Flagellin-Induced Immune Response in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
:1. Introduction
2. Results
2.1. Generation of HiPSC-CM
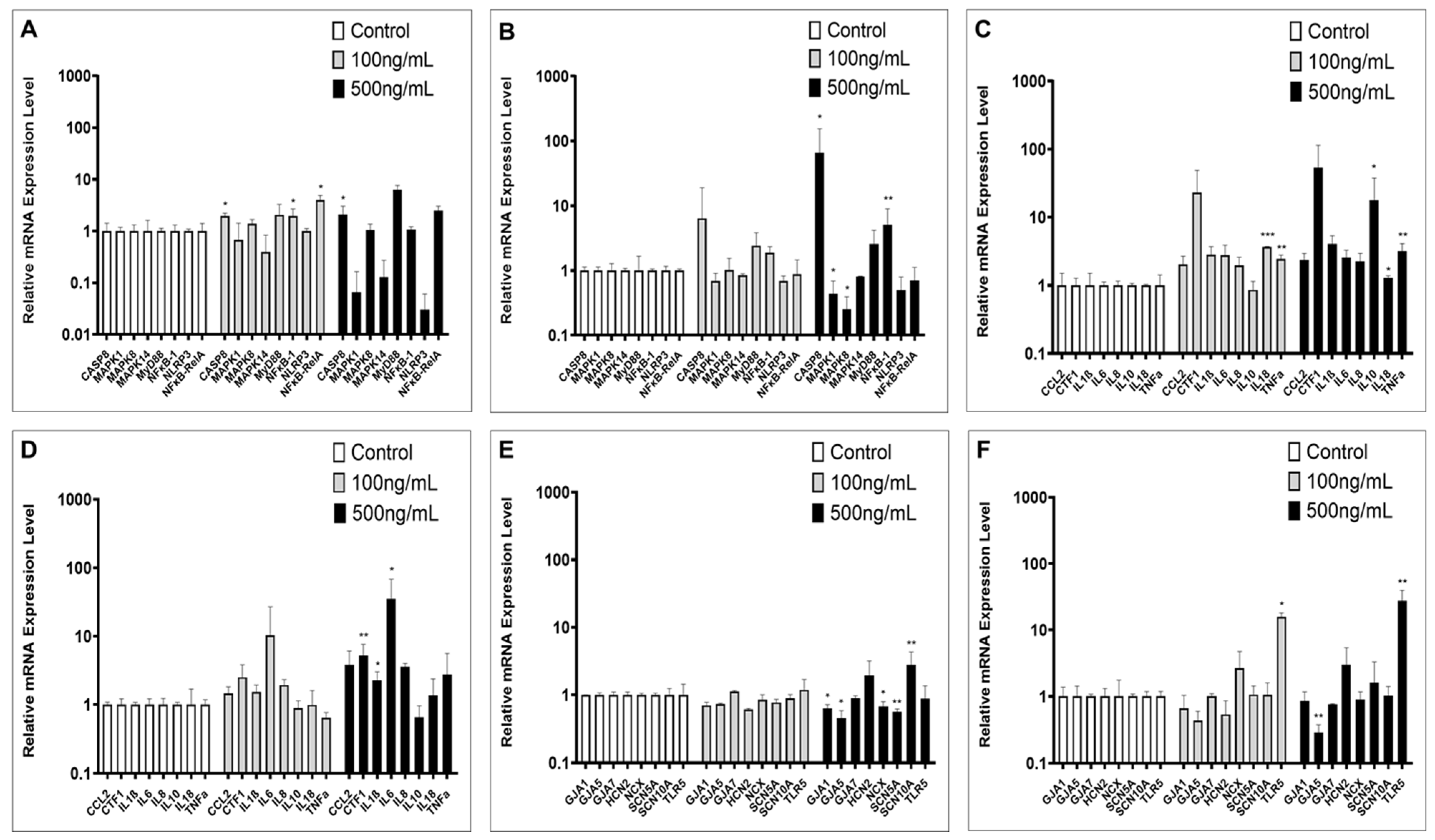
2.2. Inflammation-Associated Gene Expression
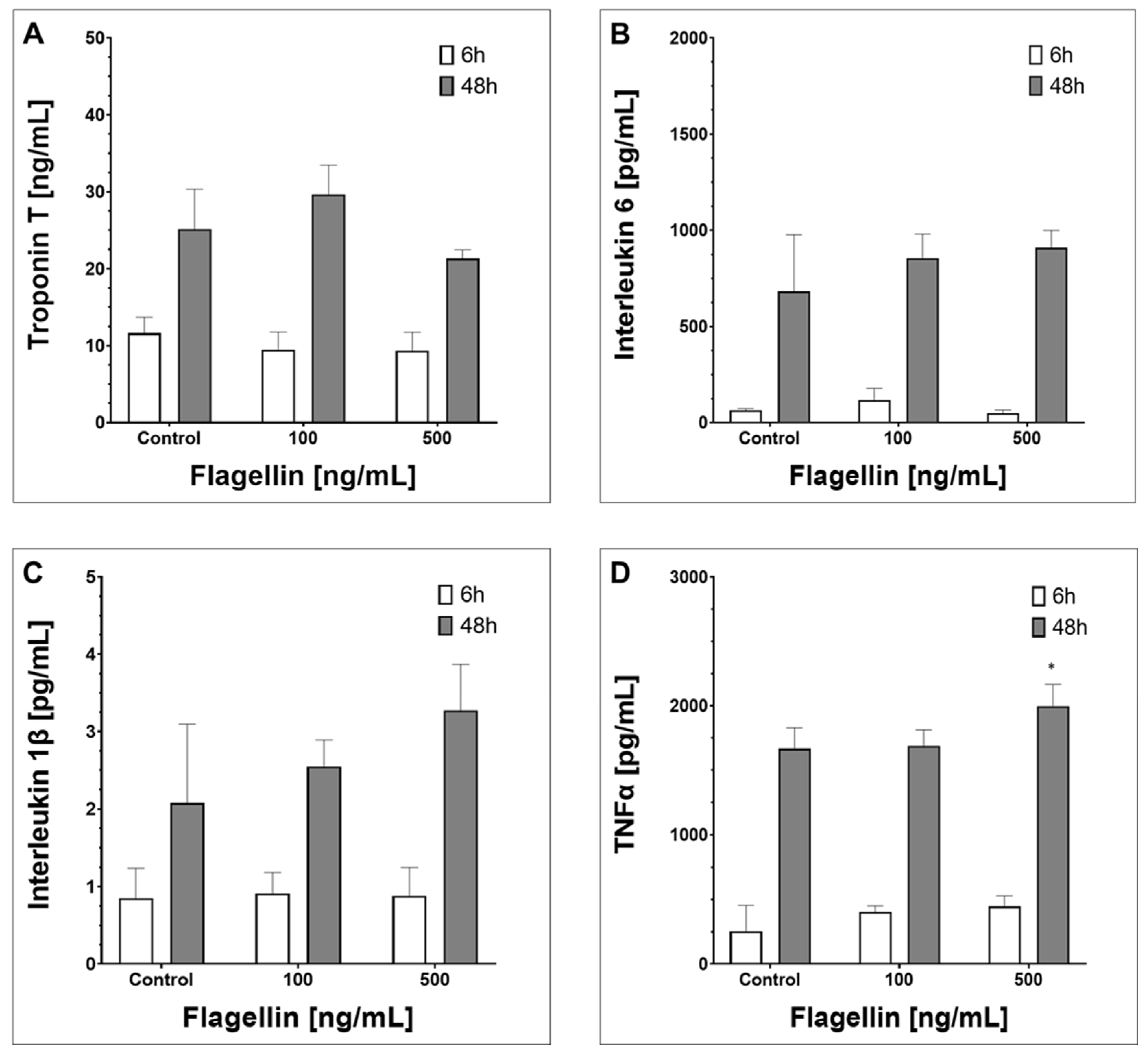
2.3. Supernatant Composition
2.4. Surface and Intracellular Protein Levels and ROS Induction
3. Material and Methods
3.1. Ethics Statement
3.2. Cell Culture with hiPSC and hiPSC-CMs
3.3. Inflammatory Studies
3.4. Polymerase Chain Reaction
3.5. ELISA
3.6. Immunohistochemistry and Fluorescence-Activated Cell Sorting
3.7. Statistical Analysis
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [CrossRef]
- Frantz, S.; Kobzik, L.; Kim, Y.D.; Fukazawa, R.; Medzhitov, R.; Lee, R.T.; Kelly, R.A. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J. Clin. Invest. 1999, 104, 271–280. [Google Scholar] [CrossRef]
- Boyd, J.H.; Mathur, S.; Wang, Y.; Bateman, R.M.; Walley, K.R. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc. Res. 2006, 72, 384–393. [Google Scholar] [CrossRef]
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest 2019, 155, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Veronese, G.; Cipriani, M.; Moroni, F.; Garascia, A.; Brambatti, M.; Adler, E.D.; Frigerio, M. Acute and Fulminant Myocarditis: A Pragmatic Clinical Approach to Diagnosis and Treatment. Curr. Cardiol. Rep. 2018, 20, 114. [Google Scholar] [CrossRef]
- Dickson, K.; Lehmann, C. Inflammatory Response to Different Toxins in Experimental Sepsis Models. Int. J. Mol. Sci. 2019, 20, 4341. [Google Scholar] [CrossRef] [PubMed]
- Liaudet, L.; Szabó, C.; Evgenov, O.V.; Murthy, K.G.; Pacher, P.; Virág, L.; Mabley, J.G.; Marton, A.; Soriano, F.G.; Kirov, M.Y.; et al. Flagellin from gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock 2003, 19, 131–137. [Google Scholar] [CrossRef]
- Frantz, S.; Ertl, G.; Bauersachs, J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 444–454. [Google Scholar] [CrossRef]
- Abram, D.; Mitchen, J.R.; Koffler, H.; Vatter, A.E. Differentiation within the bacterial flagellum and isolation of the proximal hook. J. Bacteriol. 1970, 101, 250–261. [Google Scholar] [CrossRef]
- Percy, M.G.; Gründling, A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Vázquez-Cervantes, G.I.; Pedraza-Chaverri, J.; Gutiérrez-Venegas, G. Lipoteichoic acid reduces antioxidant enzymes in H9c2 cells. Toxicol. Rep. 2020, 7, 101–108. [Google Scholar] [CrossRef]
- Hobai, I.A.; Morse, J.C.; Siwik, D.A.; Colucci, W.S. Lipopolysaccharide and cytokines inhibit rat cardiomyocyte contractility in vitro. J. Surg. Res. 2015, 193, 888–901. [Google Scholar] [CrossRef]
- Mutig, N.; Geers-Knoerr, C.; Piep, B.; Pahuja, A.; Vogt, P.M.; Brenner, B.; Niederbichler, A.D.; Kraft, T. Lipoteichoic acid from Staphylococcus aureus directly affects cardiomyocyte contractility and calcium transients. Mol. Immunol. 2013, 56, 720–728. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yamanaka, S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ. Res. 2017, 120, 1958–1968. [Google Scholar] [CrossRef]
- Yucel, G.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Lang, S.; Li, X.; Buljubasic, F.; Zimmermann, W.H.; Cyganek, L.; Utikal, J.; et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017, 7, 2935. [Google Scholar] [CrossRef] [PubMed]
- Maherali, N.; Ahfeldt, T.; Rigamonti, A.; Utikal, J.; Cowan, C.; Hochedlinger, K. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 2008, 3, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Larribere, L.; Wu, H.; Novak, D.; Galach, M.; Bernhardt, M.; Orouji, E.; Weina, K.; Knappe, N.; Sachpekidis, C.; Umansky, L.; et al. NF1 loss induces senescence during human melanocyte differentiation in an iPSC-based model. Pigment Cell Melanoma Res. 2015, 28, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rössler, U.; Hennig, A.F.; Stelzer, N.; Bose, S.; Kopp, J.; Søe, K.; Cyganek, L.; Zifarelli, G.; Ali, S.; von der Hagen, M.; et al. Efficient generation of osteoclasts from human induced pluripotent stem cells and functional investigations of lethal CLCN7-related osteopetrosis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2021, 36, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Tiburcy, M.; Zimmermann, W.H. Modeling myocardial growth and hypertrophy in engineered heart muscle. Trends Cardiovasc. Med. 2014, 24, 7–13. [Google Scholar] [CrossRef]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Huang, M.; Fan, X.; Yang, Z.; Cyganek, L.; Li, X.; Yuecel, G.; Lan, H.; Li, Y.; Wendel, A.; Lang, S.; et al. Alpha 1-adrenoceptor signalling contributes to toxic effects of catecholamine on electrical properties in cardiomyocytes. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2021, 23, 1137–1148. [Google Scholar] [CrossRef]
- Eaves-Pyles, T.; Murthy, K.; Liaudet, L.; Virág, L.; Ross, G.; Soriano, F.G.; Szabó, C.; Salzman, A.L. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 2001, 166, 1248–1260. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Honko, A.N.; Mizel, S.B. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 2005, 33, 83–101. [Google Scholar] [CrossRef]
- Rolli, J.; Rosenblatt-Velin, N.; Li, J.; Loukili, N.; Levrand, S.; Pacher, P.; Waeber, B.; Feihl, F.; Ruchat, P.; Liaudet, L. Bacterial flagellin triggers cardiac innate immune responses and acute contractile dysfunction. PLoS ONE 2010, 5, e12687. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Z.F.; Liao, H.H.; Liu, W.; Liu, J.; Ma, Z.G.; Wu, Q.Q.; Xu, M.; Zhang, N.; Zhang, Y.; et al. Toll-like receptor 5 deficiency attenuates interstitial cardiac fibrosis and dysfunction induced by pressure overload by inhibiting inflammation and the endothelial-mesenchymal transition. Biochim. Et Biophys. Acta 2015, 1852, 2456–2466. [Google Scholar] [CrossRef]
- Fu, G.; Wang, B.; He, B.; Feng, M.; Yu, Y. LPS induces cardiomyocyte necroptosis through the Ripk3/Pgam5 signaling pathway. J. Recept. Signal Transduct. Res. 2021, 41, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ralph, P.; Nakoinz, I.; Sampson-Johannes, A.; Fong, S.; Lowe, D.; Min, H.Y.; Lin, L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J. Immunol. 1992, 148, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Damås, J.K.; Aukrust, P.; Ueland, T.; Odegaard, A.; Eiken, H.G.; Gullestad, L.; Sejersted, O.M.; Christensen, G. Monocyte chemoattractant protein-1 enhances and interleukin-10 suppresses the production of inflammatory cytokines in adult rat cardiomyocytes. Basic Res. Cardiol. 2001, 96, 345–352. [Google Scholar] [CrossRef]
- Kusunoki, T.; Hailman, E.; Juan, T.S.; Lichenstein, H.S.; Wright, S.D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J. Exp. Med. 1995, 182, 1673–1682. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, J.H.; Martin, M.; Michalek, S.M.; Nahm, M.H. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 2003, 71, 5541–5548. [Google Scholar] [CrossRef]
- Morath, S.; Geyer, A.; Hartung, T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 2001, 193, 393–397. [Google Scholar] [CrossRef]
- Ihnatovych, I.; Birkaya, B.; Notari, E.; Szigeti, K. iPSC-Derived Microglia for Modeling Human-Specific DAMP and PAMP Responses in the Context of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 9668. [Google Scholar] [CrossRef]
- Camelliti, P.; Green, C.R.; Kohl, P. Structural and functional coupling of cardiac myocytes and fibroblasts. Adv. Cardiol. 2006, 42, 132–149. [Google Scholar] [CrossRef]
- Dewing, J.M.; Saunders, V.; O’Kelly, I.; Wilson, D.I. Defining cardiac cell populations and relative cellular composition of the early fetal human heart. PLoS ONE 2022, 17, e0259477. [Google Scholar] [CrossRef]
- Zuppinger, C.; Gibbons, G.; Dutta-Passecker, P.; Segiser, A.; Most, H.; Suter, T.M. Characterization of cytoskeleton features and maturation status of cultured human iPSC-derived cardiomyocytes. Eur. J. Histochem. EJH 2017, 61, 2763. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, T.J.; Antos, C.L.; Guan, K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int. J. Cardiol. 2017, 241, 379–386. [Google Scholar] [CrossRef]
- Földes, G.; Liu, A.; Badiger, R.; Paul-Clark, M.; Moreno, L.; Lendvai, Z.; Wright, J.S.; Ali, N.N.; Harding, S.E.; Mitchell, J.A. Innate immunity in human embryonic stem cells: Comparison with adult human endothelial cells. PLoS ONE 2010, 5, e10501. [Google Scholar] [CrossRef]
- Michelsen, K.S.; Aicher, A.; Mohaupt, M.; Hartung, T.; Dimmeler, S.; Kirschning, C.J.; Schumann, R.R. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 2001, 276, 25680–25686. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Moore, X.L.; Dart, A.M.; Wang, L.M. Systemic inflammatory response following acute myocardial infarction. J. Geriatr. Cardiol. JGC 2015, 12, 305–312. [Google Scholar] [CrossRef]
- Ma, Z.G.; Kong, C.Y.; Wu, H.M.; Song, P.; Zhang, X.; Yuan, Y.P.; Deng, W.; Tang, Q.Z. Toll-like receptor 5 deficiency diminishes doxorubicin-induced acute cardiotoxicity in mice. Theranostics 2020, 10, 11013–11025. [Google Scholar] [CrossRef]
- Klockner, U.; Rueckschloss, U.; Grossmann, C.; Ebelt, H.; Muller-Werdan, U.; Loppnow, H.; Werdan, K.; Gekle, M. Differential reduction of HCN channel activity by various types of lipopolysaccharide. J. Mol. Cell. Cardiol. 2011, 51, 226–235. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuecel, G.; Zhou, X.; Terkatz, L.; Wendel, A.; Reinhardt, J.; El-Battrawy, I.; Sattler, K.; Cyganek, L.; Utikal, J.; Langer, H.; et al. Flagellin-Induced Immune Response in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Int. J. Mol. Sci. 2023, 24, 13933. https://doi.org/10.3390/ijms241813933
Yuecel G, Zhou X, Terkatz L, Wendel A, Reinhardt J, El-Battrawy I, Sattler K, Cyganek L, Utikal J, Langer H, et al. Flagellin-Induced Immune Response in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. International Journal of Molecular Sciences. 2023; 24(18):13933. https://doi.org/10.3390/ijms241813933
Chicago/Turabian StyleYuecel, Goekhan, Xiaobo Zhou, Linda Terkatz, Angela Wendel, Julius Reinhardt, Ibrahim El-Battrawy, Katherine Sattler, Lukas Cyganek, Jochen Utikal, Harald Langer, and et al. 2023. "Flagellin-Induced Immune Response in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes" International Journal of Molecular Sciences 24, no. 18: 13933. https://doi.org/10.3390/ijms241813933
APA StyleYuecel, G., Zhou, X., Terkatz, L., Wendel, A., Reinhardt, J., El-Battrawy, I., Sattler, K., Cyganek, L., Utikal, J., Langer, H., Scharf, R., Duerschmied, D., & Akin, I. (2023). Flagellin-Induced Immune Response in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. International Journal of Molecular Sciences, 24(18), 13933. https://doi.org/10.3390/ijms241813933







