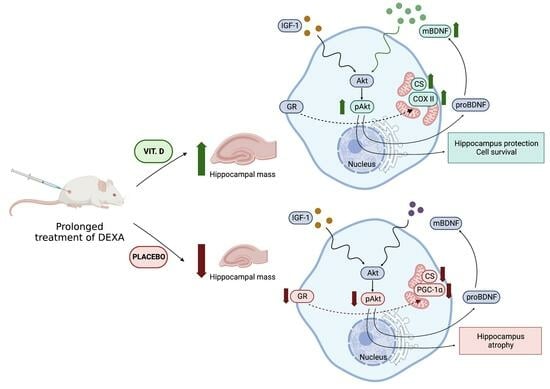
Supplementation with Vitamin D3 Protects against Mitochondrial Dysfunction and Loss of BDNF-Mediated Akt Activity in the Hippocampus during Long-Term Dexamethasone Treatment in Rats
Abstract
1. Introduction
2. Results
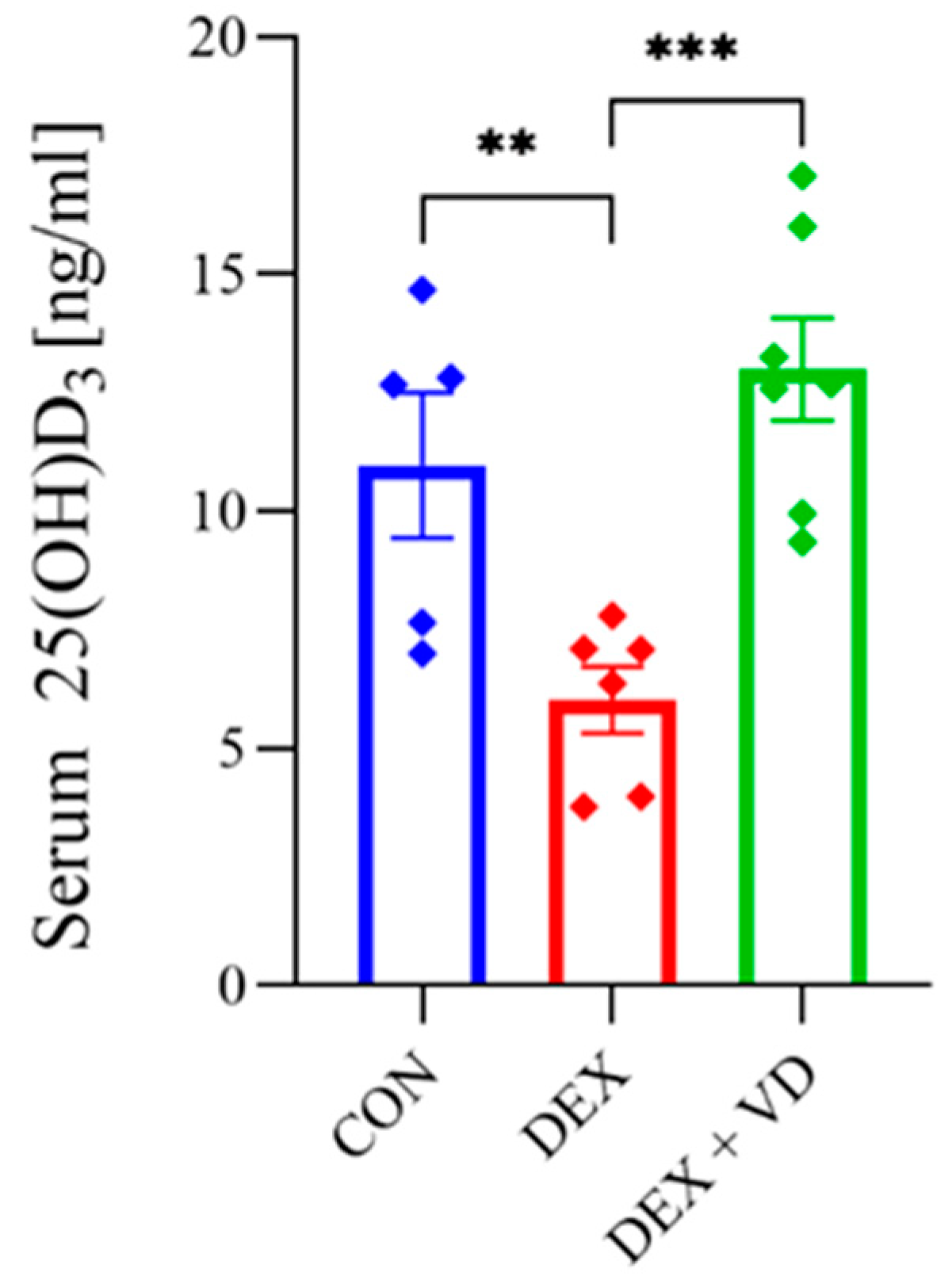
2.1. Vitamin D Concentration
2.2. Body and Hippocampus Mass Changes
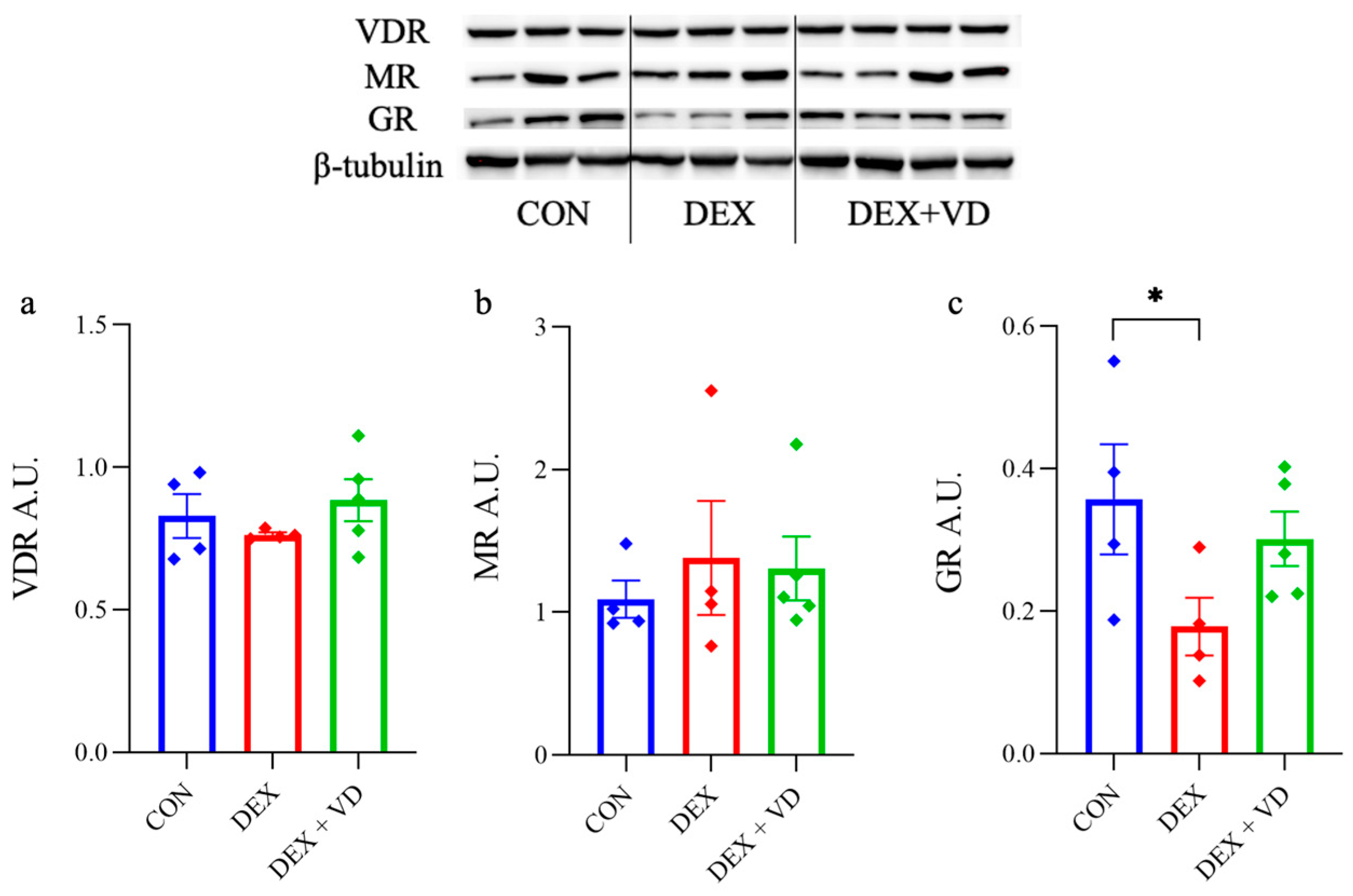
2.3. Hippocampal GR, MR, and VDR Levels
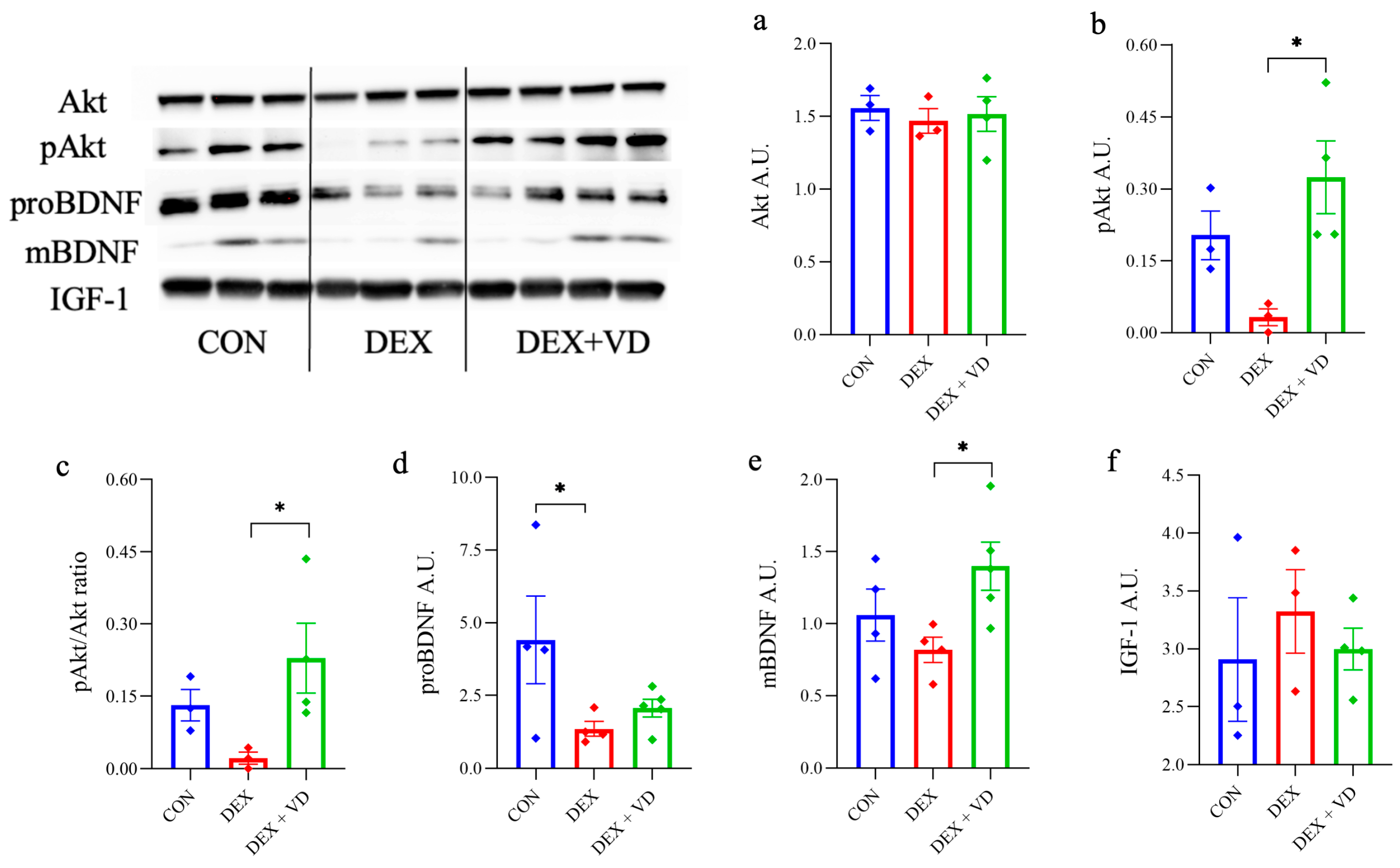
2.4. Assessment of BDNF-Akt Signaling Molecules
2.5. Assessment of Mitochondrial Biogenesis and Oxidative Metabolism
2.6. Mitochondrial Oxidative Metabolism Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Procedure
4.3. Tissue Preparation
4.3.1. Blood Collection
4.3.2. Western Blot
4.3.3. Enzymes Activity
4.4. Vitamin D3 Metabolite Concentration
4.5. Protein Expression
4.6. Enzyme Activities
4.6.1. Citrate synthase Activity
4.6.2. Cytochrome c Oxidase Activity
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giles, A.J.; Hutchinson, M.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-Induced Immunosuppression: Mechanisms and Implications for Immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, E.; Watanabe, S.; Okawa, E.; Ono, S. Regulation of Intracellular Copper by Induction of Endogenous Metallothioneins Improves the Disease Course in a Mouse Model of Amyotrophic Lateral Sclerosis. Neurotherapeutics 2015, 12, 461–476. [Google Scholar] [CrossRef]
- Johnson, D.B.; Lopez, M.J.; Kelley, B. Dexamethasone. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as Key Components of the Stress Response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Son, G.H.; Kim, K. Circadian Rhythm of Adrenal Glucocorticoid: Its Regulation and Clinical Implications. Biochim. Biophys. Acta 2011, 1812, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar]
- Karnia, M.J.; Korewo, D.; Myslinska, D.; Ciepielewski, Z.M.; Puchalska, M.; Konieczna-Wolska, K.; Kowalski, K.; Kaczor, J.J. The Positive Impact of Vitamin D on Glucocorticoid-Dependent Skeletal Muscle Atrophy. Nutrients 2021, 13, 936. [Google Scholar] [CrossRef]
- Karnia, M.J.; Myslinska, D.; Dzik, K.P.; Flis, D.J.; Ciepielewski, Z.M.; Podlacha, M.; Kaczor, J.J. The Electrical Stimulation of the Bed Nucleus of the Stria Terminalis Causes Oxidative Stress in Skeletal Muscle of Rats. Oxid. Med. Cell. Longev. 2018, 2018, 4671213. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Hunter, R.; Wei, Y.; Blumenthal, R.; Falke, C.; Khairova, R.; Zhou, R.; Yuan, P.; Machado-Vieira, R.; et al. Dynamic Regulation of Mitochondrial Function by Glucocorticoids. Proc. Natl. Acad. Sci. USA 2009, 106, 3543–3548. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- van Wamelen, D.J.; Leta, V.; Johnson, J.; Ocampo, C.L.; Podlewska, A.M.; Rukavina, K.; Rizos, A.; Martinez-Martin, P.; Chaudhuri, K.R. Drooling in Parkinson’s Disease: Prevalence and Progression from the Non-Motor International Longitudinal Study. Dysphagia 2020, 35, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, R.; Liu, Y.; Wang, W.; Liu, D.; Jiang, H.; Pan, F. Short- and Long-Term Alterations of Fkbp5-Gr and Specific Micrornas in the Prefrontal Cortex and Hippocampus of Male Rats Induced by Adolescent Stress Contribute to Depression Susceptibility. Psychoneuroendocrinology 2019, 101, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Ciriaco, M.; Ventrice, P.; Russo, G.; Scicchitano, M.; Mazzitello, G.; Scicchitano, F.; Russo, E. Corticosteroid-Related Central Nervous System Side Effects. J. Pharmacol. Pharmacother. 2013, 4 (Suppl. 1), S94–S98. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.N.; Ogg, R.J.; Scoggins, M.A.; Brinkman, T.M.; Sabin, N.D.; Pui, C.H.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Dexamethasone Exposure and Memory Function in Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Sjlife Cohort. Pediatr. Blood Cancer 2013, 60, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Stress and the Brain: Individual Variability and the Inverted-U. Nat. Neurosci. 2015, 18, 1344–1346. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Glucocorticoids and Hippocampal Atrophy in Neuropsychiatric Disorders. Arch. Gen. Psychiatry 2000, 57, 925–935. [Google Scholar] [CrossRef]
- Starkman, M.N.; Gebarski, S.S.; Berent, S.; Schteingart, D.E. Hippocampal Formation Volume, Memory Dysfunction, and Cortisol Levels in Patients with Cushing’s Syndrome. Biol. Psychiatry 1992, 32, 756–765. [Google Scholar] [CrossRef]
- Levone, B.R.; Codagnone, M.G.; Moloney, G.M.; Nolan, Y.M.; Cryan, J.F.; OF, L. Adult-Born Neurons from the Dorsal, Intermediate, and Ventral Regions of the Longitudinal Axis of the Hippocampus Exhibit Differential Sensitivity to Glucocorticoids. Mol. Psychiatry 2021, 26, 3240–3252. [Google Scholar] [CrossRef]
- Silva-Gómez, A.B.; Aguilar-Salgado, Y.; Reyes-Hernández, D.O.; Flores, G. Dexamethasone Induces Different Morphological Changes in the Dorsal and Ventral Hippocampus of Rats. J. Chem. Neuroanat. 2013, 47, 71–78. [Google Scholar] [CrossRef]
- MacPherson, A.; Dinkel, K.; Sapolsky, R. Glucocorticoids Worsen Excitotoxin-Induced Expression of Pro-Inflammatory Cytokines in Hippocampal Cultures. Exp. Neurol. 2005, 194, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, N.; Renthal, W.; Kumar, A.; Nestler, E.J. Author Correction: Epigenetic Regulation in Psychiatric Disorders. Nat. Rev. Neurosci. 2019, 20, 187–188. [Google Scholar] [CrossRef]
- Numata, S.; Ishii, K.; Tajima, A.; Iga, J.; Kinoshita, M.; Watanabe, S.; Umehara, H.; Fuchikami, M.; Okada, S.; Boku, S.; et al. Blood Diagnostic Biomarkers for Major Depressive Disorder Using Multiplex DNA Methylation Profiles: Discovery and Validation. Epigenetics 2015, 10, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential Role of Bdnf in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Taliaz, D.; Loya, A.; Gersner, R.; Haramati, S.; Chen, A.; Zangen, A. Resilience to Chronic Stress Is Mediated by Hippocampal Brain-Derived Neurotrophic Factor. J. Neurosci. 2011, 31, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell. Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef]
- Baldini, S.; Restani, L.; Baroncelli, L.; Coltelli, M.; Franco, R.; Cenni, M.C.; Maffei, L.; Berardi, N. Enriched Early Life Experiences Reduce Adult Anxiety-Like Behavior in Rats: A Role for Insulin-Like Growth Factor 1. J. Neurosci. 2013, 33, 11715–11723. [Google Scholar] [CrossRef]
- Santi, A.; Bot, M.; Aleman, A.; Penninx, B.; Aleman, I.T. Circulating Insulin-Like Growth Factor I Modulates Mood and Is a Biomarker of Vulnerability to Stress: From Mouse to Man. Transl. Psychiatry 2018, 8, 142. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Thompson, V.M.; Kitt, M.M.; Amat, J.; Hale, M.W.; Frank, M.G.; Crysdale, N.Y.; Stamper, C.E.; Hennessey, P.A.; Watkins, L.R.; et al. Greater Glucocorticoid Receptor Activation in Hippocampus of Aged Rats Sensitizes Microglia. Neurobiol. Aging 2015, 36, 1483–1495. [Google Scholar] [CrossRef]
- Herman, J.P.; Patel, P.D.; Akil, H.; Watson, S.J. Localization and Regulation of Glucocorticoid and Mineralocorticoid Receptor Messenger Rnas in the Hippocampal Formation of the Rat. Mol. Endocrinol. 1989, 3, 1886–1894. [Google Scholar] [CrossRef]
- Zhe, D.; Fang, H.; Yuxiu, S. Expressions of Hippocampal Mineralocorticoid Receptor (Mr) and Glucocorticoid Receptor (Gr) in the Single-Prolonged Stress-Rats. Acta Histochem. Cytochem. 2008, 41, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Stress Down-Regulates Corticosterone Receptors in a Site-Specific Manner in the Brain. Endocrinology 1984, 114, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulou, I.; Moutsatsou, P. Mitochondrial Glucocorticoid Receptors and Their Actions. Int. J. Mol. Sci. 2021, 22, 6054. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- An, B.S.; Tavera-Mendoza, L.E.; Dimitrov, V.; Wang, X.; Calderon, M.R.; Wang, H.J.; White, J.H. Stimulation of Sirt1-Regulated Foxo Protein Function by the Ligand-Bound Vitamin D Receptor. Mol. Cell. Biol. 2010, 30, 4890–4900. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef]
- Son, G.; Han, J. Roles of Mitochondria in Neuronal Development. BMB Rep. 2018, 51, 549–556. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, W.Y.; Li, H.D.; Cai, H.L.; Liu, Y.P.; Chen, L.Y. Stress and Vitamin D: Altered Vitamin D Metabolism in Both the Hippocampus and Myocardium of Chronic Unpredictable Mild Stress Exposed Rats. Psychoneuroendocrinology 2013, 38, 2091–2098. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Platt, N.; Rosado, A.F.; Neis, V.B.; Zeni, A.L.B.; Kaster, M.P.; Rodrigues, A.L.S. Cholecalciferol Abolishes Depressive-Like Behavior and Hippocampal Glucocorticoid Receptor Impairment Induced by Chronic Corticosterone Administration in Mice. Pharmacol. Biochem. Behav. 2020, 196, 172971. [Google Scholar] [CrossRef]
- Al-Amin, M.; Bradford, D.; Sullivan, R.K.P.; Kurniawan, N.D.; Moon, Y.; Han, S.H.; Zalesky, A.; Burne, T.H.J. Vitamin D Deficiency Is Associated with Reduced Hippocampal Volume and Disrupted Structural Connectivity in Patients with Mild Cognitive Impairment. Hum. Brain Mapp. 2019, 40, 394–406. [Google Scholar] [CrossRef]
- Bakhtiari-Dovvombaygi, H.; Izadi, S.; Zare, M.; Hassanlouei, E.A.; Dinpanah, H.; Ahmadi-Soleimani, S.M.; Beheshti, F. Vitamin D3 Administration Prevents Memory Deficit and Alteration of Biochemical Parameters Induced by Unpredictable Chronic Mild Stress in Rats. Sci. Rep. 2021, 11, 16271. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Skrobot, W.; Kaczor, K.B.; Flis, D.J.; Karnia, M.J.; Libionka, W.; Antosiewicz, J.; Kloc, W.; Kaczor, J.J. Vitamin D Deficiency Is Associated with Muscle Atrophy and Reduced Mitochondrial Function in Patients with Chronic Low Back Pain. Oxid. Med. Cell. Longev. 2019, 2019, 6835341. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of Vitamin D on Skeletal Muscle Function: Oxidative Stress, Energy Metabolism and Anabolic State. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, M.; Viano, M.; Casarin, S.; Castagnoli, C.; Pescarmona, G.; Silvagno, F. Mitochondrial and Lipogenic Effects of Vitamin D on Differentiating and Proliferating Human Keratinocytes. Exp. Dermatol. 2015, 24, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Egawa, K.; Kanzaki, N.; Izumo, T.; Rogi, T.; Shibata, H. Quercetin Glycosides Prevent Dexamethasone-Induced Muscle Atrophy in Mice. Biochem. Biophys. Rep. 2019, 18, 100618. [Google Scholar] [CrossRef]
- Aru, M.; Alev, K.; Pehme, A.; Purge, P.; Onnik, L.; Ellam, A.; Kaasik, P.; Seene, T. Changes in Body Composition of Old Rats at Different Time Points after Dexamethasone Administration. Curr. Aging Sci. 2019, 11, 255–260. [Google Scholar] [CrossRef]
- Alev, K.; Aru, M.; Vain, A.; Pehme, A.; Kaasik, P.; Seene, T. Short-Time Recovery Skeletal Muscle from Dexamethasone-Induced Atrophy and Weakness in Old Female Rats. Clin. Biomech. 2022, 100, 105808. [Google Scholar] [CrossRef]
- Koorneef, L.L.; van der Meulen, M.; Kooijman, S.; Sanchez-Lopez, E.; Scheerstra, J.F.; Voorhoeve, M.C.; Ramesh, A.N.N.; Rensen, P.C.N.; Giera, M.; Kroon, J.; et al. Dexamethasone-Associated Metabolic Effects in Male Mice Are Partially Caused by Depletion of Endogenous Corticosterone. Front. Endocrinol. 2022, 13, 960279. [Google Scholar] [CrossRef]
- Guarnotta, V.; Di Gaudio, F.; Giordano, C. Vitamin D Deficiency in Cushing’s Disease: Before and after Its Supplementation. Nutrients 2022, 14, 973. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Feng, S.; Zhao, N.; Hu, X.; Cheng, Y.; Wu, Y.; Zhou, L.; Tong, J.; Zheng, C. Clinical Efficacy of High-Dose Dexamethasone with Sequential Prednisone Maintenance Therapy for Newly Diagnosed Adult Immune Thrombocytopenia in a Real-World Setting. J. Int. Med. Res. 2021, 49, 3000605211007322. [Google Scholar] [CrossRef]
- Eshkevari, L.; Mulroney, S.E.; Egan, R.; Lao, L. Effects of Acupuncture, Ru-486 on the Hypothalamic-Pituitary-Adrenal Axis in Chronically Stressed Adult Male Rats. Endocrinology 2015, 156, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Unemura, K.; Kume, T.; Kondo, M.; Maeda, Y.; Izumi, Y.; Akaike, A. Glucocorticoids Decrease Astrocyte Numbers by Reducing Glucocorticoid Receptor Expression in Vitro and in Vivo. J. Pharmacol. Sci. 2012, 119, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Xu, T.; Yin, Y.; Huang, R.; Wang, Y.; Zhang, J.; Huang, D.; Li, W. Chronic Dexamethasone Treatment Results in Hippocampal Neurons Injury Due to Activate Nlrp1 Inflammasome in Vitro. Int. Immunopharmacol. 2017, 49, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Skupio, U.; Tertil, M.; Sikora, M.; Golda, S.; Wawrzczak-Bargiela, A.; Przewlocki, R. Behavioral and Molecular Alterations in Mice Resulting from Chronic Treatment with Dexamethasone: Relevance to Depression. Neuroscience 2015, 286, 141–150. [Google Scholar] [CrossRef]
- Froger, N.; Palazzo, E.; Boni, C.; Hanoun, N.; Saurini, F.; Joubert, C.; Dutriez-Casteloot, I.; Enache, M.; Maccari, S.; Barden, N.; et al. Neurochemical and Behavioral Alterations in Glucocorticoid Receptor-Impaired Transgenic Mice after Chronic Mild Stress. J. Neurosci. 2004, 24, 2787–2796. [Google Scholar] [CrossRef]
- Kunugi, H.; Hori, H.; Adachi, N.; Numakawa, T. Interface between Hypothalamic-Pituitary-Adrenal Axis and Brain-Derived Neurotrophic Factor in Depression. Psychiatry Clin. Neurosci. 2010, 64, 447–459. [Google Scholar] [CrossRef]
- Yau, J.L.; Seckl, J.R. Local Amplification of Glucocorticoids in the Aging Brain and Impaired Spatial Memory. Front/Aging Neurosci. 2012, 4, 24. [Google Scholar] [CrossRef]
- Koning, A.; Habets, P.C.; Bogaards, M.; Kroon, J.; van Santen, H.M.; de Bont, J.M.; Meijer, O.C. Mineralocorticoid Receptor Status in the Human Brain after Dexamethasone Treatment: A Single Case Study. Endocr. Connect. 2022, 11, e210425. [Google Scholar] [CrossRef]
- Hidalgo, A.A.; Deeb, K.K.; Pike, J.W.; Johnson, C.S.; Trump, D.L. Dexamethasone Enhances 1alpha,25-Dihydroxyvitamin D3 Effects by Increasing Vitamin D Receptor Transcription. J. Biol. Chem. 2011, 286, 36228–36237. [Google Scholar] [CrossRef]
- Zenata, O.; Vrzal, R. Fine Tuning of Vitamin D Receptor (Vdr) Activity by Post-Transcriptional and Post-Translational Modifications. Oncotarget 2011, 8, 35390–35402. [Google Scholar] [CrossRef]
- Consiglio, M.; Destefanis, M.; Morena, D.; Foglizzo, V.; Forneris, M.; Pescarmona, G.; Silvagno, F. The Vitamin D Receptor Inhibits the Respiratory Chain, Contributing to the Metabolic Switch That Is Essential for Cancer Cell Proliferation. PLoS ONE 2014, 9, e115816. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Avadhani, N.G. Cytochrome C Oxidase Dysfunction in Oxidative Stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Desquiret, V.; Gueguen, N.; Malthièry, Y.; Ritz, P.; Simard, G. Mitochondrial Effects of Dexamethasone Imply Both Membrane and Cytosolic-Initiated Pathways in Hepg2 Cells. Int. J. Biochem. Cell Biol. 2008, 40, 1629–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scaini, G.; Rochi, N.; Benedet, J.; Ferreira, G.K.; Teodorak, B.P.; Comim, C.M.; Lde, S.C.; Vuolo, F.; Constantino, L.C.; Quevedo, J.; et al. Inhibition of Brain Citrate Synthase Activity in an Animal Model of Sepsis. Rev. Bras. De Ter. Intensiv. 2011, 23, 158–163. [Google Scholar] [CrossRef]
- Meng, L.B.; Hu, G.F.; Shan, M.J.; Zhang, Y.M.; Yu, Z.M.; Liu, Y.Q.; Xu, H.X.; Wang, L.; Gong, T.; Liu, D.P. Citrate Synthase and Ogdh as Potential Biomarkers of Atherosclerosis under Chronic Stress. Oxid. Med. Cell. Longev. 2021, 2021, 9957908. [Google Scholar] [CrossRef]
- Hajiluian, G.; Farhangi, M.A.; Nameni, G.; Shahabi, P.; Megari-Abbasi, M. Oxidative Stress-Induced Cognitive Impairment in Obesity Can Be Reversed by Vitamin D Administration in Rats. Nutr. Neurosci. 2018, 21, 744–752. [Google Scholar] [CrossRef]
- AlJohri, R.; AlOkail, M.; Haq, S.H. Neuroprotective Role of Vitamin D in Primary Neuronal Cortical Culture. eNeurologicalSci 2019, 14, 43–48. [Google Scholar] [CrossRef]
- Obradovic, D.; Gronemeyer, H.; Lutz, B.; Rein, T. Cross-Talk of Vitamin D and Glucocorticoids in Hippocampal Cells. J. Neurochem. 2006, 96, 500–509. [Google Scholar] [CrossRef]
- Hagl, S.; Asseburg, H.; Heinrich, M.; Sus, N.; Blumrich, E.M.; Dringen, R.; Frank, J.; Eckert, G.P. Effects of Long-Term Rice Bran Extract Supplementation on Survival, Cognition and Brain Mitochondrial Function in Aged Nmri Mice. Neuromolecular. Med. 2016, 18, 347–363. [Google Scholar] [CrossRef]
- Yousefian, Z.; Khaleghian, A.; Parsaei, H.; Vafaei, A.A.; Rashidy-Pour, A.; Sedaghat, K. Effect of Vitamin D on Hippocampus Brain-Derived Neurotrophic Factor Level in Chronic Mild Stress Model of Depression in Rats. Middle East J. Rehabil. Health 2018, 5, e63901. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, L. Vitamin D3/Vitamin D Receptor Signaling Mitigates Symptoms of Post-Stroke Depression in Mice by Upregulating Hippocampal Bdnf Expression. Neurosci. Res. 2021, 170, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Amazit, L.; Lombes, M.; Le Menuet, D. Crosstalk between Glucocorticoid Receptor and Early-Growth Response Protein 1 Accounts for Repression of Brain-Derived Neurotrophic Factor Transcript 4 Expression. Neuroscience 2019, 399, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Rola, R.; Kowalski, K.; Bieńkowski, T.; Studzińska, S. Improved Sample Preparation Method for Fast Lc-Ms/Ms Analysis of Vitamin D Metabolites in Serum. J. Pharm. Biomed. Anal. 2020, 190, 113529. [Google Scholar] [CrossRef] [PubMed]
- Gianni, P.; Jan, K.J.; Douglas, M.J.; Stuart, P.M.; Tarnopolsky, M.A. Oxidative Stress and the Mitochondrial Theory of Aging in Human Skeletal Muscle. Exp. Gerontol. 2004, 39, 1391–1400. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korewo-Labelle, D.; Karnia, M.J.; Myślińska, D.; Kaczor, J.J. Supplementation with Vitamin D3 Protects against Mitochondrial Dysfunction and Loss of BDNF-Mediated Akt Activity in the Hippocampus during Long-Term Dexamethasone Treatment in Rats. Int. J. Mol. Sci. 2023, 24, 13941. https://doi.org/10.3390/ijms241813941
Korewo-Labelle D, Karnia MJ, Myślińska D, Kaczor JJ. Supplementation with Vitamin D3 Protects against Mitochondrial Dysfunction and Loss of BDNF-Mediated Akt Activity in the Hippocampus during Long-Term Dexamethasone Treatment in Rats. International Journal of Molecular Sciences. 2023; 24(18):13941. https://doi.org/10.3390/ijms241813941
Chicago/Turabian StyleKorewo-Labelle, Daria, Mateusz Jakub Karnia, Dorota Myślińska, and Jan Jacek Kaczor. 2023. "Supplementation with Vitamin D3 Protects against Mitochondrial Dysfunction and Loss of BDNF-Mediated Akt Activity in the Hippocampus during Long-Term Dexamethasone Treatment in Rats" International Journal of Molecular Sciences 24, no. 18: 13941. https://doi.org/10.3390/ijms241813941
APA StyleKorewo-Labelle, D., Karnia, M. J., Myślińska, D., & Kaczor, J. J. (2023). Supplementation with Vitamin D3 Protects against Mitochondrial Dysfunction and Loss of BDNF-Mediated Akt Activity in the Hippocampus during Long-Term Dexamethasone Treatment in Rats. International Journal of Molecular Sciences, 24(18), 13941. https://doi.org/10.3390/ijms241813941