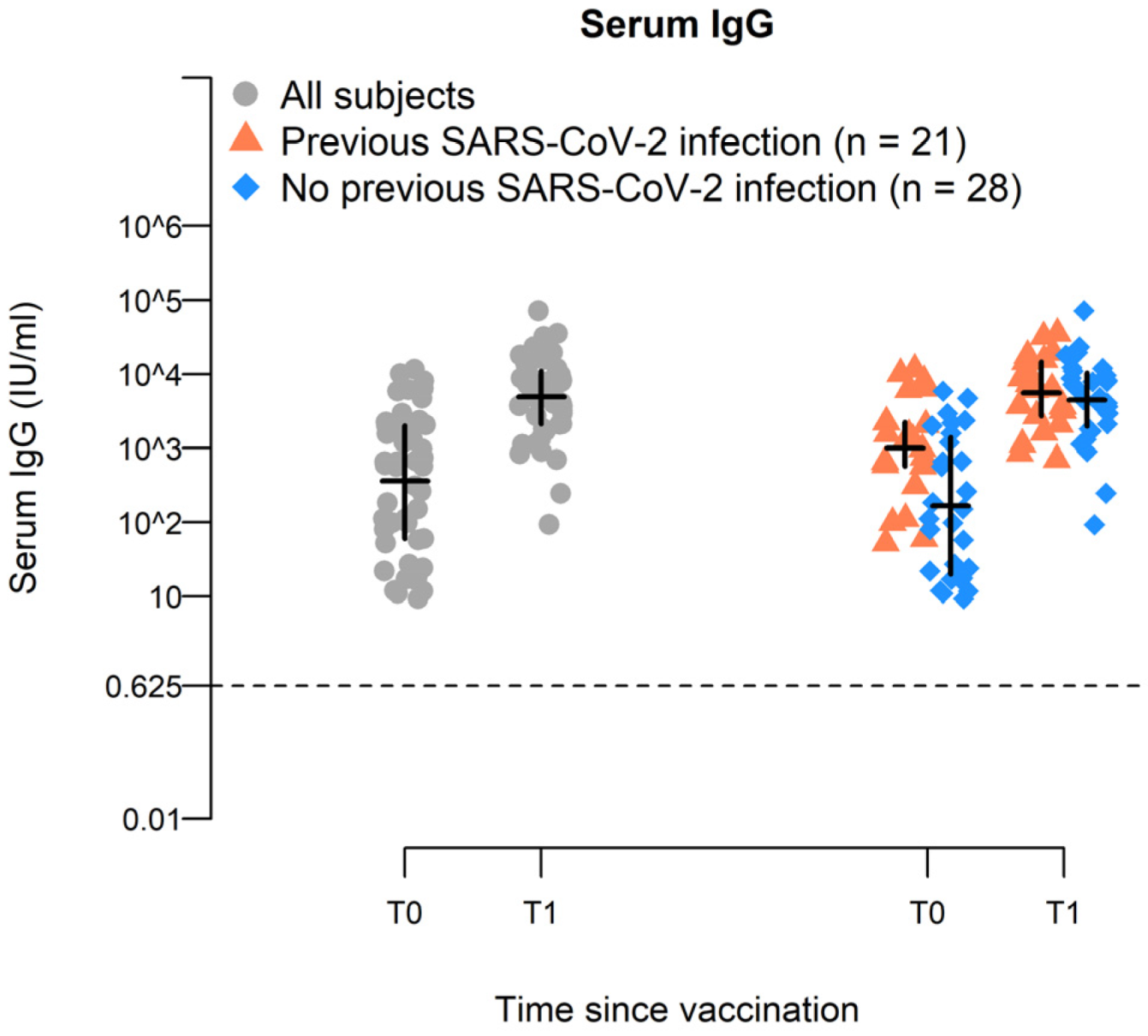
2.2. BNT162b2-Vaccine Booster Increases Spike-Specific IgG Antiboiy Titer in Elderly
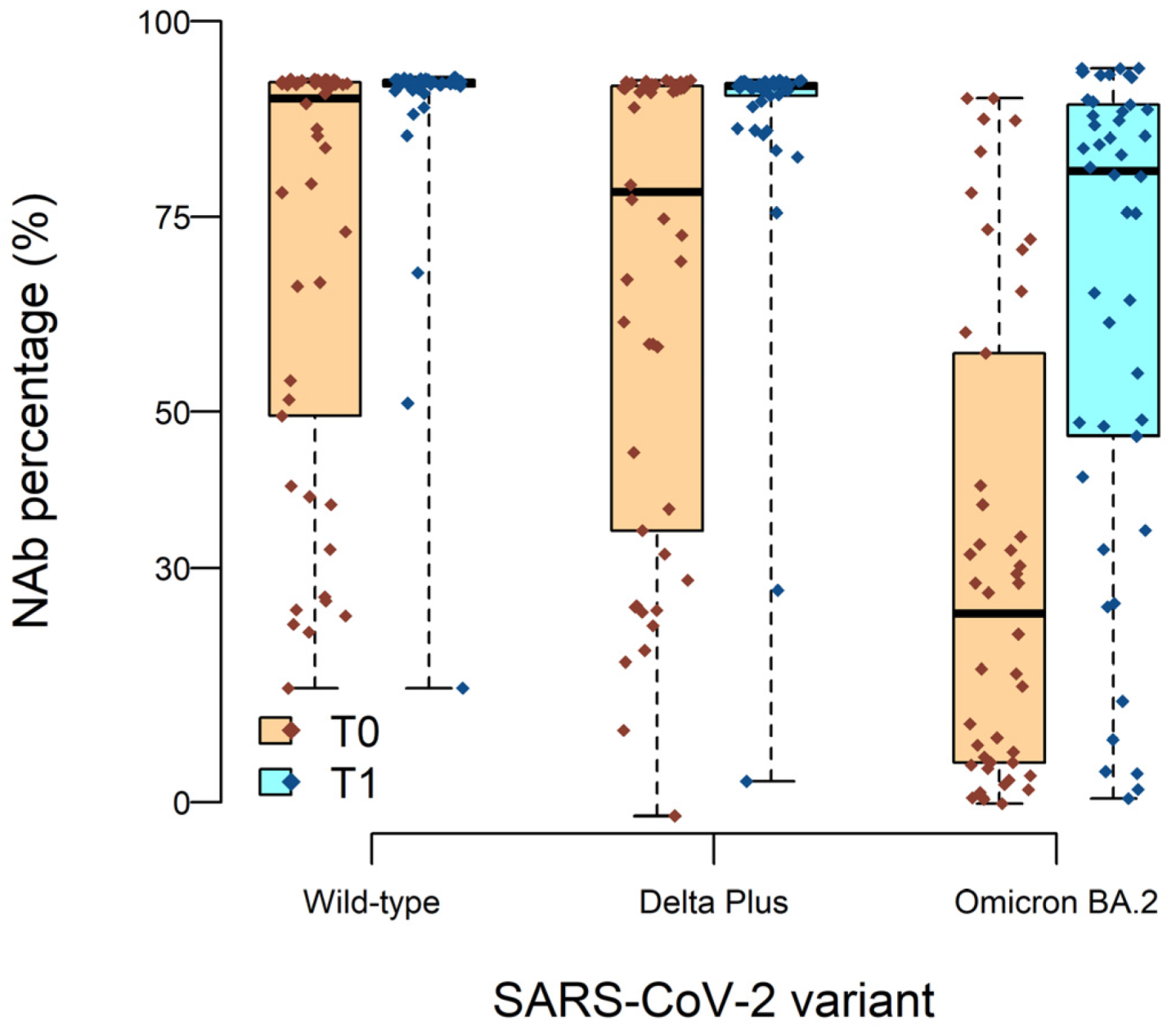
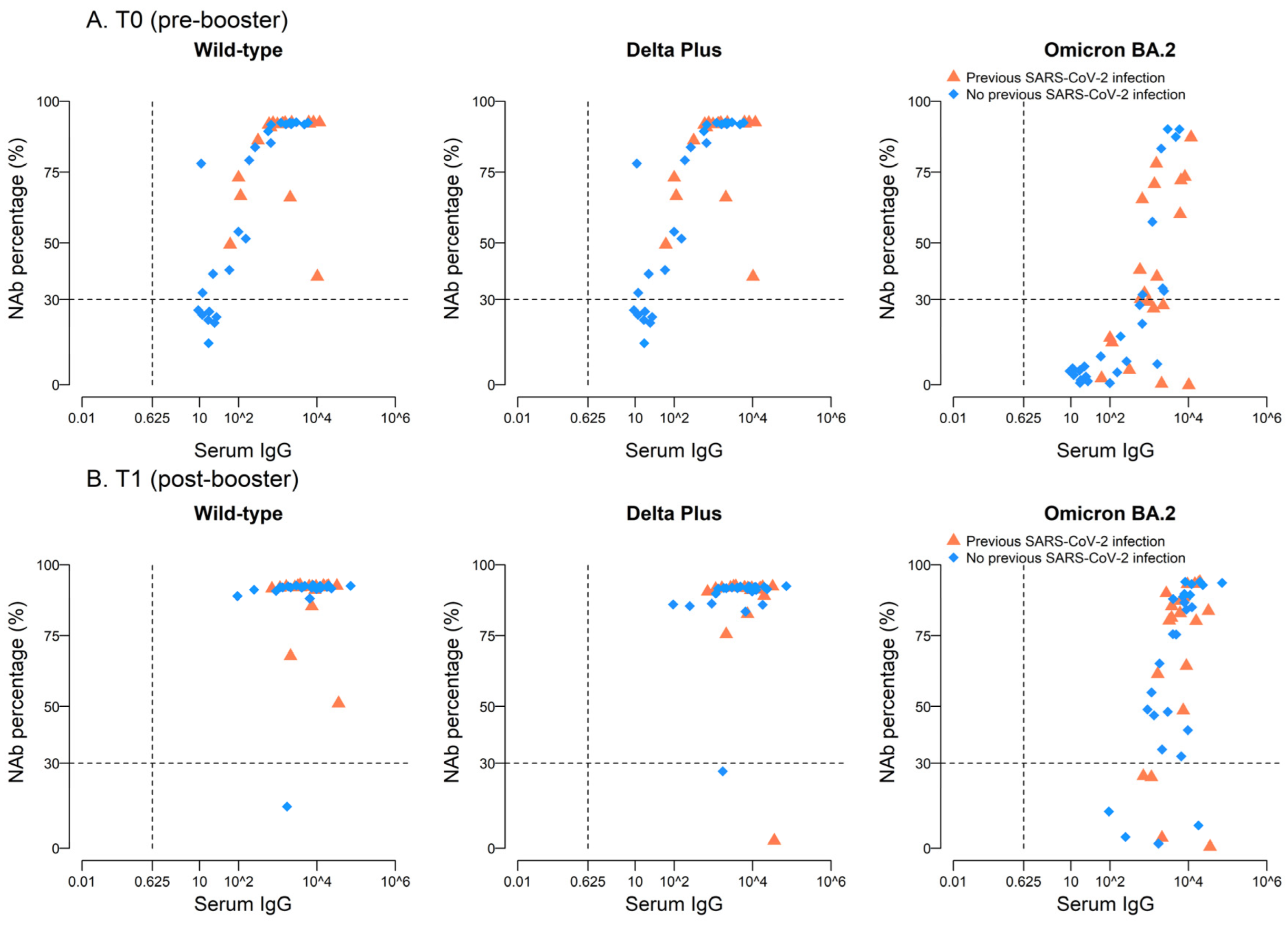
2.3. High Neutralizing Activity against Wild-Type and Delta Plus, but Not against Omicron BA.2, Variants after BNT162b2 Vaccine Boost
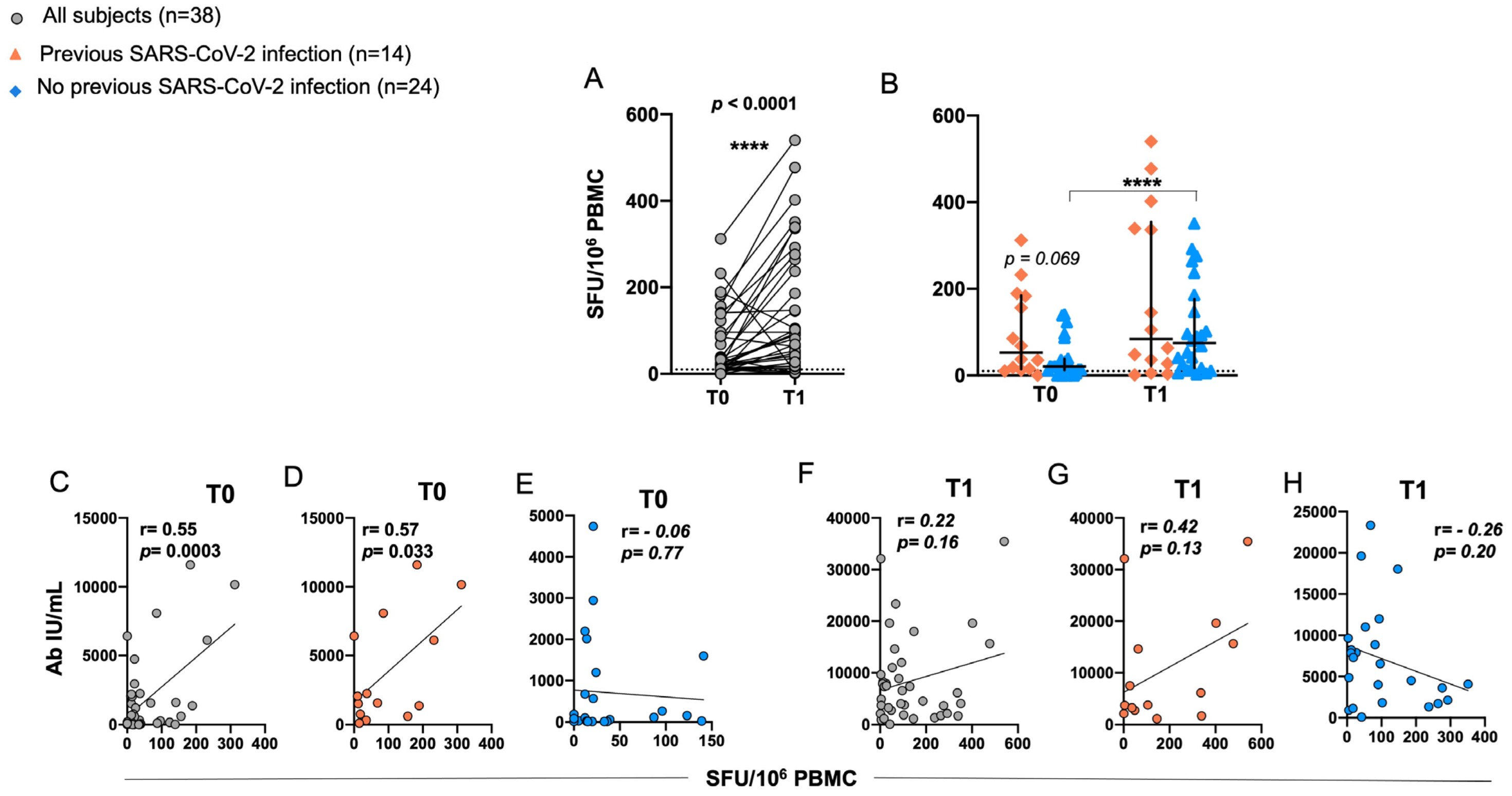
2.4. Induction of RBD-Specific T Cell Responses in Elderly after BNT162b2 Vaccination
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Antibody Measurement
4.3. Peptide Pools and Antigens
4.4. Ex Vivo Enzyme-Linked Immunospot Assay (ELISpot Assay)
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMichael, T.M.; Currie, D.W.; Clark, S.; Pogosjans, S.; Kay, M.; Schwartz, N.G.; Lewis, J.; Baer, A.; Kawakami, V.; Lukoff, M.D.; et al. Epidemiology of COVID-19 in a Long-Term Care Facility in King County, Washington. N. Engl. J. Med. 2020, 382, 2005–2011. [Google Scholar] [CrossRef]
- Crotty, F.; Watson, R.; Lim, W.K. Nursing Homes: The Titanic of Cruise Ships—Will Residential Aged Care Facilities Survive the COVID-19 Pandemic? Intern. Med. J. 2020, 50, 1033–1036. [Google Scholar] [CrossRef]
- Suetens, C.; Kinross, P.; Gallego Berciano, P.; Arroyo Nebreda, V.; Hassan, E.; Calba, C.; Fernandes, E.; Peralta-Santos, A.; Casaca, P.; Shodu, N.; et al. Increasing Risk of Breakthrough COVID-19 in Outbreaks with High Attack Rates in European Long-Term Care Facilities, July to October 2021. Eurosurveillance 2021, 26, 2101070. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Levy, M.E.; Natarajan, K.; Reese, S.E.; Naleway, A.L.; Grannis, S.J.; Klein, N.P.; DeSilva, M.B.; Ong, T.C.; Gaglani, M.; et al. Estimation of COVID-19 MRNA Vaccine Effectiveness and COVID-19 Illness and Severity by Vaccination Status during Omicron BA.4 and BA.5 Sublineage Periods. JAMA Netw. Open 2023, 6, e232598. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, COVID-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Vaccine Induces Neutralizing Antibodies and Poly-Specific T Cells in Humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and Safety of COVID-19 Vaccines in Older People. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca Vaccines on COVID-19 Related Symptoms, Hospital Admissions, and Mortality in Older Adults in England: Test Negative Case-Control Study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Muhsen, K.; Maimon, N.; Mizrahi, A.; Varticovschi, B.; Bodenheimer, O.; Gelbshtein, U.; Grotto, I.; Cohen, D.; Dagan, R. Effects of BNT162b2 COVID-19 Vaccine Booster in Long-Term Care Facilities in Israel. N. Engl. J. Med. 2022, 386, 399–401. [Google Scholar] [CrossRef]
- Muhsen, K.; Maimon, N.; Mizrahi, A.Y.; Varticovschi, B.; Bodenheimer, O.; Cohen, D.; Dagan, R. Association of BNT162b2 Vaccine Third Dose Receipt with Incidence of SARS-CoV-2 Infection, COVID-19—Related Hospitalization, and Death among Residents of Long-Term Care Facilities, August to October 2021. JAMA Netw. Open 2022, 5, e2219940. [Google Scholar] [CrossRef]
- Muhsen, K.; Maimon, N.; Mizrahi, A.Y.; Boltyansky, B.; Bodenheimer, O.; Diamant, Z.H.; Gaon, L.; Cohen, D.; Dagan, R. Association of Receipt of the Fourth BNT162b2 Dose with Omicron Infection and COVID-19 Hospitalizations among Residents of Long-Term Care Facilities. JAMA Intern. Med. 2022, 182, 859–867. [Google Scholar] [CrossRef]
- Canaday, D.H.; Oyebanji, O.A.; Keresztesy, D.; Payne, M.; Wilk, D.; Carias, L.; Aung, H.; Denis, K.S.; Lam, E.C.; Rowley, C.F.; et al. Significant Reduction in Vaccine-Induced Antibody Levels and Neutralization Activity among Healthcare Workers and Nursing Home Residents 6 Months Following Coronavirus Disease 2019 BNT162b2 MRNA Vaccination. Clin. Infect. Dis. 2022, 75, e884–e887. [Google Scholar] [CrossRef]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; Vynck, M.; De Vriese, A.S.; Reynders, M. Dynamics of the Cellular and Humoral Immune Response After BNT162b2 Messenger Ribonucleic Acid Coronavirus Disease 2019 (COVID-19) Vaccination in COVID-19-Naive Nursing Home Residents. J. Infect. Dis. 2021, 224, 1690–1693. [Google Scholar] [CrossRef]
- Canaday, D.H.; Oyebanji, O.A.; White, E.; Keresztesy, D.; Payne, M.; Wilk, D.; Carias, L.; Aung, H.; Denis, K.S.; Sheehan, M.L.; et al. COVID-19 Vaccine Booster Dose Needed to Achieve Omicron-Specific Neutralisation in Nursing Home Residents. eBioMedicine 2022, 80, 104066. [Google Scholar] [CrossRef]
- Canaday, D.H. SARS-CoV-2 Antibody Responses to the Ancestral SARS-CoV-2 Strain and Omicron BA.1 and BA.4/BA.5 Variants in Nursing Home Residents After Receipt of Bivalent COVID-19 Vaccine—Ohio and Rhode Island, September–November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 100–106. [Google Scholar] [CrossRef]
- Gianfagna, F.; Veronesi, G.; Baj, A.; Dalla Gasperina, D.; Siclari, S.; Drago Ferrante, F.; Maggi, F.; Iacoviello, L.; Ferrario, M.M. Anti-SARS-CoV-2 Antibody Levels and Kinetics of Vaccine Response: Potential Role for Unresolved Inflammation Following Recovery from SARS-CoV-2 Infection. Sci. Rep. 2022, 12, 385. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; et al. A Rapid Advice Guideline for the Diagnosis and Treatment of 2019 Novel Coronavirus (2019-NCoV) Infected Pneumonia (Standard Version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef]
- Biswas, A.; Mandal, R.S.; Chakraborty, S.; Maiti, G. Tapping the Immunological Imprints to Design Chimeric SARS-CoV-2 Vaccine for Elderly Population. Int. Rev. Immunol. 2022, 41, 448–463. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Li, Z.-N.; Chen, Z.-L.; Yue, S.-J.; Fu, R.-J.; Xu, D.-Q.; Zhang, S.; Tang, Y.-P. Are Older People Really More Susceptible to SARS-CoV-2? Aging Dis. 2022, 13, 1336–1347. [Google Scholar] [CrossRef]
- Wang, L.; Kainulainen, M.H.; Jiang, N.; Di, H.; Bonenfant, G.; Mills, L.; Currier, M.; Shrivastava-Ranjan, P.; Calderon, B.M.; Sheth, M.; et al. Differential Neutralization and Inhibition of SARS-CoV-2 Variants by Antibodies Elicited by COVID-19 MRNA Vaccines. Nat. Commun. 2022, 13, 4350. [Google Scholar] [CrossRef]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough Infections with SARS-CoV-2 Omicron despite MRNA Vaccine Booster Dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- Khandia, R.; Singhal, S.; Alqahtani, T.; Kamal, M.A.; El-Shall, N.A.; Nainu, F.; Desingu, P.A.; Dhama, K. Emergence of SARS-CoV-2 Omicron (B.1.1.529) Variant, Salient Features, High Global Health Concerns and Strategies to Counter It amid Ongoing COVID-19 Pandemic. Environ. Res. 2022, 209, 112816. [Google Scholar] [CrossRef] [PubMed]
- Abdool Karim, S.S.; de Oliveira, T. New SARS-CoV-2 Variants—Clinical, Public Health, and Vaccine Implications. N. Engl. J. Med. 2021, 384, 1866–1868. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody Responses to the BNT162b2 MRNA Vaccine in Individuals Previously Infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Demaret, J.; Corroyer-Simovic, B.; Alidjinou, E.K.; Goffard, A.; Trauet, J.; Miczek, S.; Vuotto, F.; Dendooven, A.; Huvent-Grelle, D.; Podvin, J.; et al. Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 MRNA Vaccine in Older People. Front. Immunol. 2021, 12, 778679. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Maurino, V.; Baj, A.; Gianfagna, F.; Cavallo, P.; Dentali, F.; Tettamanti, L.; et al. Mucosal Immune Response after the Booster Dose of the BNT162b2 COVID-19 Vaccine. eBioMedicine 2023, 88, 104435. [Google Scholar] [CrossRef]
- McDade, T.W.; Demonbreun, A.R.; Sancilio, A.; Mustanski, B.; D’Aquila, R.T.; McNally, E.M. Durability of Antibody Response to Vaccination and Surrogate Neutralization of Emerging Variants Based on SARS-CoV-2 Exposure History. Sci. Rep. 2021, 11, 17325. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Cheung, P.P.-H. Effectiveness of Heterologous and Homologous COVID-19 Vaccine Regimens: Living Systematic Review with Network Meta-Analysis. BMJ 2022, 377, e069989. [Google Scholar] [CrossRef]
- Maciola, A.K.; La Raja, M.; Pacenti, M.; Salata, C.; De Silvestro, G.; Rosato, A.; Pasqual, G. Neutralizing Antibody Responses to SARS-CoV-2 in Recovered COVID-19 Patients Are Variable and Correlate with Disease Severity and Receptor-Binding Domain Recognition. Front. Immunol. 2022, 13, 830710. [Google Scholar] [CrossRef] [PubMed]
- Muik, A.; Lui, B.G.; Wallisch, A.-K.; Bacher, M.; Mühl, J.; Reinholz, J.; Ozhelvaci, O.; Beckmann, N.; Güimil Garcia, R.d.l.C.; Poran, A.; et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 MRNA Vaccine—Elicited Human Sera. Science 2022, 375, 678–680. [Google Scholar] [CrossRef]
- Hurme, A.; Jalkanen, P.; Heroum, J.; Liedes, O.; Vara, S.; Melin, M.; Teräsjärvi, J.; He, Q.; Pöysti, S.; Hänninen, A.; et al. Long-Lasting T Cell Responses in BNT162b2 COVID-19 MRNA Vaccinees and COVID-19 Convalescent Patients. Front. Immunol. 2022, 13, 869990. [Google Scholar] [CrossRef]
- Mudd, P.A.; Minervina, A.A.; Pogorelyy, M.V.; Turner, J.S.; Kim, W.; Kalaidina, E.; Petersen, J.; Schmitz, A.J.; Lei, T.; Haile, A.; et al. SARS-CoV-2 MRNA Vaccination Elicits a Robust and Persistent T Follicular Helper Cell Response in Humans. Cell 2022, 185, 603–613.e15. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 Vaccination Induces Immunological T Cell Memory Able to Cross-Recognize Variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Brokstad, K.A.; Bansal, A.; Zhou, F.; Bredholt, G.; Onyango, T.B.; Sandnes, H.H.; Elyanow, R.; Madsen, A.; Trieu, M.-C.; et al. Durable Immune Responses after BNT162b2 Vaccination in Home-Dwelling Old Adults. Vaccine X 2023, 13, 100262. [Google Scholar] [CrossRef]
- Österdahl, M.F.; Christakou, E.; Hart, D.; Harris, F.; Shahrabi, Y.; Pollock, E.; Wadud, M.; Spector, T.D.; Brown, M.A.; Seow, J.; et al. Concordance of B- and T-cell Responses to SARS-CoV-2 Infection, Irrespective of Symptoms Suggestive of COVID-19. J. Med. Virol. 2022, 94, 5217–5224. [Google Scholar] [CrossRef]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal Immune Response in BNT162b2 COVID-19 Vaccine Recipients. eBioMedicine 2022, 75, 103788. [Google Scholar] [CrossRef] [PubMed]
| Variable | All Subjects (n = 49) |
|---|---|
| Age, years | 84.8 ± 10.6 |
| Sex, Female | 37 (75.5%) |
| Number of comorbidities | 1.3 ± 0.7 |
| Comorbidities -Dementia -Cardiovascular disease (CVD) -Diabetes -COPD -Autoimmune disease | 27 (55.1%) 20 (40.8%) 8 (16.3%) 7 (14.3%) 3 (6.1%) |
| Previous SARS-CoV-2 infection at T0 | 21 (42.9%) |
| Previous flu vaccination at T0 | 7 (14.3%) |
| Time | All Subjects (n = 49) | No Previous SARS-CoV-2 (n = 28) | Previous SARS-CoV-2 (n = 21) | p-Value 3 | p-Value 4 | |||
|---|---|---|---|---|---|---|---|---|
| Mean 1 | Δ(IC 95%) 2 | Mean 1 | Δ(IC 95%) 2 | Mean 1 | Δ(IC 95%) 2 | |||
| T0 | 361 | - | 166 | - | 1011 | - | 0.91 | <0.0001 |
| T1 | 4910 | 4549.2 (2778; 6320.3) | 4470 | 4304 (1877.4; 6730.6) | 5563 | 4552.8 (707; 8398.5) | 0.64 | |
| Wild-Type | Delta Plus | Omicron BA.2 | |
|---|---|---|---|
| All patients (n = 46), time | |||
| T0 | 0.79 | 0.72 | 0.72 |
| T1 | 0.06 | −0.05 | 0.53 |
| No previous SARS-CoV-2 (n = 26), time | |||
| T0 | 0.89 | 0.87 | 0.80 |
| T1 | 0.17 | 0.23 | 0.64 |
| Previous SARS-CoV-2 (n = 20), time | |||
| T0 | 0.24 | 0.16 | 0.52 |
| T1 | −0.21 | −0.34 | 0.33 |
| Variable | N | Serum IgG | Nab%, Omicron BA.2 | ||
|---|---|---|---|---|---|
| Geometric Mean (95% CI) | p-Value ^ | Mean (95% CI) | p-Value ^ | ||
| Age * | 49 | −0.01 (−0.35; 0.33) | 0.58 | 0.9 (−7.6; 9.4) | 0.84 |
| Time since T0 ** | 49 | 0.009 (−0.17; 0.18) | 0.91 | 4.3 (0.2; 8.3) | 0.04 |
| Sex | |||||
| men | 12 | 5465 (2682; 11,139) | 0.73 | 61.6 (44.4; 78.8) | 0.65 |
| women | 37 | 5092 (3380; 7670) | 63.9 (53.9; 73.9) | ||
| Diabetes | |||||
| Yes | 8 | 7231 (3016; 17,341) | 0.34 | 71.7 (50.4; 93.0) | 0.50 |
| No | 41 | 5739 (3566; 9236) | 67.7 (56.0; 79.3) | ||
| COPD | |||||
| Yes | 7 | 11,583 (4698; 28,553) | 0.04 | 57.3 (34.7; 79.9) | 0.50 |
| No | 42 | 7021 (4318; 11,416) | 61.9 (49.6; 74.1) | ||
| CVD | |||||
| Yes | 20 | 4548 (2555; 8097) | 0.71 | 64.9 (50.1; 79.7) | 0.98 |
| No | 29 | 4853 (3388; 6952) | 65.0 (55.9; 74.1) | ||
| Dementia | |||||
| Yes | 27 | 3332 (2128; 5218) | 0.01 | 63.1 (51.2; 75.1) | 0.65 |
| No | 22 | 5133 (3677; 7167) | 65.2 (56.4; 74.0) | ||
| Autoimmune Disease | |||||
| Yes | 3 | 13,210 (3235; 53,945) | 0.16 | 51.4 (16.9; 86.0) | 0.43 |
| No | 46 | 7799 (3777; 16,104) | 58.7 (40.9; 76.5) | ||
| Flu Vaccination between T0 and T1 | |||||
| Yes | 21 | 3504 (2075; 5919) | 0.10 | 60.6 (47.0; 74.2) | 0.41 |
| No | 28 | 4708 (3329; 6659) | 64.4 (55.5; 73.3) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Gasperina, D.; Veronesi, G.; Castelletti, C.M.; Varchetta, S.; Ottolini, S.; Mele, D.; Ferrari, G.; Shaik, A.K.B.; Celesti, F.; Dentali, F.; et al. Humoral and Cellular Immune Response Elicited by the BNT162b2 COVID-19 Vaccine Booster in Elderly. Int. J. Mol. Sci. 2023, 24, 13728. https://doi.org/10.3390/ijms241813728
Dalla Gasperina D, Veronesi G, Castelletti CM, Varchetta S, Ottolini S, Mele D, Ferrari G, Shaik AKB, Celesti F, Dentali F, et al. Humoral and Cellular Immune Response Elicited by the BNT162b2 COVID-19 Vaccine Booster in Elderly. International Journal of Molecular Sciences. 2023; 24(18):13728. https://doi.org/10.3390/ijms241813728
Chicago/Turabian StyleDalla Gasperina, Daniela, Giovanni Veronesi, Carlo M. Castelletti, Stefania Varchetta, Sabrina Ottolini, Dalila Mele, Giuseppe Ferrari, Amruth K. B. Shaik, Fabrizio Celesti, Francesco Dentali, and et al. 2023. "Humoral and Cellular Immune Response Elicited by the BNT162b2 COVID-19 Vaccine Booster in Elderly" International Journal of Molecular Sciences 24, no. 18: 13728. https://doi.org/10.3390/ijms241813728
APA StyleDalla Gasperina, D., Veronesi, G., Castelletti, C. M., Varchetta, S., Ottolini, S., Mele, D., Ferrari, G., Shaik, A. K. B., Celesti, F., Dentali, F., Accolla, R. S., & Forlani, G. (2023). Humoral and Cellular Immune Response Elicited by the BNT162b2 COVID-19 Vaccine Booster in Elderly. International Journal of Molecular Sciences, 24(18), 13728. https://doi.org/10.3390/ijms241813728






