First-Generation Synthetic Cathinones Produce Arrhythmia in Zebrafish Eleutheroembryos: A New Approach Methodology for New Psychoactive Substances Cardiotoxicity Evaluation
Abstract
:1. Introduction
2. Results
2.1. Twenty-Four-Hour Acute Toxicity Effects on Zebrafish Embryos
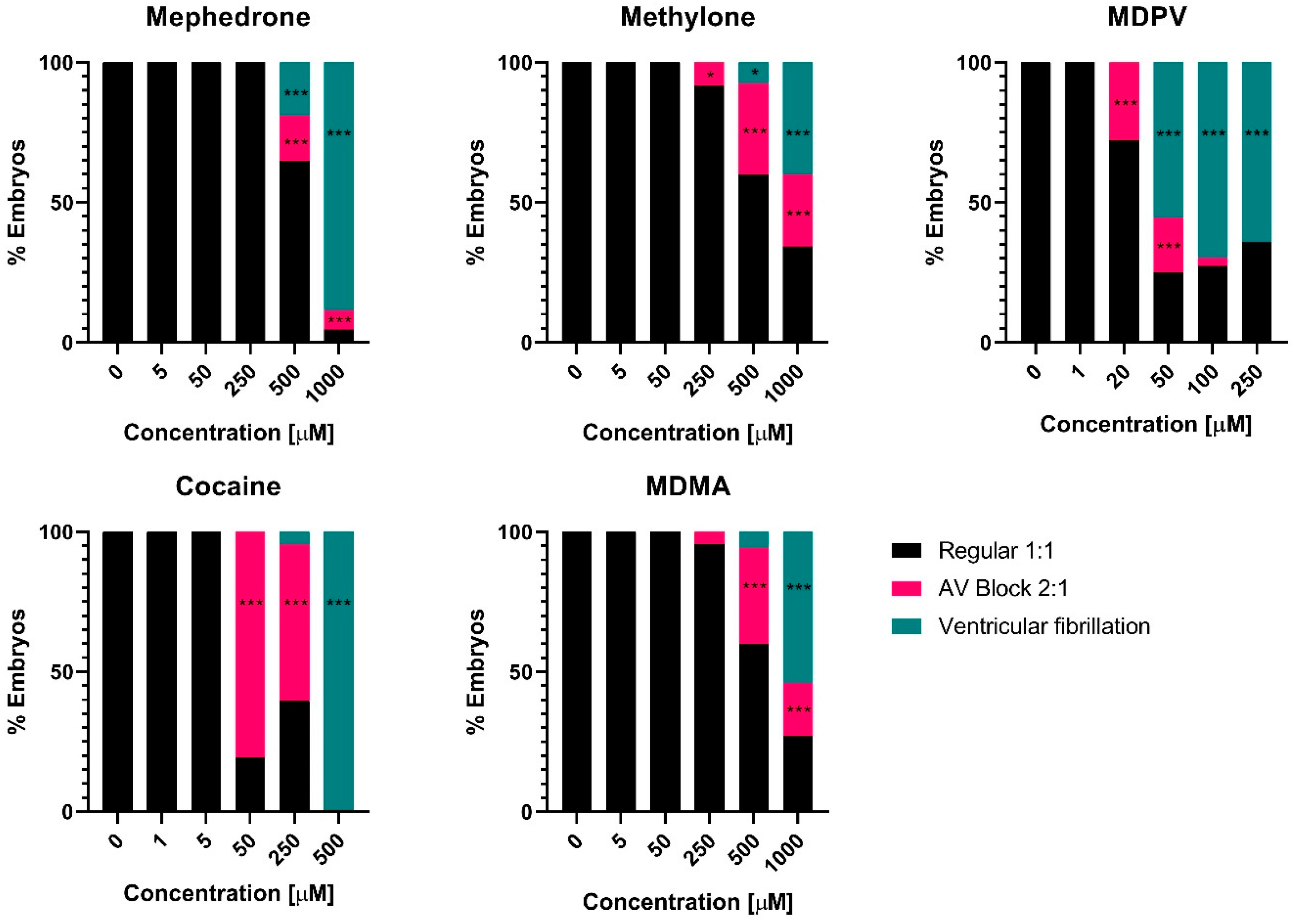
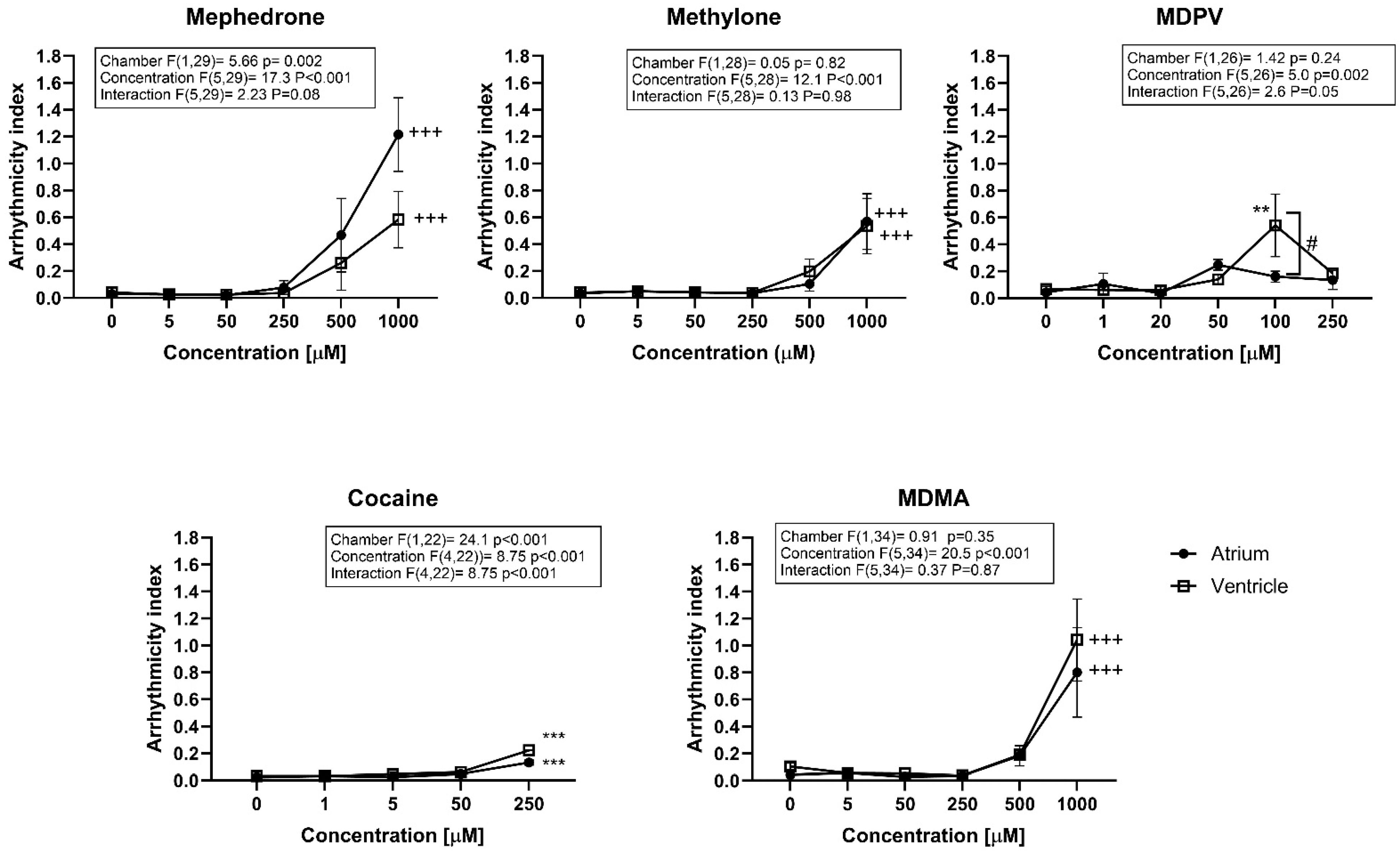
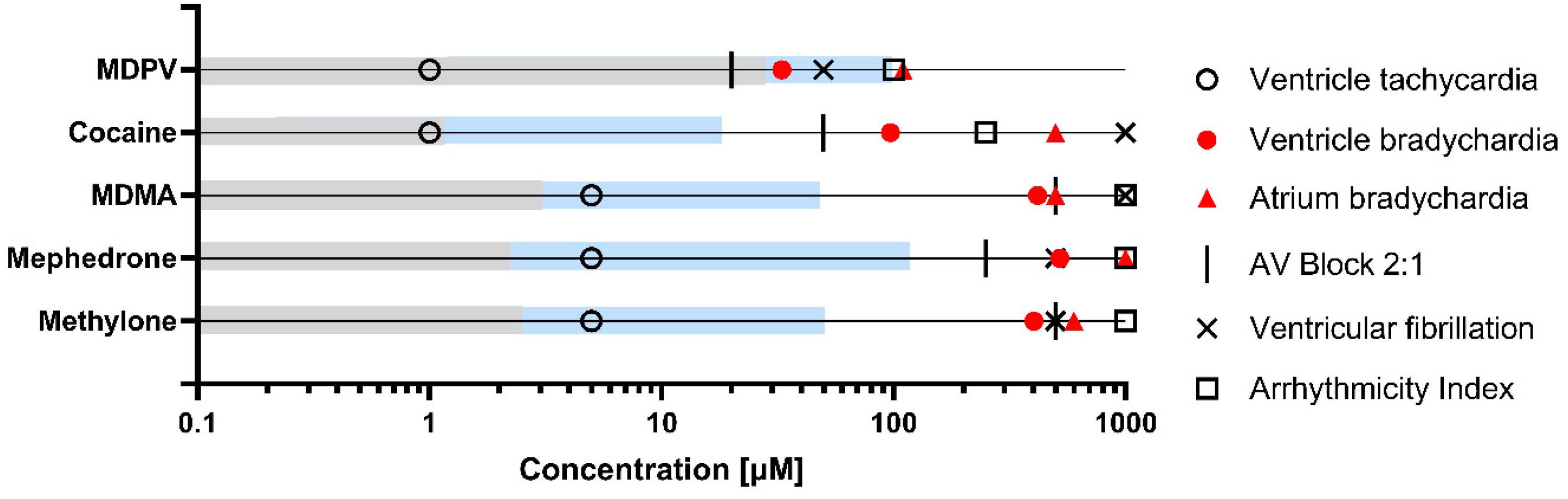
2.2. Drug-Induced Effects on Heart Rate after 2 h Exposure
3. Discussion
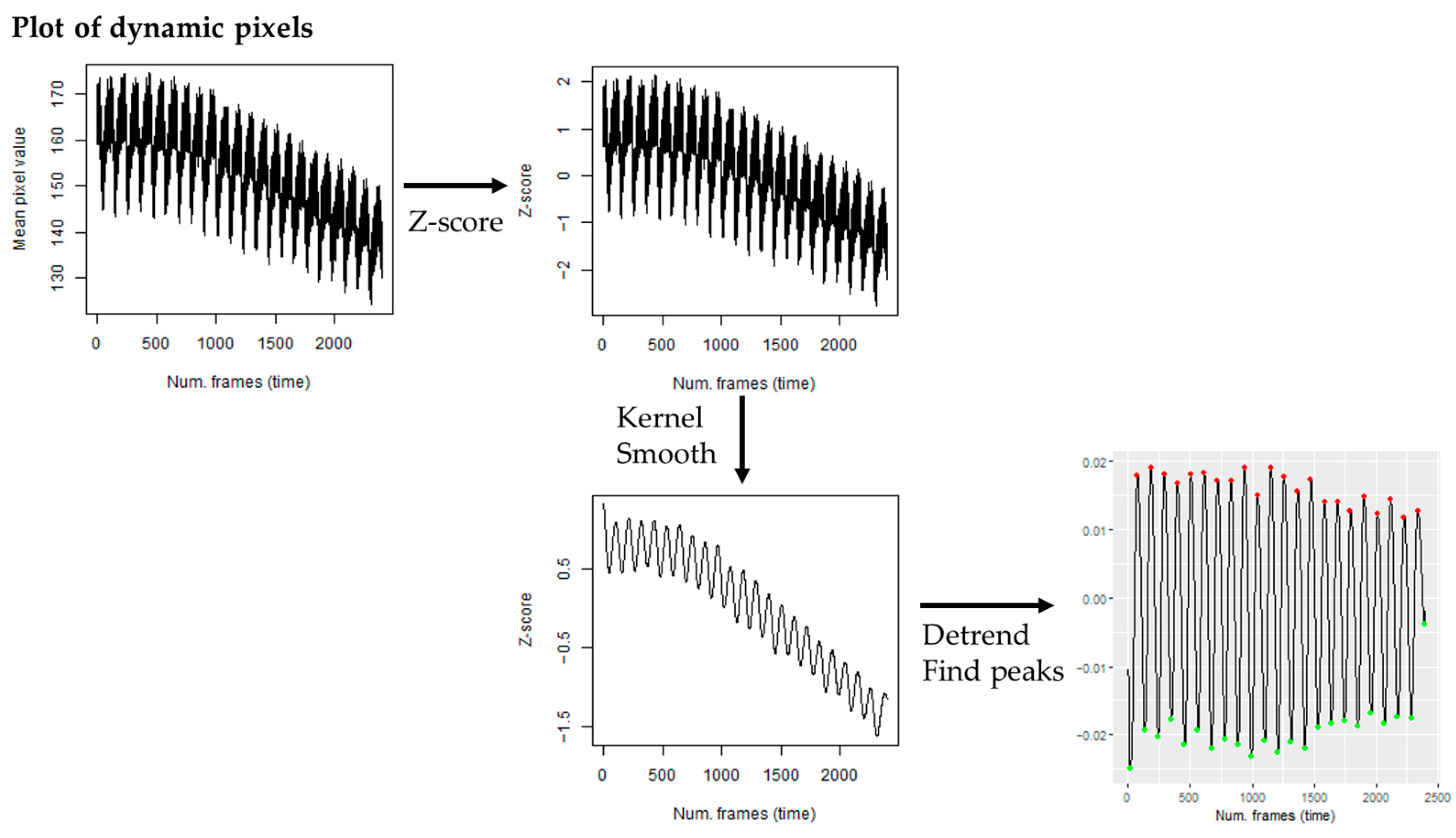
4. Materials and Methods
4.1. Chemicals and Test Solutions Preparation
4.2. Zebrafish Maintenance and Egg Production
4.3. Exposure to Drugs
4.4. Preparation of Embryos and Video Recording
4.5. Twenty-Four-Hour Acute Toxicity Test
4.6. Heart Rate Analysis
4.7. Cardiovascular Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumann, M.H.; Ayestas, M.A.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The Designer Methcathinone Analogs, Mephedrone and Methylone, Are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; Kolanos, R.; Verkariya, R.; De Felice, L.; Glennon, R.A. Mephedrone and Methylenedioxypyrovalerone (MDPV), Major Constituents of “Bath Salts”, Produce Opposite Effects at the Human Dopamine Transporter. Psychopharmacology 2013, 227, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Wolfrum, K.M.; Hatfield, M.G.; Johnson, R.A.; Murphy, K.V.; Janowsky, A. Substituted Methcathinones Differ in Transporter and Receptor Interactions. Biochem. Pharmacol. 2013, 85, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Nagarajan, S.; Wolfrum, K.M.; Reed, J.F.; Swanson, T.L.; Nilsen, A.; Janowsky, A. Structure-Activity Relationships of Bath Salt Components: Substituted Cathinones and Benzofurans at Biogenic Amine Transporters. Psychopharmacology 2019, 236, 939–952. [Google Scholar] [CrossRef]
- Lõpez-Arnau, R.; Martínez-Clemente, J.; Pubill, D.; Escubedo, E.; Camarasa, J. Comparative Neuropharmacology of Three Psychostimulant Cathinone Derivatives: Butylone, Mephedrone and Methylone. Br. J. Pharmacol. 2012, 167, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological Characterization of Designer Cathinones in Vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Kolanos, R.; Partilla, J.S.; Baumann, M.H.; Hutsell, B.A.; Banks, M.L.; Negus, S.S.; Glennon, R.A. Stereoselective Actions of Methylenedioxypyrovalerone (MDPV) To Inhibit Dopamine and Norepinephrine Transporters and Facilitate Intracranial Self-Stimulation in Rats. ACS Chem. Neurosci. 2015, 6, 771–777. [Google Scholar] [CrossRef]
- Kolanos, R.; Solis, E.; Sakloth, F.; De Felice, L.J.; Glennon, R.A. “Deconstruction” of the Abused Synthetic Cathinone Methylenedioxypyrovalerone (MDPV) and an Examination of Effects at the Human Dopamine Transporter. ACS Chem. Neurosci. 2013, 4, 1524–1529. [Google Scholar] [CrossRef]
- Marusich, J.A.; Antonazzo, K.R.; Wiley, J.L.; Blough, B.E.; Partilla, J.S.; Baumann, M.H. Pharmacology of Novel Synthetic Stimulants Structurally Related to the “Bath Salts” Constituent 3,4-Methylenedioxypyrovalerone (MDPV). Neuropharmacology 2014, 87, 206–213. [Google Scholar] [CrossRef]
- Baumann, M.H.; Bukhari, M.O.; Lehner, K.R.; Anizan, S.; Rice, K.C.; Concheiro, M.; Huestis, M.A. Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs. Curr. Top. Behav. Neurosci. 2017, 32, 93–117. [Google Scholar] [CrossRef]
- Riley, A.L.; Nelson, K.H.; To, P.; López-Arnau, R.; Xu, P.; Wang, D.; Wang, Y.; Shen, H.W.; Kuhn, D.M.; Angoa-Perez, M.; et al. Abuse Potential and Toxicity of the Synthetic Cathinones (i.e., “Bath Salts”). Neurosci. Biobehav. Rev. 2020, 110, 150–173. [Google Scholar] [CrossRef] [PubMed]
- Barrios, L.; Grison-Hernando, H.; Boels, D.; Bouquie, R.; Monteil-Ganiere, C.; Clement, R. Death Following Ingestion of Methylone. Int. J. Legal Med. 2016, 130, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Borek, H.A.; Holstege, C.P. Hyperthermia and Multiorgan Failure after Abuse of “Bath Salts” Containing 3,4-Methylenedioxypyrovalerone. Ann. Emerg. Med. 2012, 60, 103–105. [Google Scholar] [CrossRef]
- Carbone, P.N.; Carbone, D.L.; Carstairs, S.D.; Luzi, S.A. Sudden Cardiac Death Associated with Methylone Use. Am. J. Forensic Med. Pathol. 2013, 34, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Pieprzyca, E.; Skowronek, R.; Czekaj, P. Toxicological Analysis of Intoxications with Synthetic Cathinones. J. Anal. Toxicol. 2022, 46, 705–711. [Google Scholar] [CrossRef]
- Radaelli, D.; Manfredi, A.; Zanon, M.; Fattorini, P.; Scopetti, M.; Neri, M.; Frisoni, P.; D’Errico, S. Synthetic Cannabinoids and Cathinones Cardiotoxicity: Facts and Perspectives. Curr. Neuropharmacol. 2021, 19, 2038–2048. [Google Scholar] [CrossRef]
- Wood, D.M.; Davies, S.; Puchnarewicz, M.; Button, J.; Archer, R.; Ovaska, H.; Ramsey, J.; Lee, T.; Holt, D.W.; Dargan, P.I. Recreational Use of Mephedrone (4-Methylmethcathinone, 4-MMC) with Associated Sympathomimetic Toxicity. J. Med. Toxicol. 2010, 6, 327–330. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R. Synthetic Cathinones: Chemistry, Pharmacology and Toxicology of a New Class of Designer Drugs of Abuse Marketed as “Bath Salts” or “Plant Food”. Toxicol. Lett. 2012, 211, 144–149. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R. 3,4-Methylenedioxypyrovalerone (MDPV): Chemistry, Pharmacology and Toxicology of a New Designer Drug of Abuse Marketed Online. Toxicol. Lett. 2012, 208, 12–15. [Google Scholar] [CrossRef]
- Prosser, J.M.; Nelson, L.S. The Toxicology of Bath Salts: A Review of Synthetic Cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef]
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular Disease Models: A Game Changing Paradigm in Drug Discovery and Screening. Biomaterials 2019, 198, 3–26. [Google Scholar] [CrossRef]
- Rihel, J.; Ghosh, M. Zebrafish. In Drug Discovery and Evaluation: Pharmacological Assay, 4th ed.; Springer International Publishing: New York, NY, USA, 2015; pp. 4071–4155. ISBN 9783319053929. [Google Scholar]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments—A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- The European Parliament; The Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes (Text with EEA Relevance). Off. J. Eur. Union 2010, 28, 276. [Google Scholar]
- Staudt, D.; Stainier, D. Uncovering the Molecular and Cellular Mechanisms of Heart Development Using the Zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef]
- Vornanen, M.; Hassinen, M. Zebrafish Heart as a Model for Human Cardiac Electrophysiology. Channels 2016, 10, 101–110. [Google Scholar] [CrossRef]
- Langheinrich, U.; Vacun, G.; Wagner, T. Zebrafish Embryos Express an Orthologue of HERG and Are Sensitive toward a Range of QT-Prolonging Drugs Inducing Severe Arrhythmia. Toxicol. Appl. Pharmacol. 2003, 193, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Peterson, T.A.; Ruskin, J.N.; Peterson, R.T.; MacRae, C.A. Drugs That Induce Repolarization Abnormalities Cause Bradycardia in Zebrafish. Circulation 2003, 107, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Haffter, P.; Odenthal, J.; Vogelsang, E.; Brand, M.; Van Eeden, F.J.M.; Furutani-Seiki, M.; Granato, M.; Hammerschmidt, M.; Heisenberg, C.P.; et al. Mutations Affecting the Cardiovascular System and Other Internal Organs in Zebrafish. Development 1996, 123, 293–302. [Google Scholar] [CrossRef]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.J.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid Behavior-Based Identification of Neuroactive Small Molecules in the Zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Rinkwitz, S.; Mourrain, P.; Becker, T.S. Zebrafish: An Integrative System for Neurogenomics and Neurosciences. Prog. Neurobiol. 2011, 93, 231–243. [Google Scholar] [CrossRef]
- Chaudhari, G.H.; Chennubhotla, K.S.; Chatti, K.; Kulkarni, P. Optimization of the Adult Zebrafish ECG Method for Assessment of Drug-Induced QTc Prolongation. J. Pharmacol. Toxicol. Methods 2013, 67, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Jones, I.L.; Ellinor, P.T.; MacRae, C.A. In Vivo Recording of Adult Zebrafish Electrocardiogram and Assessment of Drug-Induced QT Prolongation. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H269–H273. [Google Scholar] [CrossRef]
- EMCDDA. New Psychoactive Substances: 25 Years of Early Warning and Response in Europe—An Update from the EU Early Warning System; EMCDDA: Lisbon, Portugal, 2022. [Google Scholar]
- Schwerte, T.; Prem, C.; Mairösl, A.; Pelster, B. Development of the Sympatho-Vagal Balance in the Cardiovascular System in Zebrafish (Danio rerio) Characterized by Power Spectrum and Classical Signal Analysis. J. Exp. Biol. 2006, 209, 1093–1100. [Google Scholar] [CrossRef]
- Wasel, O.; Freeman, J.L. Chemical and Genetic Zebrafish Models to Define Mechanisms of and Treatments for Dopaminergic Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5981. [Google Scholar] [CrossRef]
- Ghuran, A.; van der Wieken, L.R.; Nolan, J. Cardiovascular Complications of Recreational Drugs. BMJ 2001, 323, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, D.W.; Lutz, W.K.; Conolly, R.B. Statistical Analysis of Nonmonotonic Dose-Response Relationships: Research Design and Analysis of Nasal Cell Proliferation in Rats Exposed to Formaldehyde. Toxicol. Sci. 2004, 77, 158–164. [Google Scholar] [CrossRef]
- Margiotta-Casaluci, L.; Owen, S.F.; Rand-Weaver, M.; Winter, M.J. Testing the Translational Power of the Zebrafish: An Interspecies Analysis of Responses to Cardiovascular Drugs. Front. Pharmacol. 2019, 10, 893. [Google Scholar] [CrossRef]
- Sarmah, S.; Marrs, J.A. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. Int. J. Mol. Sci. 2016, 17, 2123. [Google Scholar] [CrossRef]
- Piao, Y.S.; Hall, F.S.; Moriya, Y.; Ito, M.; Ohara, A.; Kikura-Hanajiri, R.; Goda, Y.; Lesch, K.P.; Murphy, D.L.; Uhl, G.R.; et al. Methylone-Induced Hyperthermia and Lethal Toxicity: Role of the Dopamine and Serotonin Transporters. Behav. Pharmacol. 2015, 26, 345–352. [Google Scholar] [CrossRef]
- Dominic, P.; Ahmad, J.; Awwab, H.; Bhuiyan, M.S.; Kevil, C.G.; Goeders, N.E.; Murnane, K.S.; Patterson, J.C.; Sandau, K.E.; Gopinathannair, R.; et al. Stimulant Drugs of Abuse and Cardiac Arrhythmias. Circ. Arrhythm. Electrophysiol. 2022, 15, e010273. [Google Scholar] [CrossRef]
- Fonseca, D.A.; Ribeiro, D.M.; Tapadas, M.; Cotrim, M.D. Ecstasy (3,4-Methylenedioxymethamphetamine): Cardiovascular Effects and Mechanisms. Eur. J. Pharmacol. 2021, 903, 174156. [Google Scholar] [CrossRef] [PubMed]
- Pieprzyca, E.; Skowronek, R.; Nižnanský, Ľ.; Czekaj, P. Synthetic Cathinones—From Natural Plant Stimulant to New Drug of Abuse. Eur. J. Pharmacol. 2020, 875, 173012. [Google Scholar] [CrossRef]
- Zwartsen, A.; de Korte, T.; Nacken, P.; de Lange, D.W.; Westerink, R.H.S.; Hondebrink, L. Cardiotoxicity Screening of Illicit Drugs and New Psychoactive Substances (NPS) in Human IPSC-Derived Cardiomyocytes Using Microelectrode Array (MEA) Recordings. J. Mol. Cell. Cardiol. 2019, 136, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, P. Blood Concentrations of Synthetic Cathinones. Clin. Toxicol. 2021, 59, 648–654. [Google Scholar] [CrossRef]
- Fernando, T.; Gilbert, J.D.; Carroll, C.M.; Byard, R.W. Ecstasy and Suicide. J. Forensic Sci. 2012, 57, 1137–1139. [Google Scholar] [CrossRef]
- Papaseit, E.; Olesti, E.; de la Torre, R.; Torrens, M.; Farre, M. Mephedrone Concentrations in Cases of Clinical Intoxication. Curr. Pharm. Des. 2017, 23, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Kontak, A.; Vongpatanasin, W.; Victor, R.G. Cocaine Overdose. In Primer on the Autonomic Nervous System; Academic Press: Cambridge, MA, USA, 2012; pp. 577–581. [Google Scholar] [CrossRef]
- O’Leary, M.E.; Hancox, J.C. Role of Voltage-Gated Sodium, Potassium and Calcium Channels in the Development of Cocaine-Associated Cardiac Arrhythmias. Br. J. Clin. Pharmacol. 2010, 69, 427–442. [Google Scholar] [CrossRef]
- Magnano, A.R.; Talathoti, N.B.; Hallur, R.; Jurus, D.T.; Dizon, J.; Holleran, S.; Bloomfield, D.M.; Collins, E.; Garan, H. Effect of Acute Cocaine Administration on the QTc Interval of Habitual Users. Am. J. Cardiol. 2006, 97, 1244–1246. [Google Scholar] [CrossRef]
- Fischbach, P. The Role of Illicit Drug Use in Sudden Death in the Young. Cardiol. Young 2017, 27, S75–S79. [Google Scholar] [CrossRef]
- Meredith, I.T.; Broughton, A.; Jennings, G.L.; Esler, M.D. Evidence of a Selective Increase in Cardiac Sympathetic Activity in Patients with Sustained Ventricular Arrhythmias. N. Engl. J. Med. 1991, 325, 618–624. [Google Scholar] [CrossRef]
- Vicente, M.; Salgado-Almario, J.; Collins, M.M.; Martínez-Sielva, A.; Minoshima, M.; Kikuchi, K.; Domingo, B.; Llopis, J. Cardioluminescence in Transgenic Zebrafish Larvae: A Calcium Imaging Tool to Study Drug Effects and Pathological Modeling. Biomedicines 2021, 9, 1294. [Google Scholar] [CrossRef]
- Novellas, J.; López-Arnau, R.; Carbó, M.L.; Pubill, D.; Camarasa, J.; Escubedo, E. Concentrations of MDPV in Rat Striatum Correlate with the Psychostimulant Effect. J. Psychopharmacol. 2015, 29, 1209–1218. [Google Scholar] [CrossRef]
- Barenys, M.; Álvarez, S.; Santamaria, A.; Teixidó, E.; Gómez-Catalán, J. Developmental Exposure to MDMA (Ecstasy) in Zebrafish Embryos Reproduces the Neurotoxicity Adverse Outcome “lower Motor Activity” Described in Humans. Neurotoxicology 2022, 88, 116–123. [Google Scholar] [CrossRef]
- Bittner, L.; Teixido, E.; Seiwert, B.; Escher, B.I.; Klüver, N. Influence of PH on the Uptake and Toxicity of β-Blockers in Embryos of Zebrafish, Danio Rerio. Aquat. Toxicol. 2018, 201, 129–137. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.K.; Sorabella, R.; Tercek, A.; Isler, J.R.; Targoff, K.L. Nkx2.5 Is Essential to Establish Normal Heart Rate Variability in the Zebrafish Embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R265–R271. [Google Scholar] [CrossRef] [PubMed]
- Zabihihesari, A.; Khalili, A.; Hilliker, A.J.; Rezai, P. Open Access Tool and Microfluidic Devices for Phenotypic Quantification of Heart Function of Intact Fruit Fly and Zebrafish Larvae. Comput. Biol. Med. 2021, 132, 104314. [Google Scholar] [CrossRef] [PubMed]
- Berthold, M.R.; Cebron, N.; Dill, F.; Gabriel, T.R.; Kötter, T.; Meinl, T.; Ohl, P.; Sieb, C.; Thiel, K.; Wiswedel, B. KNIME: The Konstanz Information Miner. In Data Analysis, Machine Learning and Applications, Studies in Classification, Data Analysis, and Knowledge Organization, Proceedings of the 31st Annual Conference of the Gesellschaft für Klassifikation E.V., Albert-Ludwigs-Universität Freiburg, Freiburg im Breisgau, Germany, 7–9 March 2007; Springer: Berlin/Heidelberg, Germany, 2008; pp. 319–326. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics Notes: The Normal Distribution. BMJ 1995, 310, 298. [Google Scholar] [CrossRef] [PubMed]


| Compound | LC50 (CI95) µM | Hill Slope |
|---|---|---|
| Cocaine | 172.9 (153.3–210.3) | 2.81 |
| MDPV | 135.2 (114.6–155.7) | 6.88 |
| MDMA | 179.8 (125.6–234.0) | 2.65 |
| Methylone | 126.6 (92.1–161.1) | 15.3 |
| Mephedrone | 147.4 (92.7–202.0) | 1.86 |
| COMPOUND | EC50 (CI95) µM ATRIUM | EC50 (CI95) µM VENTRICLE |
|---|---|---|
| Cocaine | >500 | 97.2 (29.9–129.0) |
| MDPV | 110.5 (74.0–127.0) | 32.7 (18.3–46.1) |
| MDMA | 500 (412.8–586.1) | 418.3 (359.7–476.9) |
| Methylone | >1000 | 522.3 (414.8–664.1) |
| Mephedrone | 600.6 (522.5–693.2) | 403 (377.5–423.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixidó, E.; Riera-Colomer, C.; Raldúa, D.; Pubill, D.; Escubedo, E.; Barenys, M.; López-Arnau, R. First-Generation Synthetic Cathinones Produce Arrhythmia in Zebrafish Eleutheroembryos: A New Approach Methodology for New Psychoactive Substances Cardiotoxicity Evaluation. Int. J. Mol. Sci. 2023, 24, 13869. https://doi.org/10.3390/ijms241813869
Teixidó E, Riera-Colomer C, Raldúa D, Pubill D, Escubedo E, Barenys M, López-Arnau R. First-Generation Synthetic Cathinones Produce Arrhythmia in Zebrafish Eleutheroembryos: A New Approach Methodology for New Psychoactive Substances Cardiotoxicity Evaluation. International Journal of Molecular Sciences. 2023; 24(18):13869. https://doi.org/10.3390/ijms241813869
Chicago/Turabian StyleTeixidó, Elisabet, Clara Riera-Colomer, Demetrio Raldúa, David Pubill, Elena Escubedo, Marta Barenys, and Raul López-Arnau. 2023. "First-Generation Synthetic Cathinones Produce Arrhythmia in Zebrafish Eleutheroembryos: A New Approach Methodology for New Psychoactive Substances Cardiotoxicity Evaluation" International Journal of Molecular Sciences 24, no. 18: 13869. https://doi.org/10.3390/ijms241813869
APA StyleTeixidó, E., Riera-Colomer, C., Raldúa, D., Pubill, D., Escubedo, E., Barenys, M., & López-Arnau, R. (2023). First-Generation Synthetic Cathinones Produce Arrhythmia in Zebrafish Eleutheroembryos: A New Approach Methodology for New Psychoactive Substances Cardiotoxicity Evaluation. International Journal of Molecular Sciences, 24(18), 13869. https://doi.org/10.3390/ijms241813869







