The Hemostatic System in Newborns and the Risk of Neonatal Thrombosis
Abstract
:1. Introduction
2. Neonatal Hemostasis System Peculiarities and Neonatal Thrombosis
2.1. Epidemiology of Thrombosis in Neonates
2.2. Hemostasis System in the Neonate
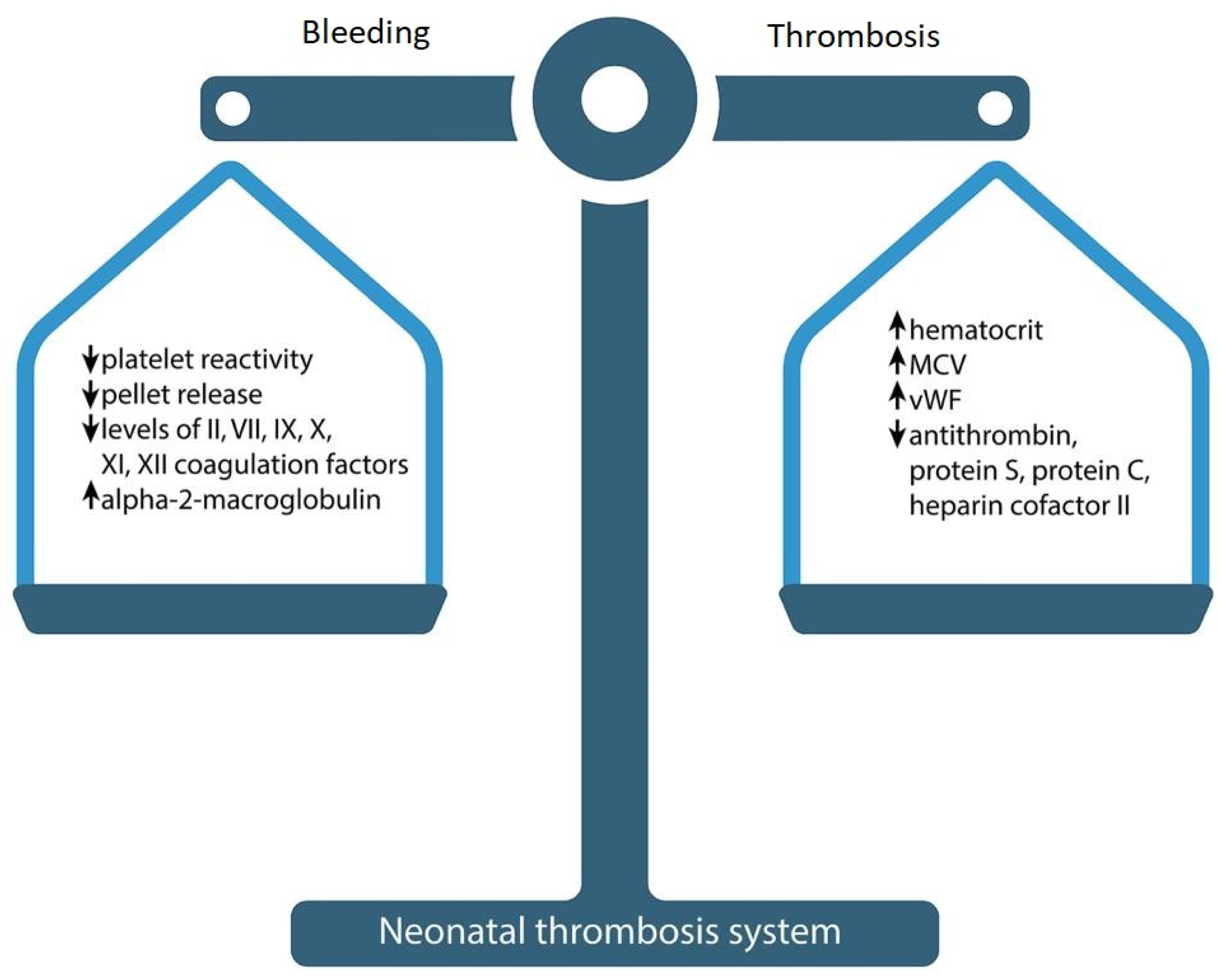
2.2.1. Neonatal Hemostatic Balance
2.2.2. Platelet Count and Function in the Neonate
- Platelet hyporeactivity to epinephrine is caused by a reduced number of alpha-2 (α2) adrenergic receptors on the cell surface.
- Decreased thrombin platelet response occurs due to the lack of protease-activated receptor 1 (PAR-1) and PAR-4 receptors on the neonatal platelets [33].
- Reduced signal transduction results in thromboxane hyporeactivity in the newborn.
- Reduced platelet activation after collagen inducing is associated with a lack of GPVI receptors, combined with defects in intracellular signaling pathways. Evidence may be seen in an insufficient phosphorylation of Syk and CLEC-2 in neonatal platelets [32].
2.3. Clinical Features of Neonatal Thrombosis
2.4. Risk Factors for Neonatal Thrombosis
- (1)
- Catheter-associated risk factors for neonatal thrombosis.
- (2)
- Neonatal thrombosis risk factors associated with the neonatal condition.
- (3)
- Neonatal thrombosis risk factors associated with maternal condition.
2.4.1. Catheter-Associated Risk Factors for Neonatal Thrombosis
2.4.2. Neonatal Thrombosis Risk Factors Associated with the Neonatal Condition
2.4.3. Neonatal Thrombosis Risk Factors Associated with Maternal Conditions
2.5. Neonatal Thrombosis Management
2.6. Prevention of Neonatal Thrombosis
2.7. COVID-19 and Neonatal Thrombosis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haley, K.M. Neonatal venous thromboembolism. Front. Pediatr. 2017, 5, 136. [Google Scholar] [CrossRef]
- Bhat, R.; Kumar, R.; Kwon, S.; Murthy, K.; Liem, R.I. Risk Factors for Neonatal Venous and Arterial Thromboembolism in the Neonatal Intensive Care Unit—A Case Control Study. J. Pediatr. 2018, 195, 28–32. [Google Scholar] [CrossRef]
- van Ommen, C.H.; Heijboer, H.; Büller, H.R.; Hirasing, R.A.; Heijmans, H.S.; Peters, M. Venous thromboembolism in childhood: A prospective two-year registry in The Netherlands. J. Pediatr. 2001, 139, 676–681. [Google Scholar] [CrossRef]
- Chalmers, E.A. Neonatal thrombosis. J. Clin. Pathol. 2000, 53, 419–423. [Google Scholar] [CrossRef]
- Robinson, V.; Achey, M.A.; Nag, U.P.; Reed, C.R.; Pahl, K.S.; Greenberg, R.G.; Clark, R.H.; Tracy, E.T. Thrombosis in infants in the neonatal intensive care unit: Analysis of a large national database. J. Thromb. Haemost. 2021, 19, 400–407. [Google Scholar] [CrossRef]
- Saracco, P.; Bagna, R.; Gentilomo, C.; Magarotto, M.; Viano, A.; Magnetti, F.; Giordano, P.; Luciani, M.; Molinari, A.C.; Suppiej, A.; et al. Clinical Data of Neonatal Systemic Thrombosis. J. Pediatr. 2016, 171, 60–66.e1. [Google Scholar] [CrossRef] [PubMed]
- Makatsariya, A.; Bitsadze, V.; Khizroeva, J.; Vorobev, A.; Makatsariya, N.; Egorova, E.; Mischenko, A.; Mashkova, T.; Antonova, A. Neonatal thrombosis. J. Matern. Fetal Neonatal Med. 2022, 35, 1169–1177. [Google Scholar] [CrossRef]
- Song, S.; Li, Z.; Zhao, G.; Li, X.; Wang, R.; Li, B.; Liu, Q. Epidemiology and risk factors for thrombosis in children and newborns: Systematic evaluation and meta-analysis. BMC Pediatr. 2023, 23, 292. [Google Scholar] [CrossRef]
- Andrew, M.; David, M.; Adams, M.; Ali, K.; Anderson, R.; Barnard, D.; Bernstein, M.; Brisson, L.; Cairney, B.; DeSai, D.; et al. Venous thromboembolic complications (VTE) in children: First analyses of the Canadian Registry of VTE. Blood 1994, 83, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Andrew, M. Neonatal thrombosis: Report of a prospective Canadian and international registry. Pediatrics 1995, 96 Pt 1, 939–943. [Google Scholar] [CrossRef]
- Nowak-Göttl, U.; von Kries, R.; Göbel, U. Neonatal symptomatic thromboembolism in Germany: Two-year survey. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 76, F163–F167. [Google Scholar] [CrossRef] [PubMed]
- Tuckuviene, R.; Christensen, A.L.; Helgestad, J.; Johnsen, S.P.; Kristensen, S.R. Pediatric venous and arterial noncerebral thromboembolism in Denmark: A nationwide population-based study. J. Pediatr. 2011, 159, 663–669. [Google Scholar] [CrossRef]
- Andrew, M.; Paes, B.; Milner, R.; Johnston, M.; Mitchell, L.; Tollefsen, D.M.; Powers, P. Development of the human coagulation system in the full-term infant. Blood 1987, 70, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Achey, M.A.; Nag, U.P.; Robinson, V.L.; Reed, C.R.; Arepally, G.M.; Levy, J.H.; Tracy, E.T. The Developing Balance of Thrombosis and Hemorrhage in Pediatric Surgery: Clinical Implications of Age-Related Changes in Hemostasis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620929092. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Latini, G.; Henry, E.; Christensen, R.D. Template bleeding times of 240 neonates born at 24 to 41 weeks gestation. J. Perinatol. 2008, 28, 427–431. [Google Scholar] [CrossRef]
- Andrew, M.; Paes, B.; Bowker, J.; Vegh, P. Evaluation of an automated bleeding time device in the newborn. Am. J. Hematol. 1990, 35, 275–277. [Google Scholar] [CrossRef]
- Boudewijns, M.; Raes, M.; Peeters, V.; Mewis, A.; Cartuyvels, R.; Magerman, K.; Rummens, J.L. Evaluation of platelet function on cord blood in 80 healthy term neonates using the Platelet Function Analyser (PFA-100); shorter in vitro bleeding times in neonates than adults. Eur. J. Pediatr. 2003, 162, 212–213. [Google Scholar] [CrossRef]
- Andrew, M.; Vegh, P.; Johnston, M.; Bowker, J.; Ofosu, F.; Mitchell, L. Maturation of the hemostatic system during childhood. Blood 1992, 80, 1998–2005. [Google Scholar] [CrossRef]
- Cvirn, G.; Gallistl, S.; Leschnik, B.; Muntean, W. Low tissue factor pathway inhibitor (TFPI) together with low antithrombin allows sufficient thrombin generation in neonates. J. Thromb. Haemost. 2003, 1, 263–268. [Google Scholar] [CrossRef]
- Cvirn, G.; Gallistl, S.; Rehak, T.; Jürgens, G.; Muntean, W. Elevated thrombin-forming capacity of tissue factor-activated cord compared with adult plasma. J. Thromb. Haemost. 2003, 1, 1785–1790. [Google Scholar] [CrossRef]
- Andrew, M.; Paes, B.; Milner, R.; Johnston, M.; Mitchell, L.; Tollefsen, D.M.; Castle, V.; Powers, P. Development of the Human Coagulation System in the Healthy Premature Infant. Blood 1988, 72, 1651–1657. [Google Scholar] [CrossRef]
- Neary, E.; McCallion, N.; Kevane, B.; Cotter, M.; Egan, K.; Regan, I.; Kirkham, C.; Mooney, C.; Coulter-Smith, S.; Ní Áinle, F. Coagulation indices in very preterm infants from cord blood and postnatal samples. J. Thromb. Haemost. 2015, 13, 2021–2030. [Google Scholar] [CrossRef]
- Nako, Y.; Ohki, Y.; Harigaya, A.; Tomomasa, T.; Morikawa, A. Plasma thrombomodulin level in very low birthweight infants at birth. Acta Paediatr. 1997, 86, 1105–1109. [Google Scholar] [CrossRef]
- Wiedmeier, S.E.; Henry, E.; Sola-Visner, M.C.; Christensen, R.D. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J. Perinatol. 2009, 29, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Sillers, L.; Van Slambrouck, C.; Lapping-Carr, G. Neonatal Thrombocytopenia: Etiology and Diagnosis. Pediatr. Ann. 2015, 44, e175–e180. [Google Scholar] [CrossRef]
- Bednarek, F.J.; Bean, S.; Barnard, M.R.; Frelinger, A.L.; Michelson, A.D. The platelet hyporeactivity of extremely low birth weight neonates is age-dependent. Thromb. Res. 2009, 124, 42–45. [Google Scholar] [CrossRef]
- Waller, A.K.; Lantos, L.; Sammut, A.; Salgin, B.; McKinney, H.; Foster, H.R.; Kriek, N.; Gibbins, J.M.; Stanworth, S.J.; Garner, S.F.; et al. Flow cytometry for near-patient testing in premature neonates reveals variation in platelet function: A novel approach to guide platelet transfusion. Pediatr. Res. 2019, 85, 874–884. [Google Scholar] [CrossRef]
- Sitaru, A.G.; Holzhauer, S.; Speer, C.P.; Singer, D.; Obergfell, A.; Walter, U.; Grossmann, R. Neonatal platelets from cord blood and peripheral blood. Platelets 2005, 16, 203–210. [Google Scholar] [CrossRef]
- Andres, O.; Schulze, H.; Speer, C.P. Platelets in neonates: Central mediators in haemostasis, antimicrobial defence and inflammation. Thromb. Haemost. 2015, 113, 3–12. [Google Scholar] [CrossRef]
- Davenport, P.; Sola-Visner, M. Platelets in the neonate: Not just a small adult. Res. Pract. Thromb. Haemost. 2022, 6, e12719. [Google Scholar] [CrossRef]
- Israels, S.J.; Cheang, T.; Roberston, C.; McMillan-Ward, E.M.; McNicol, A. Impaired Signal Transduction in Neonatal Platelets. Pediatr. Res. 1999, 45 (Suppl. S5), 687–691. [Google Scholar] [CrossRef]
- Hardy, A.T.; Palma-Barqueros, V.; Watson, S.K.; Malcor, J.D.; Eble, J.A.; Gardiner, E.E.; Blanco, J.E.; Guijarro-Campillo, R.; Delgado, J.L.; Lozano, M.L.; et al. Significant Hypo-Responsiveness to GPVI and CLEC-2 Agonists in Pre-Term and Full-Term Neonatal Platelets and following Immune Thrombocytopenia. Thromb. Haemost. 2018, 118, 1009–1020. [Google Scholar] [CrossRef]
- Schlagenhauf, A.; Schweintzger, S.; Birner-Grünberger, R.; Leschnik, B.; Muntean, W. Comparative evaluation of PAR1, GPIb-IX-V, and integrin αIIbβ3 levels in cord and adult platelets. Hamostaseologie 2010, 30 (Suppl. S1), S164–S167. [Google Scholar] [CrossRef]
- Palma-Barqueros, V.; Torregrosa, J.M.; Caparrós-Pérez, E.; Mota-Pérez, N.; Bohdan, N.; Llanos, M.D.C.; Begonja, A.J.; Sola-Visner, M.; Vicente, V.; Teruel-Montoya, R.; et al. Developmental Differences in Platelet Inhibition Response to Prostaglandin E1. Neonatology 2020, 117, 15–23. [Google Scholar] [CrossRef]
- Caparrós-Pérez, E.; Teruel-Montoya, R.; Palma-Barquero, V.; Torregrosa, J.M.; Blanco, J.E.; Delgado, J.L.; Lozano, M.L.; Vicente, V.; Sola-Visner, M.; Rivera, J.; et al. Down Regulation of the Munc18b-syntaxin-11 Complex and β1-tubulin Impairs Secretion and Spreading in Neonatal Platelets. Thromb. Haemost. 2017, 117, 2079–2091. [Google Scholar] [CrossRef]
- Pelizza, M.F.; Martinato, M.; Rosati, A.; Nosadini, M.; Saracco, P.; Giordano, P.; Luciani, M.; Ilardi, L.; Lasagni, D.; Molinari, A.C.; et al. The new Italian registry of infantile thrombosis (RITI): A reflection on its journey, challenges and pitfalls. Front. Pediatr. 2023, 11, 1094246. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Ferriero, D.M.; Cowan, F.M. Perinatal arterial ischemic stroke. Handb. Clin. Neurol. 2019, 162, 239–266. [Google Scholar] [CrossRef]
- Lynch, J.K.; Hirtz, D.G.; DeVeber, G.; Nelson, K.B. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics 2002, 109, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.W.; Inder, T.E. Perinatal and neonatal ischaemic stroke: A review. Thromb. Res. 2006, 118, 39–48. [Google Scholar] [CrossRef]
- Gacio, S.; Muñoz Giacomelli, F.; Klein, F. Presumed perinatal ischemic stroke: A review. Arch. Argent Pediatr. 2015, 113, 449–455, English, Spanish. [Google Scholar]
- Elbers, J.; Viero, S.; MacGregor, D.; deVeber, G.; Moore, A.M. Placental Pathology in Neonatal Stroke. Pediatrics 2011, 127, e722–e729. [Google Scholar] [CrossRef] [PubMed]
- Günther, G.; Junker, R.; Sträter, R.; Schobess, R.; Kurnik, K.; Heller, C.; Kosch, A.; Nowak-Göttl, U.; Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: Role of acquired and genetic prothrombotic risk factors. Stroke 2000, 31, 2437–2441, Erratum in Stroke 2001, 32, 279. [Google Scholar] [CrossRef]
- Dlamini, N.; Billinghurst, L.; Kirkham, F.J. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg. Clin. N. Am. 2010, 21, 511–527. [Google Scholar] [CrossRef]
- deVeber, G.; Andrew, M.; Adams, C.; Bjornson, B.; Booth, F.; Buckley, D.J.; Camfield, C.S.; David, M.; Humphreys, P.; Langevin, P.; et al. Cerebral sinovenous thrombosis in children. N. Engl. J. Med. 2001, 345, 417–423. [Google Scholar] [CrossRef]
- Wasay, M.; Dai, A.I.; Ansari, M.; Shaikh, Z.; Roach, E.S. Cerebral venous sinus thrombosis in children: A multicenter cohort from the United States. J. Child Neurol. 2008, 23, 26–31. [Google Scholar] [CrossRef]
- Manco-Johnson, M.J. How I treat venous thrombosis in children. Blood 2006, 107, 21–29. [Google Scholar] [CrossRef]
- Moharir, M.D.; Shroff, M.; Pontigon, A.M.; Askalan, R.; Yau, I.; Macgregor, D.; Deveber, G.A. A prospective outcome study of neonatal cerebral sinovenous thrombosis. J. Child Neurol. 2011, 26, 1137–1144. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, H.; Xing, Y. Clinical Characteristics of Venous Thrombosis Associated with Peripherally Inserted Central Venous Catheter in Premature Infants. Children 2022, 9, 1126. [Google Scholar] [CrossRef]
- Ulloa-Ricardez, A.; Romero-Espinoza, L.; Estrada-Loza Mde, J.; González-Cabello, H.J.; Núñez-Enríquez, J.C. Risk Factors for Intracardiac Thrombosis in the Right Atrium and Superior Vena Cava in Critically Ill Neonates who Required the Installation of a Central Venous Catheter. Pediatr. Neonatol. 2016, 57, 288–294. [Google Scholar] [CrossRef]
- Cholette, J.M.; Rubenstein, J.S.; Alfieris, G.M.; McDermott, M.P.; Harmon, W.G.; Vermilion, R.; Eaton, M.P.; Gangemi, J.J.; Lerner, N.B. Elevated risk of thrombosis in neonates undergoing initial palliative cardiac surgery. Ann. Thorac. Surg. 2007, 84, 1320–1325. [Google Scholar] [CrossRef]
- Fenton, K.N.; Siewers, R.D.; Rebovich, B.; Pigula, F.A. Interim mortality in infants with systemic-to-pulmonary artery shunts. Ann. Thorac. Surg. 2003, 76, 152–156, discussion 156–157. [Google Scholar] [CrossRef]
- Messinger, Y.; Sheaffer, J.W.; Mrozek, J.; Smith, C.M.; Sinaiko, A.R. Renal outcome of neonatal renal venous thrombosis: Review of 28 patients and effectiveness of fibrinolytics and heparin in 10 patients. Pediatrics 2006, 118, e1478–e1484. [Google Scholar] [CrossRef]
- Moon, C.J.; Kwon, T.H.; Lee, H.S. Portal vein thrombosis and food protein-induced allergic proctocolitis in a premature newborn with hypereosinophilia: A case report. BMC Pediatr. 2021, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Tsonis, O.; Gouvias, T.; Gkrozou, F.; Antonopoulou, I.; Giantsouli, A.; Paschopoulos, M.; Baltogianni, M. Neonatal femoral artery thrombosis at the time of birth: A case report. J. Pediatr. Neonatal Individ. Med. (JPNIM) 2020, 9, e090214. [Google Scholar]
- Mahasandana, C.; Suvatte, V.; Marlar, R.A.; Manco-Johnson, M.J.; Jacobson, L.J.; Hathaway, W.E. Neonatal purpura fulminans associated with homozygous protein S deficiency. Lancet 1990, 335, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Hattenbach, L.O.; Beeg, T.; Kreuz, W.; Zubcov, A. Ophthalmic manifestation of congenital protein C deficiency. J. AAPOS 1999, 3, 188–190. [Google Scholar] [CrossRef]
- Chalmers, E.; Cooper, P.; Forman, K.; Grimley, C.; Khair, K.; Minford, A.; Morgan, M.; Mumford, A.D. Purpura fulminans: Recognition, diagnosis and management. Arch. Dis. Child. 2011, 96, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Marlar, R.A.; Montgomery, R.R.; Broekmans, A.W. Diagnosis and treatment of homozygous protein C deficiency. Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S, International Committee on Thrombosis and Haemostasis. J. Pediatr. 1989, 114 Pt 1, 528–534. [Google Scholar] [CrossRef]
- van Ommen, C.H.; Sol, J.J. Developmental Hemostasis and Management of Central Venous Catheter Thrombosis in Neonates. Semin. Thromb. Hemost. 2016, 42, 752–759. [Google Scholar] [CrossRef]
- Thornburg, C.D.; Smith, P.B.; Smithwick, M.L.; Cotten, C.M.; Benjamin, D.K., Jr. Association between thrombosis and bloodstream infection in neonates with peripherally inserted catheters. Thromb. Res. 2008, 122, 782–785. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Chan, A.K. Venous thrombosis in neonates. Fac. Rev. 2021, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Dubbink-Verheij, G.H.; Pelsma, I.C.M.; van Ommen, C.H.; Smits-Wintjens, V.E.H.J.; Visser, R.; Steggerda, S.J.; Te Pas, A.B.; Lopriore, E. Femoral Vein Catheter is an Important Risk Factor for Catheter-related Thrombosis in (Near-)term Neonates. J. Pediatr. Hematol. Oncol. 2018, 40, e64–e68. [Google Scholar] [CrossRef]
- Amankwah, E.K.; Atchison, C.M.; Arlikar, S.; Ayala, I.; Barrett, L.; Branchford, B.R.; Streiff, M.; Takemoto, C.; Goldenberg, N.A. Risk factors for hospital-sssociated venous thromboembolism in the neonatal intensive care unit. Thromb. Res. 2014, 134, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Tuckuviene, R.; Christensen, A.L.; Helgested, J.; Hundborg, H.H.; Kristensen, S.R.; Johnsen, S.P. Infant, obstetrical and maternal characteristics associated with thromboembolism in infancy: A nationwide population-based case-control study. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F417–F422. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Kwon, S.; Zaniletti, I.; Murthy, K.; Liem, R.I. Risk factors associated with venous and arterial neonatal thrombosis in the intensive care unit: A multicentre case-control study. Lancet Haematol. 2022, 9, e200–e207. [Google Scholar] [CrossRef] [PubMed]
- Vorobev, A.; Bitsadze, V.; Khizroeva, J.; Potapkina, S.; Makatsariya, N.; Rizzo, G.; Di Renzo, G.C.; Blinov, D.V.; Pankratyeva, L.L.; Tsibizova, V.I. Neonatal thrombosis: Risk factors and principles of prophylaxis. Obstet. Gynecol. Reprod. 2021, 15, 390–403. [Google Scholar] [CrossRef]
- Walker, S.C.; Creech, C.B.; Domenico, H.J.; French, B.; Byrne, D.W.; Wheeler, A.P. A Real-time Risk-Prediction Model for Pediatric Venous Thromboembolic Events. Pediatrics 2021, 147, e2020042325. [Google Scholar] [CrossRef]
- Ovesen, P.G.; Jensen, D.M.; Damm, P.; Rasmussen, S.; Kesmodel, U.S. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. a nation-wide study. J. Matern. Fetal Neonatal Med. 2015, 28, 1720–1724. [Google Scholar] [CrossRef]
- Monagle, P.; Chan, A.K.C.; Goldenberg, N.A.; Ichord, R.N.; Journeycake, J.M.; Nowak-Göttl, U.; Vesely, S.K. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e737S–e801S. [Google Scholar] [CrossRef]
- Ting, J.; Yeung, K.; Paes, B.; Chan, A.K.C.; Petropoulos, J.A.; Banfield, L.; Bhatt, M.D.; Thrombosis and Hemostasis in Newborns (THiN) Group. How to use low-molecular-weight heparin to treat neonatal thrombosis in clinical practice. Blood Coagul. Fibrinolysis 2021, 32, 531–538. [Google Scholar] [CrossRef]
- Kenet, G.; Cohen, O.; Bajorat, T.; Nowak-Göttl, U. Insights into neonatal thrombosis. Thromb. Res. 2019, 181 (Suppl. S1), S33–S36. [Google Scholar] [CrossRef]
- Monagle, P.; Newall, F. Management of thrombosis in children and neonates: Practical use of anticoagulants in children. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 399–404. [Google Scholar] [CrossRef]
- Male, C.; Thom, K.; O’Brien, S.H. Direct oral anticoagulants: What will be their role in children? Thromb. Res. 2019, 173, 178–185. [Google Scholar] [CrossRef]
- Pągowska-Klimek, I. Perioperative thromboembolism prophylaxis in children—Is it necessary? Anaesthesiol. Intensive Ther. 2020, 52, 316–322. [Google Scholar] [CrossRef]
- Fletcher-Sandersjöö, A.; Bellander, B.M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb. Res. 2020, 194, 36–41. [Google Scholar] [CrossRef]
- Bitsadze, V.O.; Grigoreva, K.; Khizroeva, J.K.; Pervunina, T.M.; Tsibizova, V.I.; Tretyakova, M.V.; Makatsariya, A.D. Novel coronavirus infection and Kawasaki disease. J. Matern. Fetal Neonatal Med. 2022, 35, 3044–3048. [Google Scholar] [CrossRef]
- Barrero-Castillero, A.; Beam, K.S.; Bernardini, L.B.; Ramos, E.G.C.; Davenport, P.E.; Duncan, A.R.; Fraiman, Y.S.; Frazer, L.C.; Healy, H.; Herzberg, E.M. COVID-19: Neonatal-perinatal perspectives. J. Perinatol. 2021, 41, 940–951. [Google Scholar] [CrossRef]
- Leeman, R.; Shoag, J.; Borchetta, M.; Mitchell, C.; Davis, J.A.; Corrales-Medina, F.F. Clinical Implications of Hematologic and Hemostatic Abnormalities in Children with COVID-19. J. Pediatr. Hematol. Oncol. 2022, 44, e282–e286. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Campi, F.; Longo, D.; Bersani, I.; Savarese, I.; Lucignani, G.; Haass, C.; Paolino, M.C.; Vadalà, S.; De Liso, P.; Di Capua, M.; et al. Neonatal Cerebral Venous Thrombosis Following Maternal SARS-CoV-2 Infection in Pregnancy. Neonatology 2022, 119, 268–272. [Google Scholar] [CrossRef]
- Baergen, R.N.; Heller, D.S. Placental Pathology in COVID-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.J.; Barton, J.R.; Bentum, N.A.; Blackwell, S.C.; Sibai, B.M. General Guidelines in the Management of an Obstetrical Patient on the Labor and Delivery Unit during the COVID-19 Pandemic. Am. J. Perinatol. 2020, 37, 829–836. [Google Scholar] [CrossRef] [PubMed]
| Hemostasis System Parameter | At Birth | 6 Months |
|---|---|---|
| Antithrombin | 40–60% | adult level at day 90 |
| Protein S | 40–60% | adult level at day 90 |
| Protein C | low | adult level not reached |
| VWF | mean 153% | drops to ≈ 100% |
| Fibrinogen | adult level | adult level |
| Vitamin K-dependent factors (II, VII, IX, X) | mean ≈ 40–50% | 80–90% of adult level |
| Contact system factors (factors XI, XII, prekallikrein, HMWK) | mean ≈ 40–50% | 80–90% of adult level |
| Factor V and XIII (both a- and b-unit) | mean 70–80% | adult levels at day 5 |
| FVIII | mean 100% | drops slowly to ≈ 75% |
| Factor V and XIII | mean 70–80% | adult levels at day 5 |
| a2-macroglobulin | high | increasing further |
| Catheter-Associated Risk Factors for Neonatal Thrombosis | Risk Factors Associated with the Neonatal Condition | Risk Factors Associated with Maternal Condition |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khizroeva, J.; Makatsariya, A.; Vorobev, A.; Bitsadze, V.; Elalamy, I.; Lazarchuk, A.; Salnikova, P.; Einullaeva, S.; Solopova, A.; Tretykova, M.; et al. The Hemostatic System in Newborns and the Risk of Neonatal Thrombosis. Int. J. Mol. Sci. 2023, 24, 13864. https://doi.org/10.3390/ijms241813864
Khizroeva J, Makatsariya A, Vorobev A, Bitsadze V, Elalamy I, Lazarchuk A, Salnikova P, Einullaeva S, Solopova A, Tretykova M, et al. The Hemostatic System in Newborns and the Risk of Neonatal Thrombosis. International Journal of Molecular Sciences. 2023; 24(18):13864. https://doi.org/10.3390/ijms241813864
Chicago/Turabian StyleKhizroeva, Jamilya, Alexander Makatsariya, Alexander Vorobev, Victoria Bitsadze, Ismail Elalamy, Arina Lazarchuk, Polina Salnikova, Sabina Einullaeva, Antonina Solopova, Maria Tretykova, and et al. 2023. "The Hemostatic System in Newborns and the Risk of Neonatal Thrombosis" International Journal of Molecular Sciences 24, no. 18: 13864. https://doi.org/10.3390/ijms241813864
APA StyleKhizroeva, J., Makatsariya, A., Vorobev, A., Bitsadze, V., Elalamy, I., Lazarchuk, A., Salnikova, P., Einullaeva, S., Solopova, A., Tretykova, M., Antonova, A., Mashkova, T., Grigoreva, K., Kvaratskheliia, M., Yakubova, F., Degtyareva, N., Tsibizova, V., Gashimova, N., & Blbulyan, D. (2023). The Hemostatic System in Newborns and the Risk of Neonatal Thrombosis. International Journal of Molecular Sciences, 24(18), 13864. https://doi.org/10.3390/ijms241813864







