Microfluidic Isolation of Disseminated Tumor Cells from the Bone Marrow of Breast Cancer Patients
Abstract
:1. Introduction
2. Results
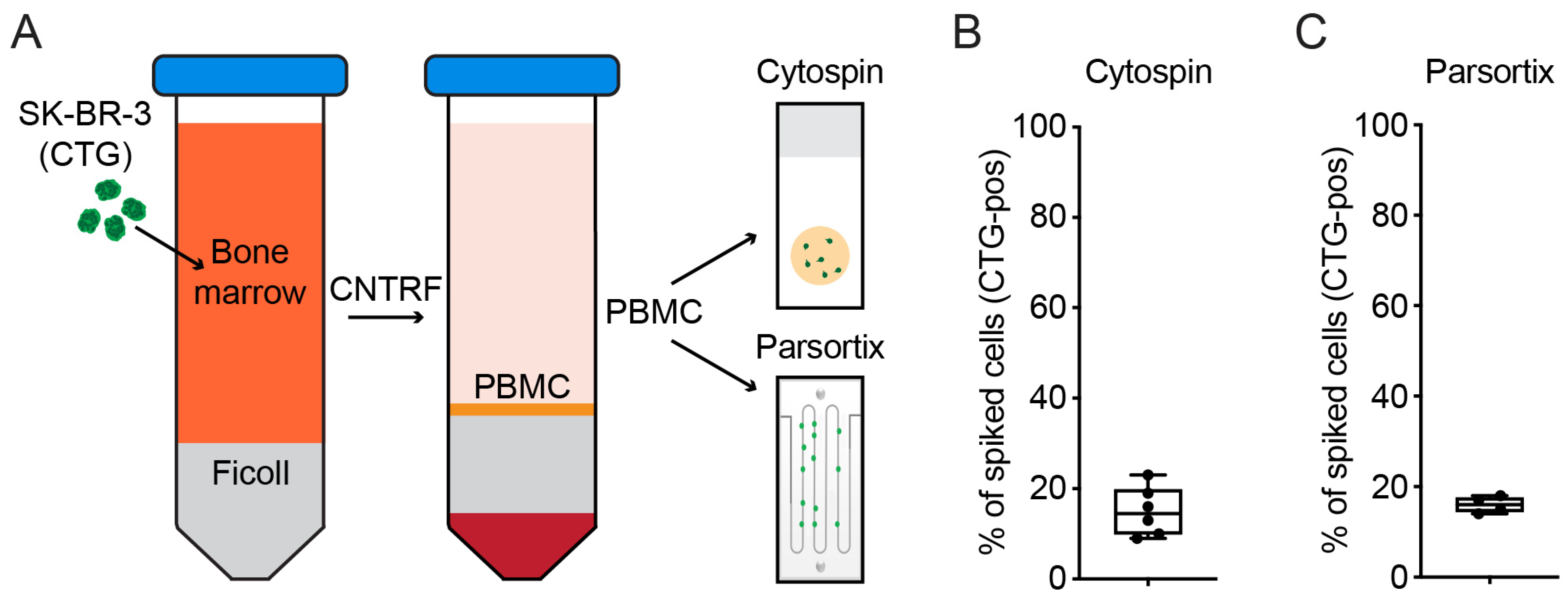
2.1. Cryopreservation of BM Enables Biobanking of DTCs
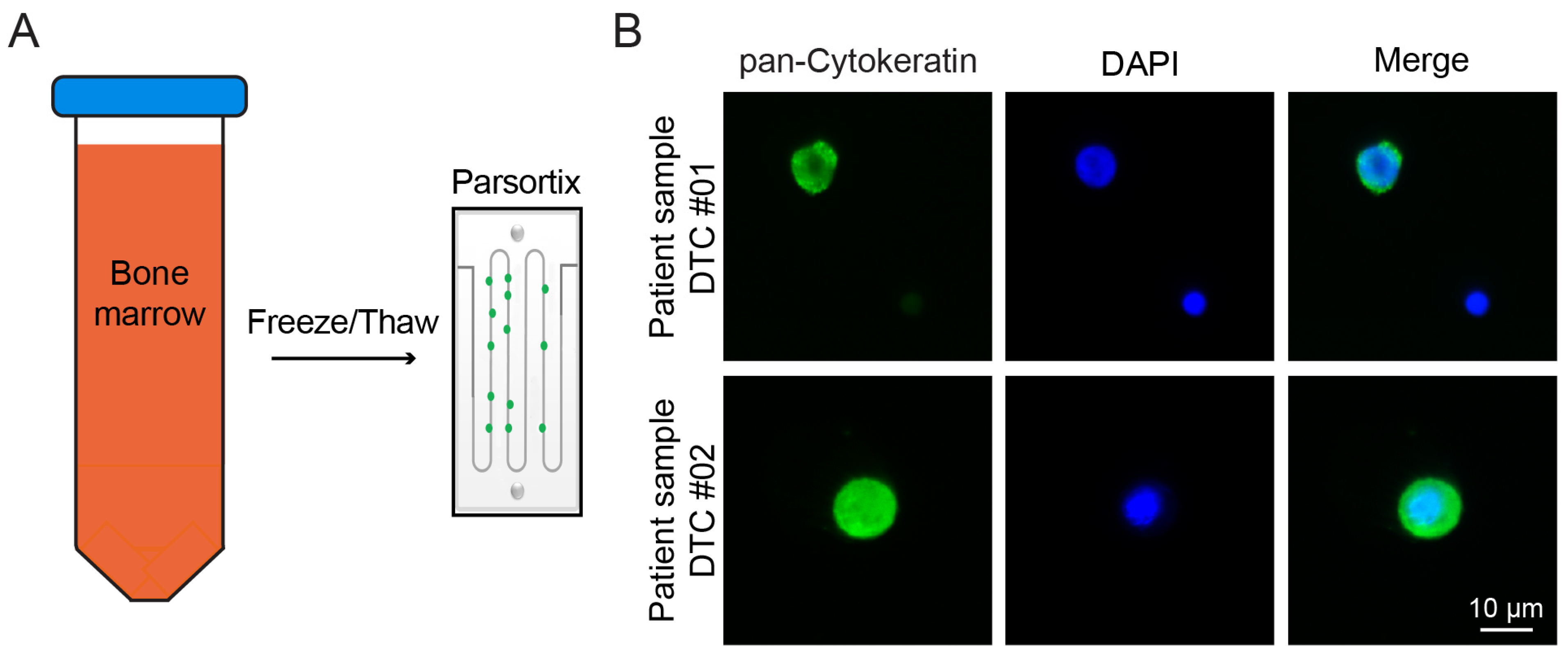
2.2. Detection of DTCs Is Possible after Parsortix Cell Separation
2.3. Microfluidic Cell Separation Displays Potential for Isolating High Numbers of DTCs from Patient Samples
2.4. Parsortix Cell Separation Enables 3D Culture of Cancer Cells after BM Cryopreservation
3. Discussion
4. Materials and Methods
4.1. Ethic Statement
4.2. Cell Culture and Reagents
4.3. Standard Method of Bone Marrow Preparation and Detection of Disseminated Tumor Cells
4.4. Bone Marrow Preparation for Cryopreservation and Microfluidic Cell Separation
4.5. Epitope-Independent Cell Separation
4.6. Cryopreservation and Thawing Procedure of Bone Marrow Cells
4.7. Evaluation of Cell Recovery after Separation Using Spiked Cells
4.8. Cell Detection via Immunofluorescence
4.9. Three-Dimensional Culture of SK-BR-3 Cells after Microfluidic Cell Separation
4.10. Imaging
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiedswang, G.; Borgen, E.; Karesen, R.; Kvalheim, G.; Nesland, J.M.; Qvist, H.; Schlichting, E.; Sauer, T.; Janbu, J.; Harbitz, T.; et al. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J. Clin. Oncol. 2003, 21, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Hartkopf, A.D.; Brucker, S.Y.; Taran, F.A.; Harbeck, N.; von Au, A.; Naume, B.; Pierga, J.Y.; Hoffmann, O.; Beckmann, M.W.; Ryden, L.; et al. International pooled analysis of the prognostic impact of disseminated tumor cells from the bone marrow in early breast cancer: Results from the PADDY study. Cancer Res. 2019, 79. [Google Scholar] [CrossRef]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Wallwiener, M.; Fehm, T.N.; Hahn, M.; Walter, C.B.; Gruber, I.; Brucker, S.Y.; Taran, F.A. Disseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapse. Ann. Oncol. 2015, 26, 1155–1160. [Google Scholar] [CrossRef]
- Chen, F.; Han, Y.; Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br. J. Cancer 2021, 124, 1912–1920. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Bone marrow as a reservoir for disseminated tumor cells: A special source for liquid biopsy in cancer patients. Bonekey Rep. 2014, 3, 584. [Google Scholar] [CrossRef] [PubMed]
- Demeulemeester, J.; Kumar, P.; Moller, E.K.; Nord, S.; Wedge, D.C.; Peterson, A.; Mathiesen, R.R.; Fjelldal, R.; Zamani Esteki, M.; Theunis, K.; et al. Tracing the origin of disseminated tumor cells in breast cancer using single-cell sequencing. Genome Biol. 2016, 17, 250. [Google Scholar] [CrossRef]
- Stoecklein, N.H.; Klein, C.A. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int. J. Cancer 2010, 126, 589–598. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Taran, F.A.; Wallwiener, M.; Hahn, M.; Becker, S.; Solomayer, E.F.; Brucker, S.Y.; Fehm, T.N.; Wallwiener, D. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients—Results from a large single-centre analysis. Eur. J. Cancer 2014, 50, 2550–2559. [Google Scholar] [CrossRef]
- Walter, V.P.; Taran, F.A.; Wallwiener, M.; Hahn, M.; Brucker, S.Y.; Hartkopf, A.D. Simultaneous detection of circulating and disseminated tumor cells in primary breast cancer patients following neoadjuvant chemotherapy. Arch. Gynecol. Obstet. 2018, 297, 785–790. [Google Scholar] [CrossRef]
- Fehm, T.; Braun, S.; Muller, V.; Janni, W.; Gebauer, G.; Marth, C.; Schindlbeck, C.; Wallwiener, D.; Borgen, E.; Naume, B.; et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 2006, 107, 885–892. [Google Scholar] [CrossRef]
- Mathiesen, R.R.; Fjelldal, R.; Liestol, K.; Due, E.U.; Geigl, J.B.; Riethdorf, S.; Borgen, E.; Rye, I.H.; Schneider, I.J.; Obenauf, A.C.; et al. High-resolution analyses of copy number changes in disseminated tumor cells of patients with breast cancer. Int. J. Cancer 2012, 131, E405–E415. [Google Scholar] [CrossRef]
- Baslan, T.; Hicks, J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat. Rev. Cancer 2017, 17, 557–569. [Google Scholar] [CrossRef]
- Schindlbeck, C.; Andergassen, U.; Jueckstock, J.; Rack, B.; Janni, W.; Jeschke, U. Disseminated and circulating tumor cells in bone marrow and blood of breast cancer patients: Properties, enrichment, and potential targets. J. Cancer Res. Clin. Oncol. 2016, 142, 1883–1895. [Google Scholar] [CrossRef]
- Cackowski, F.C.; Wang, Y.; Decker, J.T.; Sifuentes, C.; Weindorf, S.; Jung, Y.; Wang, Y.; Decker, A.M.; Yumoto, K.; Szerlip, N.; et al. Detection and isolation of disseminated tumor cells in bone marrow of patients with clinically localized prostate cancer. Prostate 2019, 79, 1715–1727. [Google Scholar] [CrossRef]
- Gires, O.; Stoecklein, N.H. Dynamic EpCAM expression on circulating and disseminating tumor cells: Causes and consequences. Cell Mol. Life Sci. 2014, 71, 4393–4402. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Kumar, A.; Golani, A.; Kumar, L.D. EMT in breast cancer metastasis: An interplay of microRNAs, signaling pathways and circulating tumor cells. Front. Biosci. 2020, 25, 979–1010. [Google Scholar] [CrossRef]
- Descamps, L.; Le Roy, D.; Deman, A.L. Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 1981. [Google Scholar] [CrossRef]
- Li, C.; He, W.; Wang, N.; Xi, Z.; Deng, R.; Liu, X.; Kang, R.; Xie, L.; Liu, X. Application of Microfluidics in Detection of Circulating Tumor Cells. Front. Bioeng. Biotechnol. 2022, 10, 907232. [Google Scholar] [CrossRef]
- Dong, Y.; Skelley, A.M.; Merdek, K.D.; Sprott, K.M.; Jiang, C.; Pierceall, W.E.; Lin, J.; Stocum, M.; Carney, W.P.; Smirnov, D.A. Microfluidics and circulating tumor cells. J. Mol. Diagn. 2013, 15, 149–157. [Google Scholar] [CrossRef]
- Cohen, E.N.; Jayachandran, G.; Hardy, M.R.; Venkata Subramanian, A.M.; Meng, X.; Reuben, J.M. Antigen-agnostic microfluidics-based circulating tumor cell enrichment and downstream molecular characterization. PLoS ONE 2020, 15, e0241123. [Google Scholar] [CrossRef]
- Hvichia, G.E.; Parveen, Z.; Wagner, C.; Janning, M.; Quidde, J.; Stein, A.; Muller, V.; Loges, S.; Neves, R.P.; Stoecklein, N.H.; et al. A novel microfluidic platform for size and deformability based separation and the subsequent molecular characterization of viable circulating tumor cells. Int. J. Cancer 2016, 138, 2894–2904. [Google Scholar] [CrossRef]
- Koch, C.; Joosse, S.A.; Schneegans, S.; Wilken, O.J.W.; Janning, M.; Loreth, D.; Muller, V.; Prieske, K.; Banys-Paluchowski, M.; Horst, L.J.; et al. Pre-Analytical and Analytical Variables of Label-Independent Enrichment and Automated Detection of Circulating Tumor Cells in Cancer Patients. Cancers 2020, 12, 442. [Google Scholar] [CrossRef]
- Pillai, S.G.; Siddappa, C.M.; Ma, C.; Snider, J.; Kaushal, M.; Watson, M.A.; Aft, R. A microfluidic-based filtration system to enrich for bone marrow disseminated tumor cells from breast cancer patients. PLoS ONE 2021, 16, e0246139. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Gruber, I.; Fehm, T.; Taran, F.A.; Wallwiener, M.; Hahn, M.; Wallwiener, D.; Krawzyck, N.; Hoffmann, J.; Hartkopf, A.D. Disseminated tumor cells as a monitoring tool for adjuvant therapy in patients with primary breast cancer. Breast Cancer Res. Treat. 2014, 144, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Nejlund, S.; Smith, J.; Kraan, J.; Stender, H.; Van, M.N.; Langkjer, S.T.; Nielsen, M.T.; Soletormos, G.; Hillig, T. Cryopreservation of Circulating Tumor Cells for Enumeration and Characterization. Biopreserv. Biobank. 2016, 14, 330–337. [Google Scholar] [CrossRef]
- Brungs, D.; Lynch, D.; Luk, A.W.; Minaei, E.; Ranson, M.; Aghmesheh, M.; Vine, K.L.; Carolan, M.; Jaber, M.; de Souza, P.; et al. Cryopreservation for delayed circulating tumor cell isolation is a valid strategy for prognostic association of circulating tumor cells in gastroesophageal cancer. World J. Gastroenterol. 2018, 24, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Rodriguez, E.; Diaz-Jimenez, N.; Ruiz-Rabelo, J.; Gomez-Luque, I.; Bascunana-Estudillo, G.; Rioja-Torres, P.; Torres-Lorite, M.; Ciria-Bru, R.; Alvarez-Benito, M.; Briceno-Delgado, J. Factors Associated with Disease Recurrence in Breast Cancer Patients with Negative Sentinel Lymph Node Biopsy. Clin. Breast Cancer 2016, 16, e181–e186. [Google Scholar] [CrossRef]
- Cianfrocca, M.; Goldstein, L.J. Prognostic and predictive factors in early-stage breast cancer. Oncologist 2004, 9, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; Russell, N.M.; Barron, S.; Wang, C.A.; Loughman, T.; Dynoodt, P.; Fender, B.; Lopez-Ruiz, C.; O’Grady, A.; Sheehan, K.M.; et al. Prognostic value of the 6-gene OncoMasTR test in hormone receptor-positive HER2-negative early-stage breast cancer: Comparative analysis with standard clinicopathological factors. Eur. J. Cancer 2021, 152, 78–89. [Google Scholar] [CrossRef]
- Cognetti, F.; Biganzoli, L.; De Placido, S.; Del Mastro, L.; Masetti, R.; Naso, G.; Pruneri, G.; Santini, D.; Tondini, C.A.; Tinterri, C.; et al. Multigene tests for breast cancer: The physician’s perspective. Oncotarget 2021, 12, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Weidele, K.; Stojanovic, N.; Feliciello, G.; Markiewicz, A.; Scheitler, S.; Alberter, B.; Renner, P.; Haferkamp, S.; Klein, C.A.; Polzer, B. Microfluidic enrichment, isolation and characterization of disseminated melanoma cells from lymph node samples. Int. J. Cancer 2019, 145, 232–241. [Google Scholar] [CrossRef]
- Kitz, J.; Goodale, D.; Postenka, C.; Lowes, L.E.; Allan, A.L. EMT-independent detection of circulating tumor cells in human blood samples and pre-clinical mouse models of metastasis. Clin. Exp. Metast. 2021, 38, 97–108. [Google Scholar] [CrossRef]
- Islam, M.; Mezencev, R.; McFarland, B.; Brink, H.; Campbell, B.; Tasadduq, B.; Waller, E.K.; Lam, W.; Alexeev, A.; Sulchek, T. Microfluidic cell sorting by stiffness to examine heterogenic responses of cancer cells to chemotherapy. Cell Death Dis. 2018, 9, 239. [Google Scholar] [CrossRef]
- Jia, Y.; Shen, P.; Yan, T.; Zhou, W.; Sun, J.; Han, X. Microfluidic Tandem Mechanical Sorting System for Enhanced Cancer Stem Cell Isolation and Ingredient Screening. Adv. Healthc. Mater. 2021, 10, e2100985. [Google Scholar] [CrossRef]
- Shields, C.W.t.; Reyes, C.D.; Lopez, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef]
- Leitao, T.P.; Corredeira, P.; Kucharczak, S.; Rodrigues, M.; Piairo, P.; Rodrigues, C.; Alves, P.; Cavaco, A.M.; Miranda, M.; Antunes, M.; et al. Clinical Validation of a Size-Based Microfluidic Device for Circulating Tumor Cell Isolation and Analysis in Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 8404. [Google Scholar] [CrossRef]
- Lee, Y.; Guan, G.; Bhagat, A.A. ClearCell(R) FX, a label-free microfluidics technology for enrichment of viable circulating tumor cells. Cytometry A 2018, 93, 1251–1254. [Google Scholar] [CrossRef]
- Sollier-Christen, E.; Renier, C.; Kaplan, T.; Kfir, E.; Crouse, S.C. VTX-1 Liquid Biopsy System for Fully-Automated and Label-Free Isolation of Circulating Tumor Cells with Automated Enumeration by BioView Platform. Cytometry A 2018, 93, 1240–1245. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, F.; Pan, X.; Wang, G.; Zhu, L.; Zhang, J.; Wen, D.; Lu, S. Xenograft tumors derived from malignant pleural effusion of the patients with non-small-cell lung cancer as models to explore drug resistance. Cancer Commun. 2018, 38, 19. [Google Scholar] [CrossRef] [PubMed]
- Addison, J.B.; Voronkova, M.A.; Fugett, J.H.; Lin, C.C.; Linville, N.C.; Trinh, B.; Livengood, R.H.; Smolkin, M.B.; Schaller, M.D.; Ruppert, J.M.; et al. Functional Hierarchy and Cooperation of EMT Master Transcription Factors in Breast Cancer Metastasis. Mol. Cancer Res. 2021, 19, 784–798. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Mader, S.; Pantel, K. Epithelial-mesenchymal plasticity in circulating tumor cells. J. Mol. Med. 2017, 95, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bednarz-Knoll, N.; Alix-Panabieres, C.; Pantel, K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metast. Rev. 2012, 31, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Franken, A.; Driemel, C.; Behrens, B.; Meier-Stiegen, F.; Endris, V.; Stenzinger, A.; Niederacher, D.; Fischer, J.C.; Stoecklein, N.H.; Ruckhaeberle, E.; et al. Label-Free Enrichment and Molecular Characterization of Viable Circulating Tumor Cells from Diagnostic Leukapheresis Products. Clin. Chem. 2019, 65, 549–558. [Google Scholar] [CrossRef]
- Zhao, N.; Powell, R.T.; Yuan, X.; Bae, G.; Roarty, K.P.; Stossi, F.; Strempfl, M.; Toneff, M.J.; Johnson, H.L.; Mani, S.A.; et al. Morphological screening of mesenchymal mammary tumor organoids to identify drugs that reverse epithelial-mesenchymal transition. Nat. Commun. 2021, 12, 4262. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]


| Experiment 1 | Experiment 2 | Experiment 3 | |
|---|---|---|---|
| Spiked cells | 124 | 163 | 188 |
| Harvested cells | 90 | 83 | 110 |
| % Harvested cells | 72.6% | 50.9% | 58.5% |
| Sample ID | CK-Classification * | Expected Number of DTCs ** | Number of DTCs Detected |
|---|---|---|---|
| DTC #01 | 1+ | 160 | 125 |
| DTC #02 | 2+ | 100 | 87 |
| DTC #03 | 2+ | 50 | 83 |
| DTC #04 | 2+ | 20 | 17 |
| Sample Classification | No. of Patient Samples (%) | Number of Tumor Cells in 3.0 × 106 Total Cells |
|---|---|---|
| DTC positive | 361 (100.0) | ≥1 |
| CK 1+ | 306 (84.7) | 1–3 |
| CK 2+ | 46 (12.7) | 4–7 |
| CK 3+ | 9 (2.5) | 8–10 |
| Sample Classification | Median Number of Cells Expected after Standard Method (n) | Median Number of Cells Expected after Parsortix *, 95% CI |
|---|---|---|
| CK 1+ | 2 | 204 (83–794) |
| CK 2+ | 5.5 | 528 (190–2073) |
| CK 3+ | 9 | 737 (405–1925) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volmer, L.L.; Önder, C.E.; Volz, B.; Singh, A.R.; Brucker, S.Y.; Engler, T.; Hartkopf, A.D.; Koch, A. Microfluidic Isolation of Disseminated Tumor Cells from the Bone Marrow of Breast Cancer Patients. Int. J. Mol. Sci. 2023, 24, 13930. https://doi.org/10.3390/ijms241813930
Volmer LL, Önder CE, Volz B, Singh AR, Brucker SY, Engler T, Hartkopf AD, Koch A. Microfluidic Isolation of Disseminated Tumor Cells from the Bone Marrow of Breast Cancer Patients. International Journal of Molecular Sciences. 2023; 24(18):13930. https://doi.org/10.3390/ijms241813930
Chicago/Turabian StyleVolmer, Léa L., Cansu E. Önder, Barbara Volz, Anjali R. Singh, Sara Y. Brucker, Tobias Engler, Andreas D. Hartkopf, and André Koch. 2023. "Microfluidic Isolation of Disseminated Tumor Cells from the Bone Marrow of Breast Cancer Patients" International Journal of Molecular Sciences 24, no. 18: 13930. https://doi.org/10.3390/ijms241813930
APA StyleVolmer, L. L., Önder, C. E., Volz, B., Singh, A. R., Brucker, S. Y., Engler, T., Hartkopf, A. D., & Koch, A. (2023). Microfluidic Isolation of Disseminated Tumor Cells from the Bone Marrow of Breast Cancer Patients. International Journal of Molecular Sciences, 24(18), 13930. https://doi.org/10.3390/ijms241813930





