Performance of Zr-Based Metal–Organic Framework Materials as In Vitro Systems for the Oral Delivery of Captopril and Ibuprofen
Abstract
:1. Introduction
2. Results
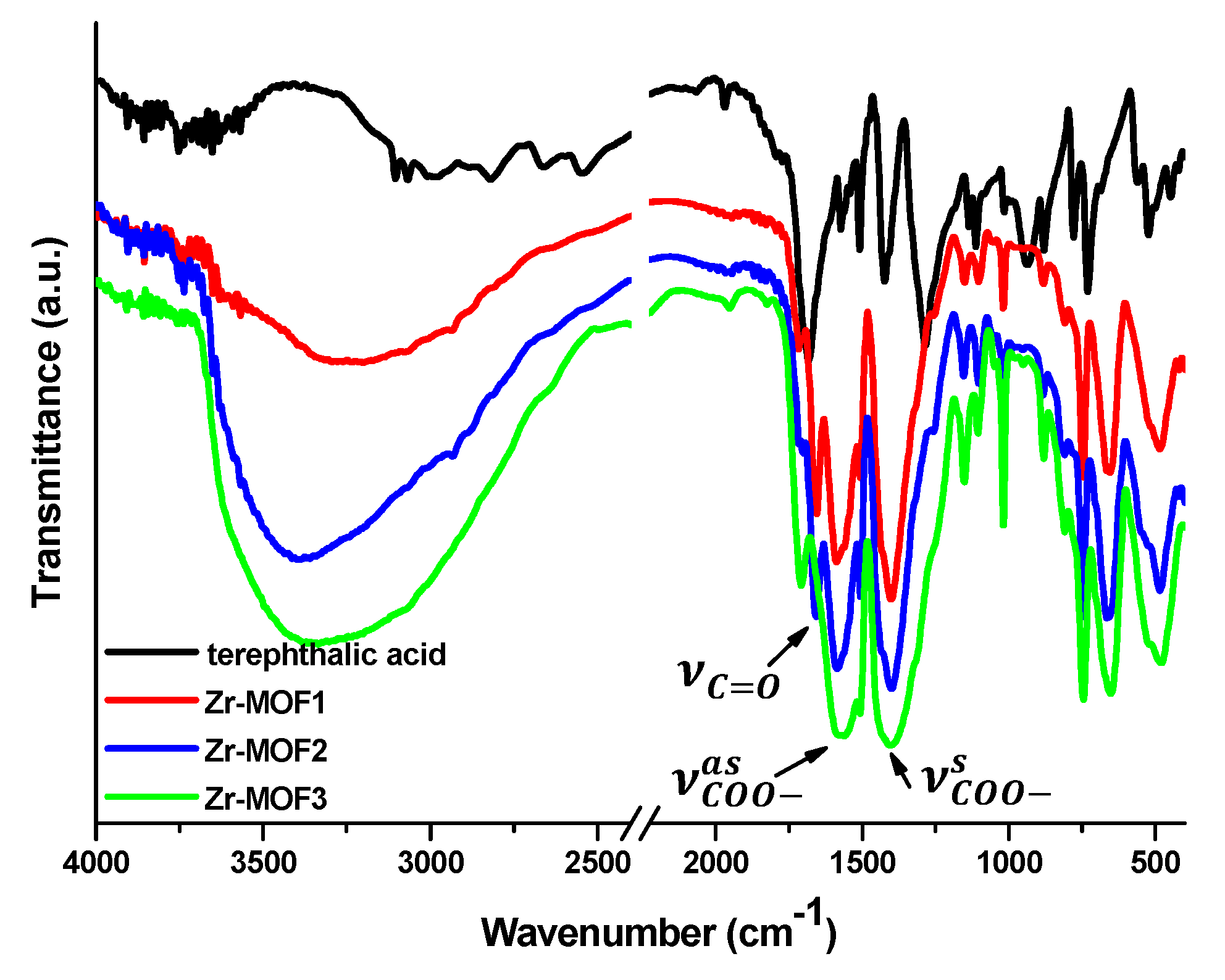
2.1. FT-IR
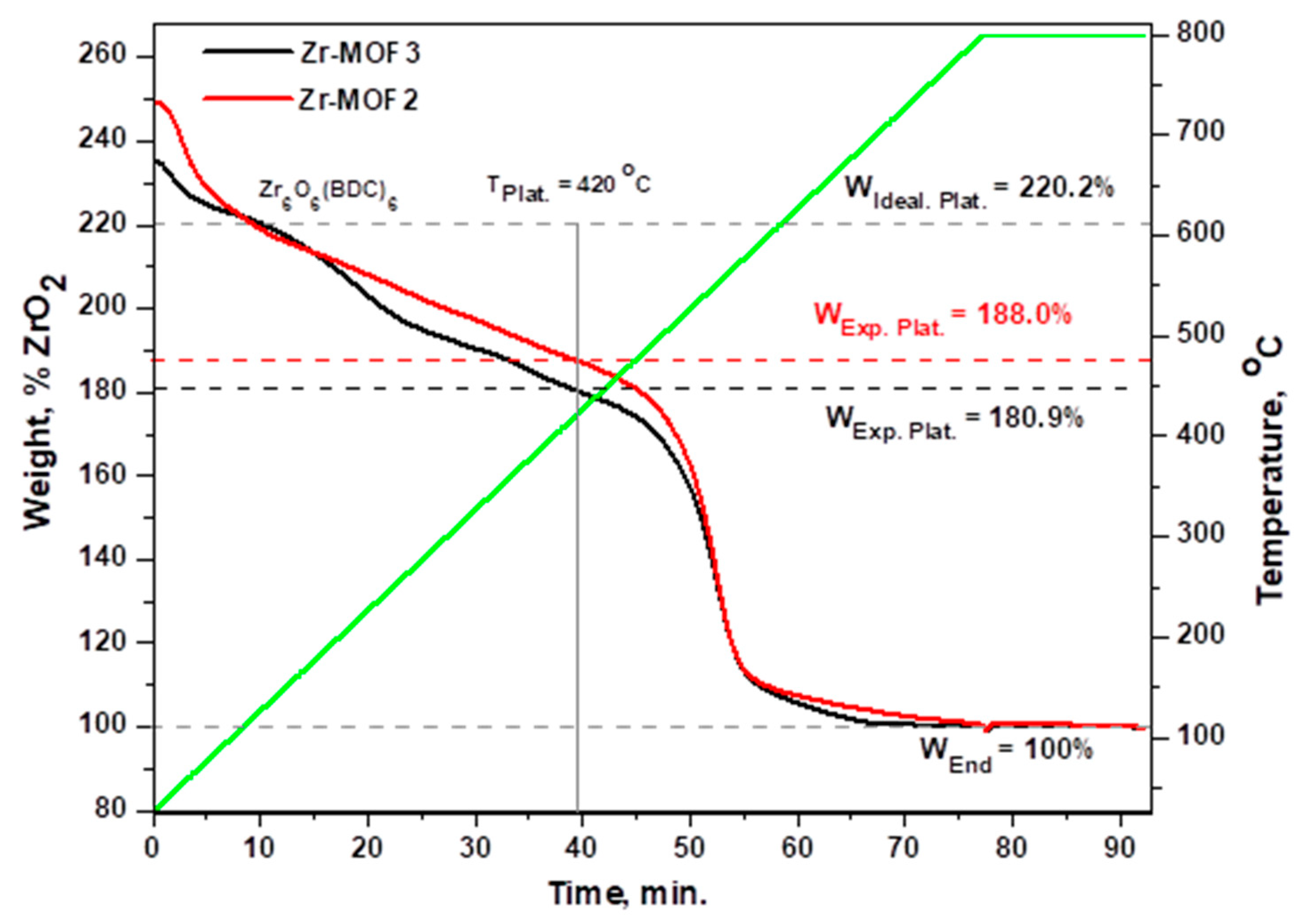
2.2. Thermal Stability
2.3. Textural Characterization of Zr-MOFs by Nitrogen Sorption Method
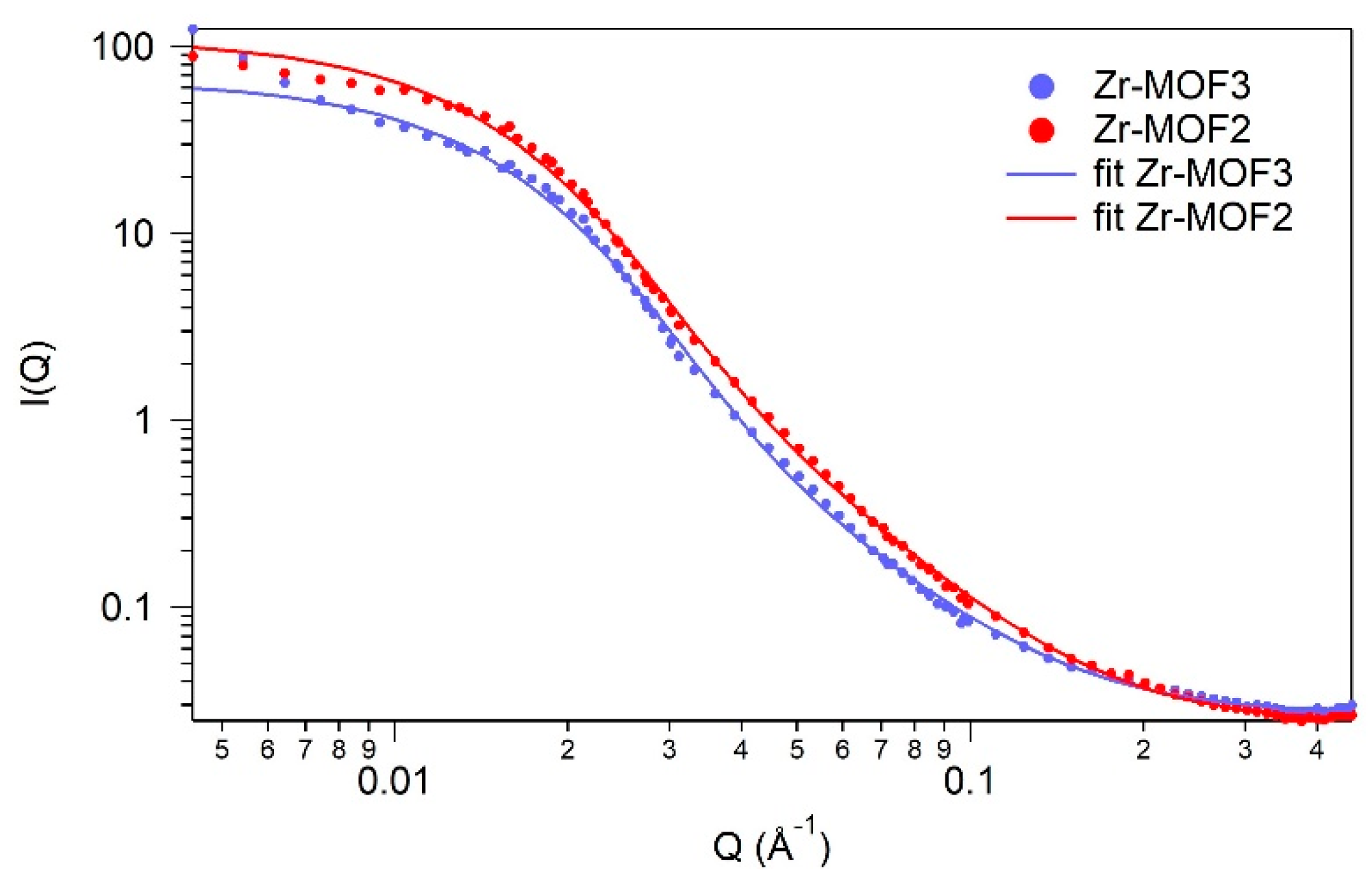
2.4. SANS (Small-Angle Neutron Scattering)
2.5. X-ray Diffraction
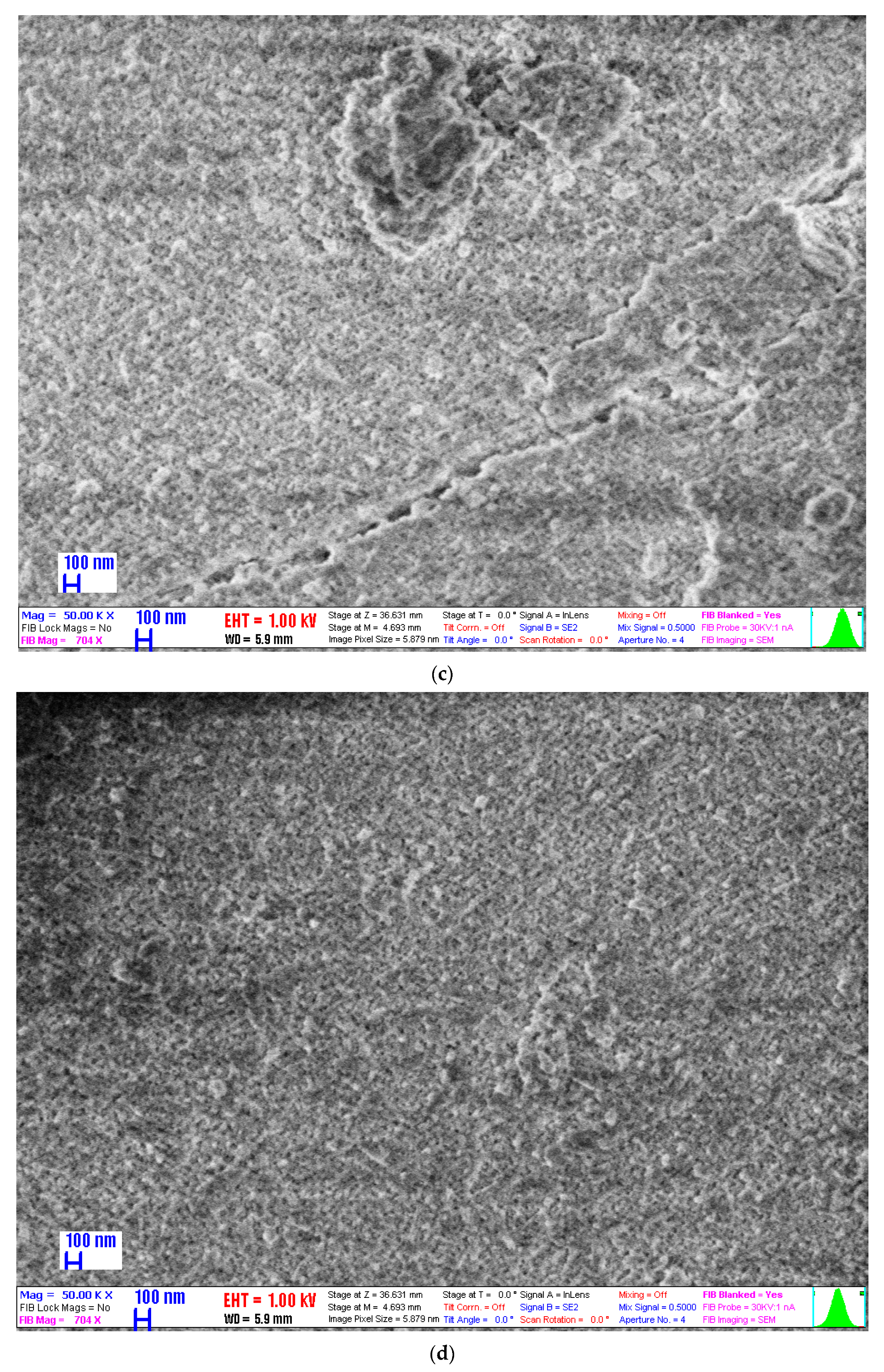
2.6. Scanning Electron Microscopy
2.7. Drug Loading
2.8. Drug Release
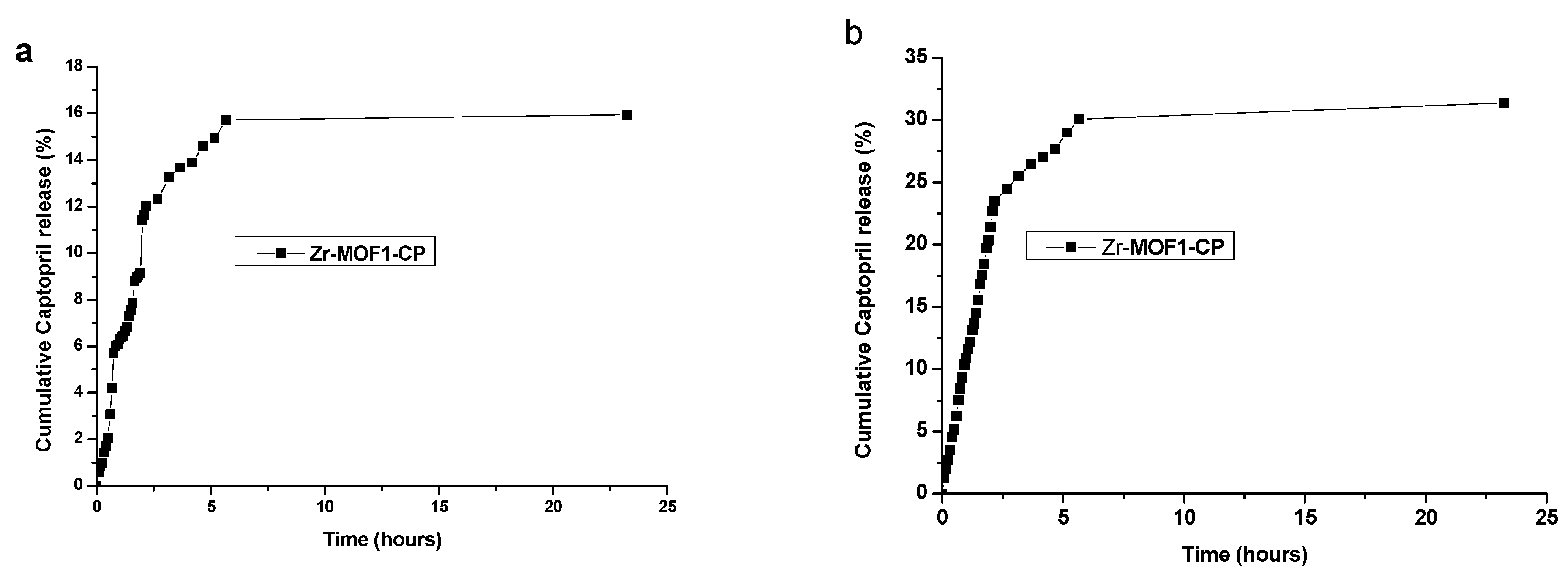
2.8.1. Captopril Release from Zr-MOF1-CP Sample Material Synthetized Using the Chemical Method
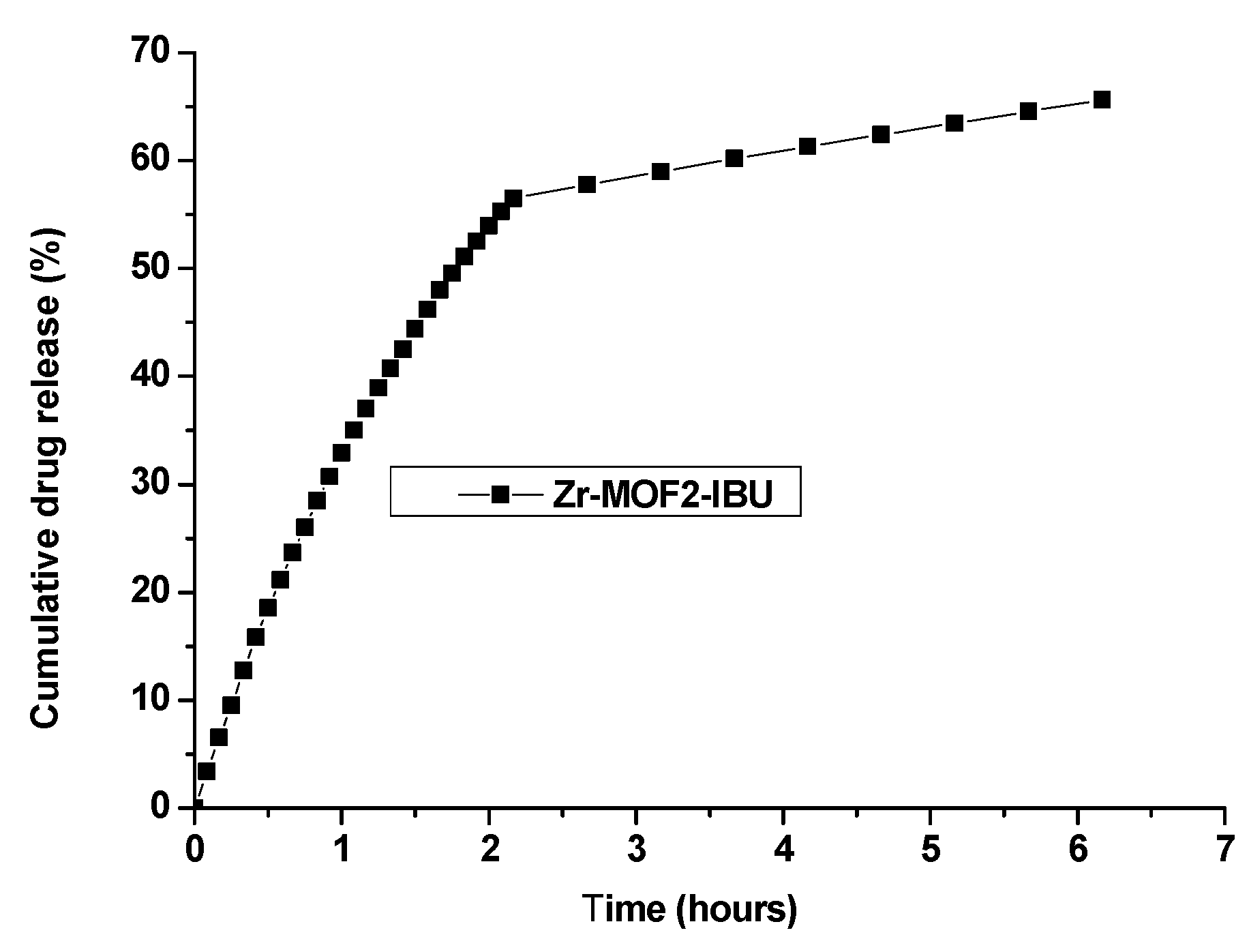
2.8.2. Ibuprofen Release from Zr-MOF2-IBU
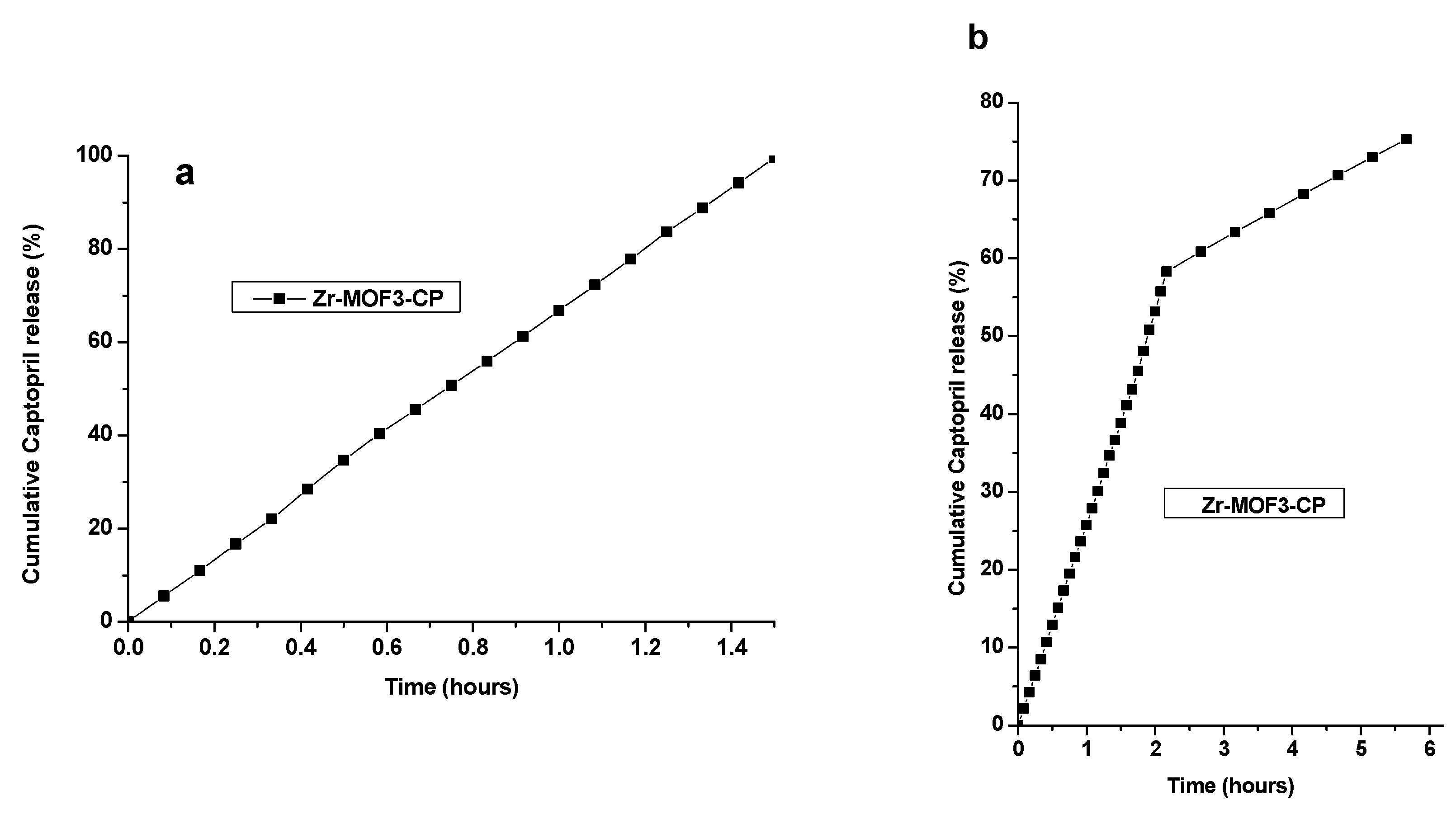
2.8.3. Captopril Release Using the Dialysis Membrane from Zr-MOF3-CP
2.9. Kinetics Models Applied for the First Hours of Release
3. Discussion
3.1. Drug Loading
3.2. Drug Release
3.3. Kinetic Modelling
3.4. The Biosafety of Zr4+, Terephthalic Acid and the Resulted Zr-MOF
4. Materials and Methods
4.1. Synthesis
4.1.1. Chemical Synthesis Procedure
4.1.2. Solvothermal Method of Synthesis
4.2. Characterization
4.3. Drug Loading and Release Procedures
4.3.1. Chemicals for Captopril Loading and Release Procedure
4.3.2. Captopril Loaded by Adsorption
4.3.3. In Vitro Captopril Release Procedures
4.3.4. Captopril Release Procedure Using the Dialysis Membrane (Regenerate Cellulose Tubular Membrane Zellu Trans MWCO: 12,000–14,000; Pore Size = 25 Å; Wall Thickness = 40 µm; Karlsruhe, Germany)
4.3.5. Chemicals for Ibuprofen Loading and Release Procedure
4.3.6. Ibuprofen Loaded by Adsorption
4.3.7. In Vitro Ibuprofen Release Procedures
4.3.8. Solutions Prepared for the Drug Loading and Release Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuyama, K. Supercritical Fluid Processing for Metal–Organic Frameworks, Porous Coordination Polymers, and Covalent Organic Frameworks. J. Supercrit. Fluids 2018, 134, 197–203. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Forgan, R.S. Application of Zirconium MOFs in Drug Delivery and Biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-Based Metal–Organic Frameworks: Design, Synthesis, Structure, and Applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Balassa, J.J. Abnormal Trace Metals in Man: Zirconium. J. Chronic Dis. 1966, 19, 573–586. [Google Scholar] [CrossRef]
- Piciorus, M.E.; Popa, A.; Ianasi, C.; Szerb, E.I.; Cretu, C. Zr (IV) Mofs Based on Terephtalic Acid and Acetic Acid Modulator. Sci. Tech. Bull.-Chem. Food Sci. Eng. 2020, 17, 33–41. [Google Scholar]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of zr-based metal-organic frameworks: From nano to single crystals. Chem.-Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Gutov, V.O.; Hevia, M.-G.; Escudero-Adan, E.C.; Shafir, A. Metal-Organic Framework (MOF) Defects under Control: Insights into the Missing Linker Sites and Their Implication in the Reactivity of Zirconium-Based Frameworks. Inorg. Chem. 2015, 54, 8396–8400. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect Engineering: Tuning the Porosity and Composition of the Metal–Organic Framework UiO-66 via Modulated Synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Hu, Q.; Zhang, X.; Zhang, J.; Cui, Y.; Yang, Y.; Li, B.; Qian, G. A Zirconium-based Metal-organic Framework with Encapsulated Anionic Drug for Uncommonly Controlled Oral Drug Delivery. Microporous Mesoporous Mater. 2019, 275, 229–234. [Google Scholar] [CrossRef]
- Egu, J.C.; Moldován, K.; Herman, P.; István, F.; Kalmár, J.; Fenyvesi, F. 6ER-017 Prevention of Extravasation by the Local Application of Hybrid Aerogel Microparticles as Drug Delivery Systems for Cervical Cancer Chemotherapy. Eur. J. Hosp. Pharm. 2022, 29, A172. [Google Scholar] [CrossRef]
- Chen, T.; Wang, M.; Tan, K.; Chen, C.; Li, M.; Mao, C. Ibuprofen Loaded into Metal–Organic Framework Shells Coated on Fe3O4 Nanoparticles for the Removal of Protein-Bound Uremic Toxins in Blood. ACS Appl. Nano Mater. 2022, 5, 5838–5846. [Google Scholar] [CrossRef]
- National Library of Medicine. Captopril. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Captopril (accessed on 28 August 2023).
- National Library of Medicine. Ibuprofen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ibuprofen (accessed on 28 August 2023).
- Popovici, R.F.; Seftel, E.M.; Mihai, G.D.; Popovici, E.; Voicu, V.A. Controlled Drug Delivery System Based on Ordered Mesoporous Silica Matrices of Captopril as Angiotensin-Converting Enzyme Inhibitor Drug. J. Pharm. Sci. 2011, 100, 704–714. [Google Scholar] [CrossRef]
- Gohel, M.; Nagori, S.A. Fabrication and Evaluation of Captopril Modified-Release Oral Formulation. Pharm. Dev. Technol. 2009, 14, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Hassan-Zadeh, D.; Monajjem-Zadeh, F.; Taghi-Zadeh, N. Effect of Various Surfactants and Their Concentration on Controlled Release of Captopril from Polymeric Matrices. Acta Pharm. 2008, 58, 151–162. [Google Scholar] [CrossRef]
- Shanthi, N.; Gupta, R.; Mahato, A. A Review on Captopril Oral Sustained/Controlled Release Formulations. Int. J. Drug Dev. Res. 2010, 2, 257–264. [Google Scholar]
- ChBEI. Available online: http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:5855 (accessed on 16 June 2023).
- Sánchez-Sánchez, Á.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. pH-Responsive Ordered Mesoporous Carbons for Controlled Ibuprofen Release. Carbon N. Y. 2015, 94, 152–159. [Google Scholar] [CrossRef]
- Sarker, M.; Shin, S.; Jhung, S.H. Synthesis and Functionalization of Porous Zr-Diaminostilbenedicarboxylate Metal-Organic Framework for Storage and Stable Delivery of Ibuprofen. ACS Omega 2019, 4, 9860–9867. [Google Scholar] [CrossRef]
- Wang, G.; Otuonye, A.N.; Blair, E.A.; Denton, K.; Tao, Z.; Asefa, T. Functionalized Mesoporous Materials for Adsorption and Release of Different Drug Molecules: A Comparative Study. J. Solid State Chem. 2009, 182, 1649–1660. [Google Scholar] [CrossRef]
- McCarthy, C.A.; Ahern, R.J.; Dontireddy, R.; Ryan, K.B.; Crean, A.M. Mesoporous Silica Formulation Strategies for Drug Dissolution Enhancement: A Review. Expert Opin. Drug Deliv. 2016, 13, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Remuiñán-Pose, P.; López-Iglesias, C.; Iglesias-Mejuto, A.; Mano, J.F.; García-González, C.A.; Rial-Hermida, M.I. Preparation of Vancomycin-Loaded Aerogels Implementing Inkjet Printing and Superhydrophobic Surfaces. Gels 2022, 8, 417. [Google Scholar] [CrossRef]
- Bahrani, S.; Hashemi, S.A.; Mousavi, S.M.; Azhdari, R. Zinc-Based Metal–Organic Frameworks as Nontoxic and Biodegradable Platforms for Biomedical Applications: Review Study. Drug Metab. Rev. 2019, 51, 356–377. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Omidifar, N.; Zarei, M.; Bahrani, S.; Yousefi, K.; Chiang, W.-H.; Babapoor, A. Bioinorganic Synthesis of Polyrhodanine Stabilized Fe3O4/Graphene Oxide in Microbial Supernatant Media for Anticancer and Antibacterial Applications. Bioinorg. Chem. Appl. 2021, 2021, 9972664. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.-H. Recent Advancements in Polythiophene-based Materials And Their Biomedical, Geno Sensor and DNA Detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Mazraedoost, S.; Yousefi, K.; Gholami, A.; Behbudi, G.; Ramakrishna, S.; Omidifar, N.; Alizadeh, A.; Chiang, W.-H. Multifunctional Gold Nanorod for Therapeutic Applications and Pharmaceutical Delivery Considering Cellular Metabolic Responses, Oxidative Stress and Cellular Longevity. Nanomaterials 2021, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Kalashgrani, M.Y.; Omidifar, N.; Bahrani, S.; Vijayakameswara Rao, N.; Babapoor, A.; Gholami, A.; Chiang, W.-H. Bioactive Graphene Quantum Dots Based Polymer Composite for Biomedical Applications. Polymers 2022, 14, 617. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile Synthesis of Morphology and Size-Controlled Zirconium Metal–Organic Framework UiO-66: The Role of Hydrofluoric Acid in Crystallization. Cryst. Eng. Comm. 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Beaucage, G. Small-Angle Scattering from Polymeric Mass Fractals of Arbitrary Mass-Fractal Dimension. J. Appl. Crystallogr. 1996, 29, 134–146. [Google Scholar] [CrossRef]
- Kline, S.R. Reduction and Analysis of SANS and USANS Data Using IGOR Pro. J. Appl. Crystallogr. 2006, 39, 895–900. [Google Scholar] [CrossRef]
- Sang, X.; Zhang, J.; Xiang, J.; Cui, J.; Zheng, L.; Zhang, J.; Wu, Z.; Li, Z.; Mo, G.; Xu, Y.; et al. Ionic Liquid Accelerates the Crystallization of Zr-Based Metal–Organic Frameworks. Nat. Commun. 2017, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.W. Small-Angle Scattering Studies of Disordered, Porous and Fractal Systems. J. Appl. Crystallogr. 1991, 24, 414–435. [Google Scholar] [CrossRef]
- Øien, S.; Wragg, D.; Reinsch, H.; Svelle, S.; Bordiga, S.; Lamberti, C.; Lillerud, K.P. Detailed Structure Analysis of Atomic Positions and Defects in Zirconium Metal–Organic Frameworks. Cryst. Growth Des. 2014, 14, 5370–5372. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Rojas, S.; Colinet, I.; Cunha, D.; Hidalgo, T.; Salles, F.; Serre, C.; Guillou, N.; Horcajada, P. Toward Understanding Drug Incorporation and Delivery from Biocompatible Metal-Organic Frameworks in View of Cutaneous Administration. ACS Omega 2018, 3, 2994–3003. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Cai, M.; Qin, L.; You, L.; Yao, Y.; Wu, H.; Zhang, Z.; Zhang, L.; Yin, X.; Ni, J. Functionalization of MOF-5 with Mono-Substituents: Effects on Drug Delivery Behavior. RSC Adv. 2020, 10, 36862–36872. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Wells, C.J.R.; Forgan, R.S. Multivariate Modulation of the Zr MOF UiO-66 for Defect-Controlled Combination Anticancer Drug Delivery. Angew. Chem.-Int. Ed. 2020, 59, 5211–5217. [Google Scholar] [CrossRef]
- Abánades Lázaro, I. The Effect of Surface Functionalisation on Cancer Cells Internalisation and Selective Cytotoxicity of Zirconium Metal Organic Frameworks. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2018. [Google Scholar]
- Huang, X.; Brazel, C.S. On the Importance and Mechanisms of Burst Release in Matrix-Controlled Drug Delivery Systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in Drug Delivery: From Design to Application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Almásy, L. New Measurement Control Software on the Yellow Submarine SANS Instrument at the Budapest Neutron Centre. J. Surf. Investig. 2021, 15, 527–531. [Google Scholar] [CrossRef]
- Len, A.; Paladini, G.; Románszki, L.; Putz, A.-M.; Almásy, L.; László, K.; Bálint, S.; Krajnc, A.; Kriechbaum, M.; Kuncser, A.; et al. Physicochemical Characterization and Drug Release Properties of Methyl-Substituted Silica Xerogels Made Using Sol–Gel Process. Int. J. Mol. Sci. 2021, 22, 9197. [Google Scholar] [CrossRef]
- Kéri, M.; Forgács, A.; Papp, V.; Bányai, I.; Veres, P.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Gelatin Content Governs Hydration Induced Structural Changes in Silica-Gelatin Hybrid Aerogels—Implications in Drug Delivery. Acta Biomater. 2020, 105, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shin, W.S.; Kim, H.T. Effects of pH, Dissolved Organic Matter, and Salinity on Ibuprofen Sorption on Sediment. Environ. Sci. Pollut. Res. Int. 2016, 23, 22882–22889. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Feng, D.; Wang, K.; Gu, Z.-Y.; Wei, Z.; Chen, Y.-P.; Zhou, H.-C. An Exceptionally Stable, Porphyrinic Zr Metal–Organic Framework Exhibiting pH-Dependent Fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Ma, X.; Duan, Y.; Yao, Y.; Cai, Q. Small Titanium-Based MOFs Prepared with the Introduction of Tetraethyl Orthosilicate and Their Potential for Use in Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 13325–13332. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Q.; Xu, Y.; Wu, D.; Sun, Y.; Shen, W.; Deng, F. Controllable Release of Ibuprofen from Size-Adjustable and Surface Hydrophobic Mesoporous Silica Spheres. Powder Technol. 2009, 191, 13–20. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, H.Y.; Zhao, D.; Wang, J. Metal–Organic Frameworks (MOFs)-Boosted Filtration Membrane Technology for Water Sustainability. APL Mater. 2020, 8, 040902. [Google Scholar] [CrossRef]
- Zong, Z.; Tian, G.; Wang, J.; Fan, C.; Yang, F.; Guo, F. Recent Advances in Metal–Organic-Framework-Based Nanocarriers for Controllable Drug Delivery and Release. Pharmaceutics 2022, 14, 2790. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Abánades Lázaro, S.; Forgan, R.S. Enhancing Anticancer Cytotoxicity through Bimodal Drug Delivery from Ultrasmall Zr MOF Nanoparticles. Chem. Commun. 2018, 54, 2792–2795. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J.M.; Marshall, R.J.; Sastre, B.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. Surface-Functionalization of Zr-Fumarate MOF for Selective Cytotoxicity and Immune System Compatibility in Nanoscale Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 31146–31157. [Google Scholar] [CrossRef]
- Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.; Wu, Y.; Li, Y. Inverse and Highly Selective Separation of CO2/C2H2 on a Thulium–Organic Framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Zhong, Y.; Peng, Z.; Peng, Y.; Li, B.; Pan, Y.; Ouyang, Q.; Sakiyama, H.; Muddassir, M.; Liu, J. Construction of Fe-Doped ZIF-8/DOX Nanocomposites for Ferroptosis Strategy in the Treatment of Breast Cancer. J. Mater. Chem. B 2023, 11, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Lin, M.; Lu, C.; Kumar, A.; Pan, Y.; Liu, J.; Peng, Y. Current and Promising Applications of Hf(Iv)-Based MOFs in Clinical Cancer Therapy. J. Mater. Chem. B 2023, 11, 5693–5714. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Ma, J.; Nezamzadeh-Ejhieh, A.; Lu, C.; Pan, Y.; Liu, J.; Bai, Z. Current Status and Prospects of MOFs in Controlled Delivery of Pt Anticancer Drugs. Dalt. Trans. 2023, 52, 6226–6238. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption Mechanisms of Ibuprofen and Naproxen to UiO-66 and UiO-66-NH2: Batch Experiment and DFT Calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]
- Wang, H.L.; Yeh, H.; Li, B.H.; Lin, C.H.; Hsiao, T.C.; Tsai, D.H. Zirconium-Based Metal-Organic Framework Nanocarrier for the Controlled Release of Ibuprofen. ACS Appl. Nano Mater. 2019, 2, 3329–3334. [Google Scholar] [CrossRef]
- Fayyazi, M.; Solaimany Nazar, A.R.; Farhadian, M.; Tangestaninejad, S. Adsorptive Removal of Ibuprofen to Binary and Amine-Functionalized UiO-66 in the Aquatic Environment: Synergistic/Antagonistic Evaluation. Environ. Sci. Pollut. Res. 2022, 29, 69502–69516. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Yan, M.; Lin, X.; Lin, R.; Wu, Y. Biomimetic Dual-Layer Imprinted UiO-66-Based Basswood Membranes for Ibuprofen Recognition and Separation. ACS Sustain. Chem. Eng. 2023, 11, 6373–6384. [Google Scholar] [CrossRef]
- Modi, S.; Anderson, B.D. Determination of Drug Release Kinetics from Nanoparticles: Overcoming Pitfalls of the Dynamic Dialysis Method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef]
- Farnworth, F.; Jones, S.L.; McAlpine, I. The Production, Properties and Uses of Zirconium Chemicals. In Speciality Inorganic Chemicals; Thompson, R., Ed.; Special Publication No. 40; Royal Society of Chemistry: London, UK, 1980; pp. 248–279. [Google Scholar]
- Nikoofar, K.; Khademi, Z. A Review on Green Lewis Acids: Zirconium(IV) Oxydichloride Octahydrate (ZrOCl2·8H2O) and Zirconium(IV) Tetrachloride (ZrCl4) in Organic Chemistry. Res. Chem. Intermed. 2016, 42, 3929–3977. [Google Scholar] [CrossRef]
- Ball, G.L.; McLellan, C.J.; Bhat, V.S. Toxicological Review and Oral Risk Assessment of Terephthalic Acid (TPA) and Its Esters: A Category Approach. Crit. Rev. Toxicol. 2012, 42, 28–67. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, W.; Yu, S.; Huang, X.; Lang, X.; He, Q.; Gao, L.; Zhu, H.; Chen, J. A biocompatible Zr-Based metal-organic framework UIO-66_PDC as an oral drug carrier for pH-response release. J. Solid State Chem. 2021, 293, 121805. [Google Scholar] [CrossRef]
- Nasrabadi, M.; Ghezamzadeh, M.A.; Monfared, M.R.Z. Preparation and characterization of UiO-66 Metal-Organic Frameworks for the drug delivery of ciprofloxacin and evaluation of their antibacterial activities. New J. Chem. 2019, 43, 16033–16040. [Google Scholar] [CrossRef]
- Li, L.; Han, S.; Zhao, S.; Li, X.; Liu, B.; Liu, Y. Chittosan modified metal-organic frameworks as a promising carrier for oral drug delivery. RSC Adv. 2020, 10, 45130. [Google Scholar] [CrossRef]
- Jarai, B.M.; Stillman, Z.; Attia, L.; Decker, G.E.; Bloch, E.D.; Fromen, C.A. Evaluating UiO-66 metal-organic framework nanoparticles as acid-sensitive carriers for pulmonary drug delivery applications. ACS Appl. Mater. Interfaces 2020, 12, 38989–39004. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, H.; Peng, Y.; Tan, Z.; Tang, B. Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surf. B 2019, 178, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farboudi, A.; Mahboobnia, K.; Chogan, F.; Karimi, M.; Askari, A.; Banihashem, S.; Daravan, S.; Irani, M. UiO-66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core-shell nanofibers for controlled release of doxorubicin and folic acid. Int. J. Biol. Macromol. 2020, 150, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, A.; Amerizadeh, F.; Asgharzadeh, F.; Drummen, G.P.C.; Hassanian, S.M.; Landarani, M.; Avan, A.; Sabouri, Z.; Darroudi, M.; Khazaei, M. Magnetic Amine-Functionalized UiO-66 for Oxaliplatin Delivery to Colon Cancer Cells: In Vitro Studies. J. Clust. Sci. 2022, 33, 2345–2361. [Google Scholar] [CrossRef]
- Tai, S.; Zhang, W.; Zhang, J.; Luo, G.; Jia, Y.; Deng, M.; Ling, Y. Facile preparation of UiO-66 nanoparticles with tunable sizes in a continuous flow microreactor and its application in drug delivery. Microporous Mesoporous Mater. 2016, 220, 148–154. [Google Scholar] [CrossRef]




| Sample | Surface Area, BET, m2/g | Pore Size Distribution, BJH Ads, nm | Pore Size Distribution BJH Des, nm | Pore Width, DFT, nm | Average Pore Size, nm | Total Pore Volume, cm3/g | Micropore Surface Area, m2/g | Total Micropore Volume, cm3/g |
|---|---|---|---|---|---|---|---|---|
| Zr-MOF1 | 1123 | 3.09 | 24.36 | 1.77 | 3.78 | 1.06 | 904 | 0.38 |
| Zr-MOF2 | 912 | 3.47 | 3.28 | 2.35 | 4.04 | 0.92 | 678 | 0.29 |
| Zr-MOF3 | 731 | 3.41 | 12.50 | 2.19 | 3.91 | 0.71 | 482 | 0.20 |
| Drug Loading | Drug Release | |||||
|---|---|---|---|---|---|---|
| Drug Used for Entrapment | Drug Effectively Entrapped | Loading Efficiency (%) | Loading Capacity (mg Drug/g of Carrier) | Calibration Curve | Release Buffer | Cumulative Drug Release |
| Captopril | ||||||
| Captopril 200 mg | 199.56 mg | 99.78 | 997.8 | HCl buffer pH = 1.2 | HCl buffer pH = 1.2 | 15.9% (in 23.5 h) |
| Captopril 200 mg | 199.612 mg | 99.8 | 998.08 | NaCl 0.9% | Phosphate Buffer pH = 7.4 | 31.38% (in 23.5 h) |
| Ibuprofen | ||||||
| 55 mg | 53.1565 mg | 96.65 | 65.46 | NaCl 0.9% | Phosphate Buffer pH = 7.4 | 65.62% (in 6.17 h) |
| Captopril (calculated for the dialysis experiment) | ||||||
| 199.9 mg | 198.9198 mg | 99.51 | 994.6 | HCl buffer pH = 1.2 | HCl buffer pH = 1.2 | 99.51% (in 1.5 h) |
| NaCl 0.9% | Phosphate Buffer pH = 7.4 | 75.29% (in 23.5 h) | ||||
| Sample | Time of Release (h) | % of Captopril Release | |
|---|---|---|---|
| pH = 1.2 | pH = 7.4 | ||
| Zr-MOF1-CP | 5.6 h | 15.72 | 30.08 |
| Zr-MOF1-CP | 23.25 h | 15.94 | 31.38 |
| Sample | Time of Release (h) | % of Ibuprofen Release pH = 7.4 |
|---|---|---|
| Zr-MOF2-IBU | 5.6 h | 64.54 |
| Zr-MOF2-IBU | 6.17 h | 65.62 |
| Sample | Time of Release (h) | % of Captopril Release | |
|---|---|---|---|
| pH = 1.2 | pH = 7.4 | ||
| Zr-MOF3-CP | 1.5 h | 99.51 | - |
| Zr-MOF3-CP | 5.6 h | - | 75.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cretu, C.; Nicola, R.; Marinescu, S.-A.; Picioruș, E.-M.; Suba, M.; Duda-Seiman, C.; Len, A.; Illés, L.; Horváth, Z.E.; Putz, A.-M. Performance of Zr-Based Metal–Organic Framework Materials as In Vitro Systems for the Oral Delivery of Captopril and Ibuprofen. Int. J. Mol. Sci. 2023, 24, 13887. https://doi.org/10.3390/ijms241813887
Cretu C, Nicola R, Marinescu S-A, Picioruș E-M, Suba M, Duda-Seiman C, Len A, Illés L, Horváth ZE, Putz A-M. Performance of Zr-Based Metal–Organic Framework Materials as In Vitro Systems for the Oral Delivery of Captopril and Ibuprofen. International Journal of Molecular Sciences. 2023; 24(18):13887. https://doi.org/10.3390/ijms241813887
Chicago/Turabian StyleCretu, Carmen, Roxana Nicola, Sorin-Alin Marinescu, Elena-Mirela Picioruș, Mariana Suba, Corina Duda-Seiman, Adel Len, Levente Illés, Zsolt Endre Horváth, and Ana-Maria Putz. 2023. "Performance of Zr-Based Metal–Organic Framework Materials as In Vitro Systems for the Oral Delivery of Captopril and Ibuprofen" International Journal of Molecular Sciences 24, no. 18: 13887. https://doi.org/10.3390/ijms241813887








