A Novel Probiotic-Based Oral Vaccine against SARS-CoV-2 Omicron Variant B.1.1.529
Abstract
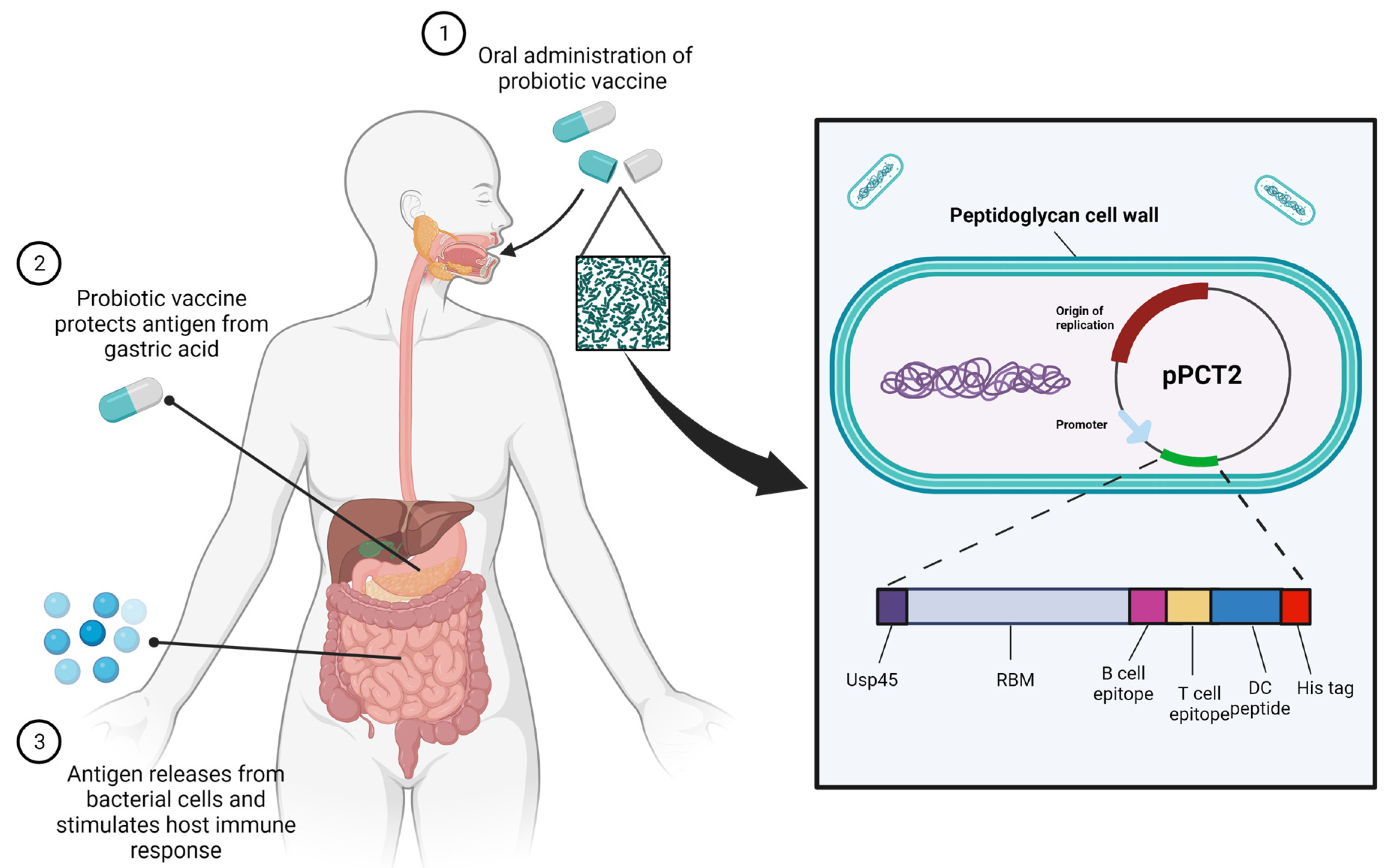
:1. Introduction
2. Results
2.1. Concept of Study and Construction of OMGVac
2.2. Validation of Recombinant Protein Expression and Plasmid Stability
2.3. OMGVac-Induced Immune Response after Two-Dose Regimen
2.4. Safe Administration of OMGVac Based on Histo- and Physio-Pathological Finding
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Animals
4.2. Visualization and Statistical Analysis
4.3. Construction of pPCT2-Usp45-RBM-B-T-DC and OMGVac
4.4. Growth Kinetics, Protein Expression, and Plasmid Stability of OMGVac
4.4.1. Growth Kinetics, Protein Extraction, and Western Blot Analysis
4.4.2. Plasmid Stability Test of OMGVac
4.5. In Vivo Study of OMGVac on Golden Syrian Hamsters
4.5.1. Bacteria Preparation
4.5.2. Immunization and Sample Collection
4.5.3. SARS-CoV-2 SpikeRBD Omicron B.1.1.529 Strain-Specific Serum IgG and IgA ELISA
4.5.4. Hamster Alanine Aminotransferase 1 (ALT1) and Glutamate Oxaloacetate Transaminase 1 (GOT1) ELISA
4.5.5. Physio- and Histo-Pathological Examination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef]
- Dhar Chowdhury, S.A.-O.; Oommen, A.M. Epidemiology of COVID-19. Dig. Endosc. 2020, 11, 3–7. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 14 June 2023).
- Division of Viral Diseases under the National Center for Immunization and Respiratory Diseases (NCIRD), SARS-CoV-2 Variant Classifications and Definitions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 14 June 2023).
- Manjunath, R.; Gaonkar, S.L.; Saleh, E.A.M.; Husain, K. A comprehensive review on COVID-19 Omicron (B.1.1.529) variant. Saudi J. Biol. Sci. 2022, 29, 103372. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines with WHO Emergency Use Listing. Available online: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (accessed on 29 June 2023).
- Dehghani, J.; Movafeghi, A.; Mathieu-Rivet, E.; Mati-Baouche, N.; Calbo, S.; Lerouge, P.; Bardor, M. Microalgae as an Efficient Vehicle for the Production and Targeted Delivery of Therapeutic Glycoproteins against SARS-CoV-2 Variants. Mar. Drugs 2022, 20, 657. [Google Scholar] [CrossRef]
- Kiefer, A.M.; Niemeyer, J.; Probst, A.; Erkel, G.; Schroda, M. Production and secretion of functional SARS-CoV-2 spike protein in Chlamydomonas reinhardtii. Front. Plant Sci. 2022, 13, 988870. [Google Scholar] [CrossRef]
- Landete, J.M. A review of food-grade vectors in lactic acid bacteria: From the laboratory to their application. Crit. Rev. Biotechnol. 2017, 37, 296–308. [Google Scholar] [CrossRef]
- Adiyoga, R.; Arief, I.I.; Budiman, C.; Abidin, Z. In vitro anticancer potentials of Lactobacillus plantarum IIA-1A5 and Lactobacillus acidophilus IIA-2B4 extracts against WiDr human colon cancer cell line. Food Sci. Technol. 2022, 42, e87221. [Google Scholar] [CrossRef]
- Yeo, S.; Park, H.; Seo, E.; Kim, J.; Kim, B.K.; Choi, I.S.; Huh, C.S. Anti-Inflammatory and Gut Microbiota Modulatory Effect of Lactobacillus rhamnosus Strain LDTM 7511 in a Dextran Sulfate Sodium-Induced Colitis Murine Model. Microorganisms 2020, 8, 845. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Z.; Zhang, Y.; Pan, L. Lactic acid bacteria as mucosal delivery vehicles: A realistic therapeutic option. Appl. Microbiol. Biotechnol. 2016, 100, 5691–5701. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Angulo, C.; Meza, B. Food-Grade Organisms as Vaccine Biofactories and Oral Delivery Vehicles. Trends Biotechnol. 2016, 34, 124–136. [Google Scholar] [CrossRef]
- Moradi-kalbolandi, S.; Majidzadeh, A.K.; Abdolvahab, M.H.; Jalili, N.; Farahmand, L. The Role of Mucosal Immunity and Recombinant Probiotics in SARS-CoV2 Vaccine Development. Probiotics Antimicrob. Proteins 2021, 13, 1239–1253. [Google Scholar]
- Taghinezhad, S.S.; Mohseni, A.H.; Bermúdez-Humarán, L.G.; Casolaro, V.; Cortes-Perez, N.G.; Keyvani, H.; Simal-Gandara, J. Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease. Vaccines 2021, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, L.; Guo, Y.; Li, X.; Ma, L.; Sun, R.; Han, X.; Liu, J.; Huang, J. Oral SARS-CoV-2 Spike Protein Recombinant Yeast Candidate Prompts Specific Antibody and Gut Microbiota Reconstruction in Mice. Front. Microbiol. 2022, 13, 792532. [Google Scholar] [CrossRef]
- Wang, M.; Fu, T.; Hao, J.; Li, L.; Tian, M.; Jin, N.; Ren, L.; Li, C. A Recombinant Lactobacillus Plantarum Strain Expressing the Spike Protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020, 160, 736. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The Role of Cell Surface Architecture of Lactobacilli in Host-Microbe Interactions in the Gastrointestinal Tract. Mediat. Inflamm. 2013, 2013, 237921. [Google Scholar] [CrossRef] [PubMed]
- Shida, K.; Kiyoshima-Shibata, J.; Nagaoka, M.; Watanabe, K.; Nanno, M. Induction of Interleukin-12 by Lactobacillus Strains Having a Rigid Cell Wall Resistant to Intracellular Digestion. J. Dairy Sci. 2006, 89, 3306–3317. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. FAO/WHO Guidance to Governments on the Application of HACCP in Small And/or Less-Developed Food Businesses. In FAO and Food Nutrition Paper; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; Volume 86, pp. 1–74. [Google Scholar]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef]
- Cano-Garrido, O.; Seras-Franzoso, J.; Garcia-Fruitós, E. Lactic acid bacteria: Reviewing the potential of a promising delivery live vector for biomedical purposes. Microb. Cell Factories 2015, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- del Rio, B.; Redruello, B.; Fernandez, M.; Cruz Martin, M.; Ladero, V.; Alvarez, M.A. Lactic Acid Bacteria as a Live Delivery System for the in situ Production of Nanobodies in the Human Gastrointestinal Tract. Front. Microbiol. 2019, 9, 3179. [Google Scholar] [CrossRef]
- Vesa, T.; Pochart, P.; Marteau, P. Pharmacokinetics of Lactobacillus Plantarum NCIMB 8826, Lactobacillus Fermentum KLD, and Lactococcus Lactis MG 1363 in the Human Gastrointestinal Tract. Aliment. Pharmacol. Ther. 2000, 14, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Szatraj, K.; Szczepankowska, A.K.; Chmielewska-Jeznach, M. Lactic acid bacteria—Promising vaccine vectors: Possibilities, limitations, doubts. J. Appl. Microbiol. 2017, 123, 325–339. [Google Scholar] [CrossRef]
- Yadav, A.K.; Espaillat, A.; Cava, F. Bacterial Strategies to Preserve Cell Wall Integrity against Environmental Threats. Front. Microbiol. 2018, 9, 2064. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Yang, G.L.; Yang, X.; Shonyela, S.M.; Zhao, L.; Jiang, Y.L.; Huang, H.B.; Shi, C.W.; Wang, J.Z.; Wang, G.; et al. Recombinant Lactobacillus plantarum expressing HA2 antigen elicits protective immunity against H9N2 avian influenza virus in chickens. Appl. Microbiol. Biotechnol. 2017, 101, 8475–8484. [Google Scholar] [CrossRef]
- Jee, P.-F.; Tiong, V.; Shu, M.-H.; Khoo, J.-J.; Wong, W.F.; Rahim, R.A.; AbuBakar, S.; Chang, L.-Y. Oral immunization of a non-recombinant Lactococcus lactis surface displaying influenza hemagglutinin 1 (HA1) induces mucosal immunity in mice. PLoS ONE 2017, 12, e0187718. [Google Scholar] [CrossRef]
- Lee, J.-S.; Poo, H.; Han, D.P.; Hong, S.-P.; Kim, K.; Cho, M.W.; Kim, E.; Sung, M.-H.; Kim, C.-J. Mucosal Immunization with Surface-Displayed Severe Acute Respiratory Syndrome Coronavirus Spike Protein on Lactobacillus casei Induces Neutralizing Antibodies in Mice. J. Virol. 2006, 80, 4079–4087. [Google Scholar] [CrossRef]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.J.; Lee, Y.-K.; Cho, S. Oral Vaccine Delivery for Intestinal Immunity—Biological Basis, Barriers, Delivery System, and M Cell Targeting. Polymers 2018, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.-T.; Daniell, H. Plant-Made Oral Vaccines against Human Infectious diseases—Are We There Yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA Dominates the Early Neutralizing Antibody Response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Yoshida, R.; Yokoyama, A.; Miyamoto, H.; Kajihara, M.; Maruyama, J.; Nao, N.; Manzoor, R.; Takada, A. Comparison of Antiviral Activity between IgA and IgG Specific to Influenza Virus Hemagglutinin: Increased Potential of IgA for Heterosubtypic Immunity. PLoS ONE 2014, 9, e85582. [Google Scholar] [CrossRef]
- Zhuang, Z.; Lai, X.; Sun, J.; Chen, Z.; Zhang, Z.; Dai, J.; Liu, D.; Li, Y.; Li, F.; Wang, Y.; et al. Mapping and role of T cell response in SARS-CoV-2–infected mice. J. Exp. Med. 2021, 218, e20202187. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; et al. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb. Cell Factories 2018, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Ma, C.; Yang, X.; Pan, X.; Li, G.; Ma, D. Oral Immunization of Recombinant Lactococcus lactis and Enterococcus faecalis Expressing Dendritic Cell Targeting Peptide and Hexon Protein of Fowl Adenovirus 4 Induces Protective Immunity Against Homologous Infection. Front. Veter. Sci. 2021, 8, 632218. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Duong, T.; Sandwick, S.J.; Hoover, T.; Klaenhammer, T.R. Dendritic Cell Targeting of Bacillus Anthracis Protective Antigen Expressed by Lactobacillus Acidophilus Protects Mice from Lethal Challenge. Proc. Natl. Acad. Sci. USA 2009, 106, 4331–4336. [Google Scholar] [CrossRef]
- Ou, B.; Yang, Y.; Lv, H.; Lin, X.; Zhang, M. Current Progress and Challenges in the Study of Adjuvants for Oral Vaccines. BioDrugs 2023, 37, 143–180. [Google Scholar] [CrossRef]
- Damas, J.; Hughes, G.M.; Keough, K.C.; Painter, C.A.; Persky, N.S.; Corbo, M.; Hiller, M.; Koepfli, K.-P.; Pfenning, A.R.; Zhao, H.; et al. Broad Host Range of SARS-CoV-2 Predicted by Comparative and Structural Analysis of ACE2 in Vertebrates. Proc. Natl. Acad. Sci. USA 2020, 117, 22311–22322. [Google Scholar] [CrossRef]
- Parolin, C.; Virtuoso, S.; Giovanetti, M.; Angeletti, S.; Ciccozzi, M.; Borsetti, A. Animal Hosts and Experimental Models of SARS-CoV-2 Infection. Chemotherapy 2021, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian Hamsters as a Small Animal Model for SARS-CoV-2 Infection and Countermeasure Development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, L.; Liu, S.; Wang, Q.; Zhang, D.; Xu, Z.; Han, P.; Li, X.; Peng, Q.; Su, C.; et al. Receptor Binding and Complex Structures of Human ACE2 to Spike RBD from Omicron and Delta SARS-CoV-2. Cell 2022, 185, 630–640.e10. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Raza, S.; Zohaib, A.; Riaz, M.I.; Amin, A.; Awais, M.; Khan, S.U.; Ijaz Khan, M.; Chu, Y.-M. Immunoinformatics Based Prediction of Recombinant Multi-Epitope Vaccine for the Control and Prevention of SARS-CoV-2. Alex. Eng. J. 2021, 60, 3087–3097. [Google Scholar] [CrossRef]
- Jain, R.; Jain, A.; Verma, S.K. Prediction of Epitope based Peptides for Vaccine Development from Complete Proteome of Novel Corona Virus (SARS-COV-2) Using Immunoinformatics. Int. J. Pept. Res. Ther. 2021, 27, 1729–1740. [Google Scholar] [CrossRef]
- Curiel, T.J.; Morris, C.; Brumlik, M.; Landry, S.J.; Finstad, K.; Nelson, A.; Joshi, V.; Hawkins, C.; Alarez, X.; Lackner, A.; et al. Peptides Identified through Phage Display Direct Immunogenic Antigen to Dendritic Cells. J. Immunol. 2004, 172, 7425–7431. [Google Scholar] [CrossRef] [PubMed]
- Welker, D.L.; Hughes, J.E.; Steele, J.L.; Broadbent, J.R. High efficiency electrotransformation of Lactobacillus casei. FEMS Microbiol. Lett. 2015, 362, 1–6. [Google Scholar] [CrossRef]
- Ohgane, k.; Yoshioka, H. Quantification of Gel Bands by an Image J Macro, Band/Peak Quantification Tool. Protocols Io 2019. [Google Scholar] [CrossRef]
- Shete, A.; Mohandas, S.; Jain, R.; Yadav, P.D. A Qualitative IgG ELISA for Detection of SARS-CoV-2-Specific Antibodies in Syrian Hamster Serum Samples. STAR Protoc. 2021, 2, 100573. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chau, E.C.T.; Kwong, T.C.; Pang, C.K.; Chan, L.T.; Chan, A.M.L.; Yao, X.; Tam, J.S.L.; Chan, S.W.; Leung, G.P.H.; Tai, W.C.S.; et al. A Novel Probiotic-Based Oral Vaccine against SARS-CoV-2 Omicron Variant B.1.1.529. Int. J. Mol. Sci. 2023, 24, 13931. https://doi.org/10.3390/ijms241813931
Chau ECT, Kwong TC, Pang CK, Chan LT, Chan AML, Yao X, Tam JSL, Chan SW, Leung GPH, Tai WCS, et al. A Novel Probiotic-Based Oral Vaccine against SARS-CoV-2 Omicron Variant B.1.1.529. International Journal of Molecular Sciences. 2023; 24(18):13931. https://doi.org/10.3390/ijms241813931
Chicago/Turabian StyleChau, Eddie Chung Ting, Tsz Ching Kwong, Chun Keung Pang, Lee Tung Chan, Andrew Man Lok Chan, Xiaoqiang Yao, John Siu Lun Tam, Shun Wan Chan, George Pak Heng Leung, William Chi Shing Tai, and et al. 2023. "A Novel Probiotic-Based Oral Vaccine against SARS-CoV-2 Omicron Variant B.1.1.529" International Journal of Molecular Sciences 24, no. 18: 13931. https://doi.org/10.3390/ijms241813931
APA StyleChau, E. C. T., Kwong, T. C., Pang, C. K., Chan, L. T., Chan, A. M. L., Yao, X., Tam, J. S. L., Chan, S. W., Leung, G. P. H., Tai, W. C. S., & Kwan, Y. W. (2023). A Novel Probiotic-Based Oral Vaccine against SARS-CoV-2 Omicron Variant B.1.1.529. International Journal of Molecular Sciences, 24(18), 13931. https://doi.org/10.3390/ijms241813931







