An Updated Review on Developing Small Molecule Kinase Inhibitors Using Computer-Aided Drug Design Approaches
Abstract
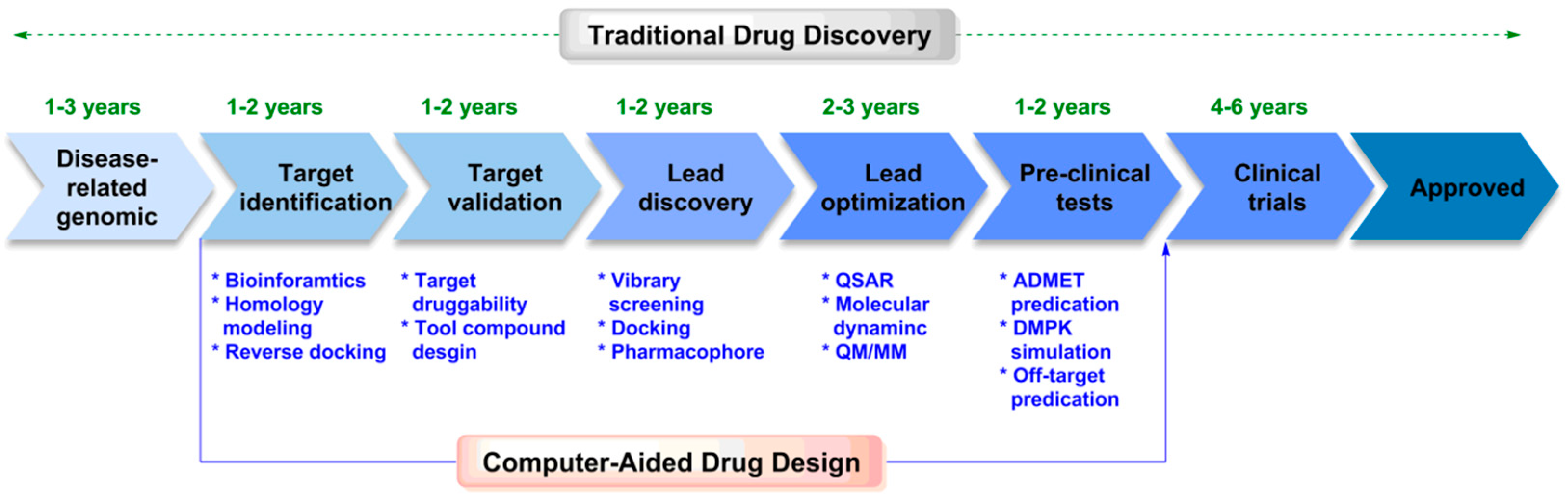
1. Introduction
2. Computer-Aided Drug Design
2.1. Structure-Based Drug Design
2.1.1. Preparation of Target Structures
2.1.2. Molecular Docking
| Software | Algorithm | Scoring Function | Website | Ref. |
|---|---|---|---|---|
| Dock | Fragment growth | Force field, Surface matching score, Environment matching score | http://dock.compbio.ucsf.edu/DOCK_6/, accessed on 16 July 2023). | [66] |
| AutoDock | Genetic algorithm | Environment matching score | http://autodock.scripps.edu/, accessed on 16 July 2023). | [67] |
| GOLD | Genetic algorithm | Empirical | http://www.biosolveit.de/FlexX/, accessed on 16 July 2023). | [68] |
| FlexX | Fragment growth | Empirical | https://github.com/flexxui/flexx, accessed on 16 July 2023). | [69] |
| Z-Dock | Geometric matching/Molecular dynamics | CAPRI+ | http://zdock.umassmed.edu/, accessed on 16 July 2023). | [70] |
| Hex | Geometric matching | CAPRI+ | http://www.csd.abdn.ac.uk/hex/, accessed on 16 July 2023). | [71] |
| SLIDE | Systematic | Force field, Empirical | http://www.bmb.msu.edu/~kuhn/software/slide/, accessed on 16 July 2023). | [72] |
| Fred | Systematic | Empirical | http://www.eyesopen.com/oedocking, accessed on 16 July 2023). | [73] |
| LeDock | Annealing–Genetic algorithm | Physics/knowledge hybrid | http://www.lephar.com/software.htm, accessed on 16 July 2023). | [74] |
| Glide | Systematic | XP/SP/HTVS | https://www.schrodinger.com, accessed on 16 July 2023). | [75] |
| Surflex-Dock | Hammerhead | Empirical | http://www.tripos.com, accessed on 16 July 2023). | [76] |
2.1.3. Molecular Dynamics
2.1.4. Quantum Chemistry
2.1.5. Molecular Docking–Molecular Dynamics–Quantum Chemistry
2.1.6. Virtual Screening
2.2. Ligand-Based Drug Design
2.2.1. Quantitative Structure–Activity Relationship
2.2.2. DFT-Based Quantitative Structure–Activity Relationship
2.2.3. Pharmacophore Modeling
2.2.4. Molecular Similarity
3. Kinases
3.1. Structure and Function of Kinases
3.2. Small Molecule Kinase Inhibitors
4. Small Molecule Kinase Inhibitors Discovered Using CADD
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, L.; Ciallella, H.L.; Aleksunes, L.M.; Zhu, H. Advancing computer-aided drug discovery (CADD) by big data and data-driven machine learning modeling. Drug Discov. Today 2020, 25, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Gomeni, R.; Bani, M.; D’angeli, C.; Corsi, M.; Bye, A. Computer-assisted drug development (CADD): An emerging technology for designing first-time-in-man and proof-of-concept studies from preclinical experiments. Eur. J. Pharm. Sci. 2001, 13, 261–270. [Google Scholar] [CrossRef]
- Finn, J. Application of SBDD to the Discovery of New Antibacterial Drugs. Methods Mol. Biol. 2012, 841, 291–319. [Google Scholar] [PubMed]
- Acharya, C.; Coop, A.; Polli, J.E.; MacKerell, A.D. Recent Advances in Ligand-Based Drug Design: Relevance and Utility of the Conformationally Sampled Pharmacophore Approach. Curr. Comput. Aided Drug Des. 2011, 7, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Sabe, V.T.; Ntombela, T.; Jhamba, L.A.; Maguire, G.E.M.; Kruger, H.G. Current trends in computer aided drug design and a highlight of drugs discovered via computational techniques: A review. Eur. J. Med. Chem. 2021, 224, 113705. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.S.; Wimalasena, J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol. Endocrinol. 1996, 10, 488–498. [Google Scholar] [PubMed]
- Haldar, S.; Chintapalli, J.; Croce, C.M. Taxol Induces bcl-2 Phosphorylation and Death of Prostate Cancer Cells. Cancer Res. 1996, 56, 1253–1255. [Google Scholar]
- Itoh, N.; Semba, S.; Ito, M.; Takeda, H.; Kawata, S.; Yamakawa, M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2010, 94, 3127–3134. [Google Scholar] [CrossRef]
- Mcdonald, P.C.; Oloumi, A.; Mills, J.; Dobreva, I.; Maidan, M.; Gray, V.; Wederell, E.D.; Bally, M.B.; Foster, L.J.; Dedhar, S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008, 68, 1618–1624. [Google Scholar] [CrossRef]
- Cordwell, S.J.; White, M.Y. Targeted proteomics for determining phosphorylation site-specific associations in cardiovascular disease. Circulation 2012, 126, 1803–1807. [Google Scholar] [CrossRef]
- Nishida, M.; Saiki, S.; Kitajima, N.; Nakaya, M.; Sato, Y.; Kurose, H. ChemInform Abstract: Regulation of Cardiovascular Functions by the Phosphorylation of TRPC Channels. Cheminform 2011, 42, 14. [Google Scholar] [CrossRef]
- Streeter, J.; Schickling, B.; Thiel, W.; Miller, F. Nox1 Phosphorylation in Cardiovascular Disease. Free Radic. Biol. Med. 2012, 53, S175. [Google Scholar] [CrossRef]
- Wieland, T.; Attwood, P.V. Alterations in reversible protein histidine phosphorylation as intracellular signals in cardiovascular disease. Front. Pharmacol. 2015, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Pospisilik, J.A.; Knauf, C.; Joza, N.; Benit, P.; Orthofer, M.; Cani, P.D.; Ebersberger, I.; Nakashima, T.; Sarao, R.; Neely, G.; et al. Targeted Deletion of AIF Decreases Mitochondrial Oxidative Phosphorylation and Protects from Obesity and Diabetes. Cell 2007, 131, 476–491. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Grundkeiqbal, I.; Iqbal, K.; Gong, C.X. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J. Neurochem. 2010, 111, 242–249. [Google Scholar] [CrossRef]
- Szendroedi, J.; Schmid, A.I.; Chmelik, M.; Toth, C.; Brehm, A.; Krssak, M.; Nowotny, P.; Wolzt, M.; Waldhausl, W.; Roden, M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med. 2007, 4, 154. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Chernishof, V.; Ogorzalek, R.R.L.; Jang, H.; Tank, S.; Yang, P.; Mink, S.; Shultz, D.; Bellone, C.J.; et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 2007, 129, 903–914. [Google Scholar] [CrossRef]
- Caudle, R.M.; Perez, F.M.; Vallepinero, A.Y.D.; Iadarola, M.J. Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Mol. Pain. 2005, 1, 25. [Google Scholar] [CrossRef]
- Suzuki, M.; Mimuro, H.; Kiga, K. Helicobacter pylori CagA Phosphorylation-Independent Function in Epithelial Proliferation and Inflammation. Cell Host Microbe 2009, 5, 23–34. [Google Scholar] [CrossRef]
- Wertz, I.E.; Newton, K.; Seshasayee, D.; Fukumatsu, M.; Ishijima, N.; Morikawa, H.; Nagai, S.; Koyasu, S.; Gilman, R.H.; Kersulyte, D.; et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 2015, 528, 370–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Wang, X.; Zhang, Y.; Xia, M. AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mp, M.; Pm, F. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007, 6, 464–479. [Google Scholar]
- Davis, M.I.; Hunt, J.P.; Herrgard, S.; Ciceri, P.; Wodicka, L.M.; Pallares, G.; Hocker, M.; Treiber, D.K.; Zarrinkar, P.P. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1046–1051. [Google Scholar] [CrossRef]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schith, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nat. Rev. Drug Discov. 2021, 20, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Macalino, S.J.Y.; Vijayakumar, G.; Sunhye, H.; Sun, C. Role of computer-aided drug design in modern drug discovery. Arch. Pharmacal Res. 2015, 38, 1686–1701. [Google Scholar] [CrossRef]
- Dimasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar]
- Patani, G.A.; Lavoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Brown, F.K.; Sherer, E.C.; Johnson, S.A.; Holloway, M.K.; Sherborne, S.B. The evolution of drug design at Merck Research Laboratories. J. Comput.-Aided Mol. Des. 2017, 31, 255–266. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Cavasotto, C.N.; Phatak, S.S. Homology modeling in drug discovery: Current trends and applications. Drug Discov. Today 2009, 14, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods Mol. Biol. 2008, 443, 365–382. [Google Scholar] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C. A genetic algorithm for flexible molecular overlay and pharmacophore elucidation. J. Comput.-Aided Mol. Des. 1995, 9, 532. [Google Scholar] [CrossRef]
- Hopfinger, A.J.; Wang, S.; Tokarski, J.S.; Jin, B.; Albuquerque, M.; Madhav, P.J.; Duraiswami, C. Construction of 3D-QSAR Models Using the 4D-QSAR Analysis Formalism. J. Am. Chem. Soc. 1997, 119, 10509–10524. [Google Scholar] [CrossRef]
- Durrant, J.D.; Andrew, M.C.J. Molecular dynamics simulations and drug discovery. BMC Biol. 2011, 9, 71. [Google Scholar] [CrossRef]
- Keseru, G.M.; Kolossváry, I. Molecular Mechanics and Conformational Analysis in Drug Design; Balckwell Science: Oxford, UK, 1999. [Google Scholar]
- Gravenstein, S.; Johnston, S.L.; Loeschel, E.; Webster, A. Zanamivir. Drug Saf. 2001, 24, 1113–1125. [Google Scholar] [CrossRef]
- Noble, S.; Faulds, D. Saquinavir. Drugs 1996, 52, 93–112. [Google Scholar] [CrossRef]
- Silver, R.T. Imatinib mesylate (Gleevec (TM)) reduces phlebotomy requirements in polycythemia vera. Leukemia 2003, 17, 1186–1187. [Google Scholar] [CrossRef]
- Chirikjian, G.S. Conformational Modeling of Continuum Structures in Robotics and Structural Biology: A Review. Adv. Robot. Int. J. Robot. Soc. Jpn. 2015, 29, 817–829. [Google Scholar] [CrossRef]
- Congreve, M.; Murray, C.W.; Blundell, T.L. Keynote review: Structural biology and drug discovery. Drug Discov. Today 2005, 10, 895–907. [Google Scholar] [CrossRef]
- Garratt, R. Structural biology and cancer. BMC Proc. 2013, 7, K15. [Google Scholar] [CrossRef]
- Holler, T.P.; Evdokimov, A.G.; Narasimhan, L. Structural biology approaches to antibacterial drug discovery. Expert Opin. Drug Discov. 2007, 2, 1085–1101. [Google Scholar] [CrossRef]
- Penin, F. Structural biology of hepatitis C virus. Clin. Liver Dis. 2003, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Iwatsubo, T. Structural Biology of Presenilins and Signal Peptide Peptidases. J. Biol. Chem. 2013, 288, 14673–14680. [Google Scholar] [CrossRef] [PubMed]
- Fenalti, G.; Buckle, A.M. Structural biology of the GAD autoantigen. Autoimmun. Rev. 2010, 9, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput.-Aided Drug Des. 2016, 7, 146–157. [Google Scholar] [CrossRef]
- Yuriev, E.; Holien, J.; Ramsland, P.A. Improvements, trends, and new ideas in molecular docking: 2012–2013 in review. J. Mol. Recognit. 2015, 28, 581–604. [Google Scholar] [CrossRef]
- Yuriev, E.; Ramsland, P.A. Latest developments in molecular docking: 2010–2011 in review. J. Mol. Recognit. 2013, 26, 215–239. [Google Scholar] [CrossRef]
- Chen, H.; Lyne, P.D.; Giordanetto, F.; Timothy, L.; Jin, L. On evaluating molecular-docking methods for pose prediction and enrichment factors. J. Chem. Inf. Model. 2006, 46, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Timmers, L.F.; Caceres, R.A. Evaluation of molecular docking using polynomial empirical scoring functions. Curr. Drug Targets 2008, 9, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.D.; Jewsbury, P.J.; Essex, J.W. A review of protein-small molecule docking methods. J. Comput.-Aided Mol. Des. 2002, 16, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Kumar, S.; Saloni, S.; Singh, H.; Kim, M.; Sharma, P.; Misra, S.; Khan, F. Molecular docking, QSAR and ADMET studies of withanolide analogs against breast cancer. Drug Des. Dev. Ther. 2017, 11, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Ma, X.; Shu, Q.; Zhang, F.; Uchil, V.; Cherukupalli, G.R. Impdh as a Biological Probe for Rna Antiviral Drug Discovery: Synthesis, Enzymology, Molecular Docking, and Antiviral Activity of New Ribonucleosides with Surrogate Bases. Cheminform 2007, 26, 651–654. [Google Scholar]
- Ding, W.; Gu, J.; Cao, L.; Li, N.; Ding, G.; Wang, Z.Z.; Chen, L.R.; Xu, X.J.; Xiao, W. Traditional Chinese herbs as chemical resource library for drug discovery of anti-infective and anti-inflammatory. J. Ethnopharmacol. 2014, 155, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Stalin, A.; Ignacimuthu, S. Molecular docking of γ-sitosterol with some targets related to diabetes. Eur. J. Med. Chem. 2012, 47, 38–43. [Google Scholar] [CrossRef]
- Rayalu, D.J.; Selvaraj, C.; Singh, S.K.; Ganeshan, R.; Seshapani, P. Homology modeling, active site prediction, and targeting the anti hypertension activity through molecular docking on endothelin-B receptor domain. Bioinformation 2012, 8, 81–86. [Google Scholar] [CrossRef][Green Version]
- Mathew, B.; Suresh, J.; Anbazhagan, S.; Dev, S. Molecular Docking Studies of Some Novel Antidepressant 5-Substituted Phenyl-3-(Thiophen-2-yl)-4, 5-Dihydro-1h-Pyrazole-1-Carboxamides against Monoamine Oxidase Isoforms. Cent. Nerv. Syst. Ag. Med. Chem. 2016, 16, 75–80. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; Eltahir, K.E.; Asiri, Y.A. Synthesis, anti-inflammatory activity and COX-1/COX-2 inhibition of novel substituted cyclic imides. Part 1: Molecular docking study. Eur. J. Med. Chem. 2011, 46, 1648–1655. [Google Scholar] [CrossRef]
- Cheng, K.; Zheng, Q.Z.; Qian, Y.; Shi, L.; Zhao, J.; Zhu, H.L. Synthesis, antibacterial activities and molecular docking studies of peptide and Schiff bases as targeted antibiotics. Bioorg. Med. Chem. 2009, 17, 7861–7871. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Chan, S.H.; Leung, C.H. Molecular docking for virtual screening of natural product databases. Chem. Sci. 2011, 2, 1656–1665. [Google Scholar] [CrossRef]
- Ruyck, J.D.; Brysbaert, G.; Blossey, R.; Lensink, M. Molecular docking as a popular tool in drug design, an in silico travel. Adv. Appl. Bioinform. Chem. 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Stark, J.L.; Powers, R. Application of NMR and molecular docking in structure-based drug discovery. Top. Curr. Chem. 2012, 326, 1–34. [Google Scholar] [PubMed]
- Lang, P.T.; Brozell, S.R.; Mukherjee, S.; Pettersen, E.F.; Meng, E.C.; Thomas, V.; Rizzo, R.C.; Case, D.A.; James, T.L.; Kuntz, I.D. DOCK 6: Combining techniques to model RNA–small molecule complexes. RNA 2009, 15, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved Protein–Ligand Docking Using GOLD. Proteins Struct. Funct. Genet. 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Rarey, M.; Lengauer, T.; Klebe, G.; Kramer, B. A Fast Flexible Docking Method using an Incremental Construction Algorithm. J. Mol. Biol. 1996, 261, 470–489. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Weng, Z.P. ZDOCK: An initial-stage protein-docking algorithm. Proteins Struct. Funct. Genet. 2003, 52, 80–87. [Google Scholar] [CrossRef]
- Macindoe, G.; Mavridis, L.; Venkatraman, V.; Devignes, M.D.; Ritchie, D.W. HexServer: An FFT-based protein docking server powered by graphics processors. Nucleic Acids Res. 2010, 38, 445–449. [Google Scholar] [CrossRef]
- Zavodszky, M.I.; Sanschagrin, P.C.; Korde, R.S.; Kuhn, L.A. Distilling the essential features of a protein surface for improving protein–ligand docking, scoring, and virtual screening. J. Comput.-Aided Mol. Des. 2002, 16, 883–902. [Google Scholar] [CrossRef] [PubMed]
- McGann, M.; Almond, H.; Nicholls, A.; Grant, J.A.; Brown, F.K. Gaussian docking functions. Biopolymers 2003, 68, 76–90. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Z.B. Using LeDock as a docking tool for computational drug design. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012143. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef]
- Danel, T.; Łęski, J.; Podlewska, S.; Podalak, I. Docking-based generative approaches in the search for new drug candidates. Drug Discov. Today 2023, 28, 103439. [Google Scholar] [CrossRef]
- Foroutan, M.; Fatemi, S.M.; Esmaeilian, F. A review of the structure and dynamics of nanoconfined water and ionic liquids via molecular dynamics simulation. Eur. Phys. J. E 2017, 40, 19. [Google Scholar] [CrossRef]
- Komanduri, R.; Raff, L.M. A review on the molecular dynamics simulation of machining at the atomic scale. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2001, 215, 1639–1672. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471. [Google Scholar] [CrossRef]
- Vivo, M.D.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef]
- Galeazzi, R. Molecular Dynamics as a Tool in Rational Drug Design: Current Status and Some Major Applications. Curr. Comput.-Aided Drug Des. 2009, 5, 225–240. [Google Scholar] [CrossRef]
- Mortier, J.; Rakers, C.; Bermudez, M.; Murgueitio, M.S.; Riniker, S.; Wolber, G. The impact of molecular dynamics on drug design: Applications for the characterization of ligand–macromolecule complexes. Drug Discov. Today 2015, 20, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Perryman, A.L.; Lin, J.H.; Mccammon, J.A. Restrained molecular dynamics simulations of HIV-1 protease: The first step in validating a new target for drug design. Biopolymers 2010, 82, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Coote, M.L.; Barakat, K. Molecular dynamics-driven drug discovery: Leaping forward with confidence. Drug Discov. Today 2017, 22, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Wereszczynski, J.; Mccammon, J.A. Accelerated molecular dynamics in computational drug design. Methods Mol. Biol. 2012, 819, 515. [Google Scholar] [PubMed]
- Case, D.A.; Darden, T.; Cheatham, T.E., III; Simmerling, C.; Wang, J.M.; Duke, R.E.; Luo, R.; Croeley, M.; Zhang, W. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Hutter, J.; Iannuzzi, M. CPMD: Car-Parrinello molecular dynamics. Z. Krist.-Cryst. Mater. 2009, 220, 65080. [Google Scholar] [CrossRef]
- Nelson, M.T.; Humphrey, W.; Gursoy, A.; Dalke, A.; Kale, L.V.; Skeel, R.D.; Schulten, K. NAMD: A parallel, object-oriented molecular dynamics program. Int. J. High Perform. Comput. Appl. 1996, 10, 251–268. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ‘t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Spoel, D.V.; Drunen, R.V. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Karplus, M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Rackers, J.A.; Wang, Z.; Lu, C.; Laury, M.L. Tinker 8: Software Tools for Molecular Design. J. Chem. Theory Comput. 2018, 14, 5273–5289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Caflisch, A. Molecular dynamics in drug design. Eur. J. Med. Chem. 2015, 91, 4–14. [Google Scholar] [CrossRef]
- Lin, J.H.; Perryman, A.L.; Schames, J.R.; McCammon, J.A. Computational drug design accommodating receptor flexibility: The relaxed complex scheme. J. Am. Chem. Soc. 2002, 124, 5632–5633. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.H.; De Groot, B.L. Ubiquitin dynamics in complexes reveal molecular recognition mechanisms beyond induced fit and conformational selection. PLoS Comput. Biol. 2012, 8, e1002704. [Google Scholar] [CrossRef] [PubMed]
- Sotriffer, C.A.; Krämer, O.; Klebe, G. Probing flexibility and “induced-fit” phenomena in aldose reductase by comparative crystal structure analysis and molecular dynamics simulations. Proteins Struct. Funct. Bioinformat. 2010, 56, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Biswa Ranjan, M.; Yixuan, W. Interaction of I50V mutant and I50L/A71V double mutant HIV-protease with inhibitor TMC114 (darunavir): Molecular dynamics simulation and binding free energy studies. J. Phys. Chem. B 2012, 116, 1884–1900. [Google Scholar]
- Zhou, T.; Georgeon, S.; Moser, R.; Moore, D.J.; Caflisch, A.; Hantschel, O. Specificity and mechanism-of-action of the JAK2 tyrosine kinase inhibitors ruxolitinib and SAR302503 (TG101348). Leukemia 2014, 28, 404–407. [Google Scholar] [CrossRef]
- Spiliotopoulos, D.; Caflisch, A. Molecular Dynamics Simulations of Bromodomains Reveal Binding-Site Flexibility and Multiple Binding Modes of the Natural Ligand Acetyl-Lysine. Israel J. Chem. 2015, 54, 1084–1092. [Google Scholar] [CrossRef]
- Bera, V.; Payghan; Pavan, V. Use of Molecular Dynamics Simulations in Structure-Based Drug Discovery. Curr. Pharm. Des. 2019, 25, 3339–3349. [Google Scholar] [CrossRef]
- Johnson, K.H. Quantum Chemistry. Annu. Rev. Phys. Chem. 1975, 26, 39–57. [Google Scholar] [CrossRef]
- Arnold, A.; Weigend, F.; Evers, F. Quantum chemistry calculations for molecules coupled to reservoirs: Formalism, implementation, and application to benzenedithiol. J. Chem. Phys. 2007, 126, 174101. [Google Scholar] [CrossRef] [PubMed]
- Chernev, P.; Zaharieva, I.; Rossini, E.; Galstyan, A.; Dau, H.; Knapp, E.W. Merging Structural Information from X-ray Crystallography, Quantum Chemistry and EXAFS Spectra: The Oxygen Evolving Complex in PSII. J. Phys. Chem. B 2016, 120, 10899–10922. [Google Scholar] [CrossRef] [PubMed]
- Hernándezvaldés, D.; Alberto, R.; Jáureguihaza, U. Quantum chemistry calculations of technetium and rhenium compounds with application in radiopharmacy: Review. RSC Adv. 2016, 6, 107127–107140. [Google Scholar] [CrossRef]
- Kim, W.Y.; Choi, Y.C.; Min, S.K.; Cho, Y.; Kim, K.S. Application of quantum chemistry to nanotechnology: Electron and spin transport in molecular devices. Cheminform 2009, 38, 2319–2333. [Google Scholar] [CrossRef]
- Zhang, L.; Min, G.; Zheng, X.; Liu, C.; Yang, J. Application of Quantum Chemistry Method in the Performance Evaluation and Mechanism Study of Corrosion Inhibitors. Corros. Prot. 2017, 38, 829–833. [Google Scholar]
- Carloni, P.; Alber, F. Quantum Medicinal Chemistry; Wiley-VCH: Berlin, Germany, 2005. [Google Scholar]
- Lyne, P.D.; Hodoscek, M.; Karplus, M. A Hybrid QM-MM Potential Employing Hartree-Fock or Density Functional Methods in the Quantum Region. J. Phys. Chem. A 1999, 103, 3462–3471. [Google Scholar] [CrossRef]
- Dinner, A.R.; Lopez, X.; Karplus, M. A charge-scaling method to treat solvent in QM/MM simulations. Theor. Chem. Acc. 2003, 109, 118–124. [Google Scholar] [CrossRef]
- Reuter, N.; Dejaegere, A.; Maigret, B.; Karplus, M. Frontier Bonds in QM/MM Methods: A Comparison of Different Approaches. J. Phys. Chem. A 2000, 104, 1720–1735. [Google Scholar] [CrossRef]
- Pezeshki, S.; Lin, H. Adaptive-Partitioning QM/MM for Molecular Dynamics Simulations: 4. Proton Hopping in Bulk Water. J. Chem. Theory Comput. 2015, 11, 2398–2411. [Google Scholar] [CrossRef]
- Zhou, Y.Q. Martin Karplus Feeling of winning. J. Seek. Knowl. Guide 2013, 2, 150–151. [Google Scholar]
- And, Q.C.; Karplus, M. Quantum Mechanical/Molecular Mechanical Studies of the Triosephosphate Isomerase-Catalyzed Reaction: Verification of Methodology and Analysis of Reaction Mechanisms. J. Phys. Chem. B 2002, 106, 1768–1798. [Google Scholar]
- Chung, L.W.; Sameera, W.M.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.F.; Liu, F.Y. The ONIOM method and Its Applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Humbel, S.; Froese, R.D.J.; Matsubara, T.; Sieber, S.; Morokuma, K. ONIOM: A Multilayered Integrated MO + MM Method for Geometry Optimizations and Single Point Energy Predictions. A Test for Diels-Alder Reactions and Pt(P(t-Bu)3)2 + H2 Oxidative Addition. J. Phys. Chem. 1996, 100, 174–186. [Google Scholar] [CrossRef]
- Vreven, T.; Byun, K.S.; Komáromi, I.; Dapprich, S.; Montgomery, J.A.; Morokuma, K.; Frisch, M.J. Combining Quantum Mechanics Methods with Molecular Mechanics Methods in ONIOM. J. Chem. Theory Comput. 2006, 2, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Vreven, T.; Morokuma, K.; Farkas, O.; Schlegel, H.B.; Frisch, M.J. Geometry optimization with QM/MM, ONIOM, and other combined methods. I. Microiterations Constraints. J. Comput. Chem. 2003, 24, 760–769. [Google Scholar] [CrossRef]
- Adeniyi, A.A.; Soliman, M.E.S. Implementing QM in docking calculations: Is it a waste of computational time? Drug Discov. Today 2017, 22, 1216–1223. [Google Scholar] [CrossRef]
- Chaskar, P.; Zoete, V.; Röhrig, U.F. On-the-fly QM/MM Docking with Attracting Cavities. J. Chem. Inf. Model. 2016, 57, 73–84. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Z.; Zhong, N.; Shen, H.J.; Tu, Z.G.; Liu, H.Q.; Lu, R.Z. QM/MM–PB/SA scoring of the interaction strength between Akt kinase and apigenin analogues. Comput. Biol. Chem. 2014, 52, 25–33. [Google Scholar] [CrossRef]
- Raha, K.; Peters, M.B.; Wang, B.; Yu, N.; Wollacott, A.M.; Westerhoff, L.M.; Merz, K.M. The role of quantum mechanics in structure-based drug design. Drug Discov. Today 2007, 12, 725–731. [Google Scholar] [CrossRef]
- Kelly, E.B.; Tuszynski, J.A.; Klobukowski, M. QM and QM/MD simulations of the Vinca alkaloids docked to tubulin. J. Mol. Gr. Model. 2011, 30, 54–66. [Google Scholar] [CrossRef]
- Adam, P.; Martin, L.; Jan, Ř.; Jiří, B.; Pavel, M.; Pavlína, Ř.; Pavel, H.; Jindřich, F. QM/MM calculations reveal the different nature of the interaction of two carborane-based sulfamide inhibitors of human carbonic anhydrase II. J. Phys. Chem. B 2013, 117, 16096–16104. [Google Scholar]
- Alzate-Morales, J.; Caballero, J. Computational study of the interactions between guanine derivatives and cyclin-dependent kinase 2 (CDK2) by CoMFA and QM/MM. J. Chem. Inf. Model. 2010, 50, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Cebriã, N.-P.A.; Rovira, T.; Saura, P.; González, À.L.; Lluch, J.M. On the Inhibition of Mammalian 15-Lipoxygenase by Three Ebselen-like Drugs. A QM/MM and MM/PBSA Comparative Study. J. Phys. Chem. A 2017, 121, 9752–9763. [Google Scholar]
- Friesner, R.A. Combined quantum and molecular mechanics (QM/MM). Drug Discov. Today Technol. 2004, 1, 253–260. [Google Scholar] [CrossRef]
- Ahmed, M.; Sadek, M.M.; Serrya, R.A.; Kafafy, A.H.N.; Abouzid, K.A.; Wang, F. Assessment of new anti-HER2 ligands using combined docking, QM/MM scoring and MD simulation. J. Mol. Gr. Model. 2013, 40, 91–98. [Google Scholar] [CrossRef]
- Schneider, G. Virtual screening: An endless staircase? Nat. Rev. Drug Discov. 2010, 9, 273–276. [Google Scholar] [CrossRef]
- Yuan, K.; Min, W.; Wang, X.; Li, J.X.; Kuang, W.B.; Zhang, F.; Xie, S.N.; Yang, P. Discovery of novel and selective CDK4/6 inhibitors by pharmacophore and structure-based virtual screening. Future Med. Chem. 2020, 12, 1121–1136. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, S.; Liu, H.; Liu, T.; Yang, Q. Discovery of Multitarget Inhibitors against Insect Chitinolytic Enzymes via Machine Learning-Based Virtual Screening. J. Agric. Food Chem. 2023, 71, 8769–8777. [Google Scholar] [CrossRef]
- Gahlawat, A.; Kumar, N.; Kumar, R.; Sandhu, H.; Singh, I.P.; Singh, S.; Sjöstedt, A.; Garg, P. Structure-based virtual screening to discover potential lead molecules for the SARS-CoV-2 main protease. J. Chem. Inf. Model. 2020, 60, 5781–5793. [Google Scholar] [CrossRef]
- Nie, F.; Kunciw, D.L.; Wilcke, D.; Stokes, J.E.; Galloway, W.R.J.D.; Bartlett, S.; Sore, H.F.; Spring, D.R. A Multidimensional Diversity-Oriented Synthesis Strategy for Structurally Diverse and Complex Macrocycles. Angew. Chem. Int. Ed. Engl. 2016, 55, 11139–11143. [Google Scholar] [CrossRef]
- Lehn, J.M. Dynamic Combinatorial Chemistry and Virtual Combinatorial Libraries. Chem. A Eur. J. 2015, 5, 2455–2463. [Google Scholar] [CrossRef]
- Szymkuć, S.; Gajewska, E.P.; Klucznik, T.; Molga, K.; Dittwald, P.; Startek, M.; Bajczyk, M.; Grzybowski, B.A. Computer-Assisted Synthetic Planning: The End of the Beginning. Angew. Chem. 2016, 55, 5904–5937. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Reddy, S.; Sardeshmukh, A.; Gautham, B.P.; Shroff, G.; Srinivasan, A. Application of Machine Learning Techniques for Inverse Prediction in Manufacturing Process Chains. In Proceedings of the 3rd World Congress on Integrated Computational Materials Engineering (ICME 2015), Colorado Springs, CO, USA, 31 May–4 June 2015. [Google Scholar]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Aron, W. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Granda, J.M.; Donina, L.; Dragone, V.; Long, D.L.; Cronin, L. Controlling an organic synthesis robot with machine learning to search for new reactivity. Nature 2018, 559, 377–381. [Google Scholar] [CrossRef]
- Segler, M.H.S.; Preuss, M.; Waller, M.P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 2018, 555, 604–610. [Google Scholar] [CrossRef]
- Dudek, A.Z.; Tomasz, A.; Jorge, G. Computational methods in developing quantitative structure-activity relationships (QSAR): A review. Comb. Chem. High Throughput Screen. 2006, 9, 213–228. [Google Scholar] [CrossRef]
- Chen, B. Development of quantitative structure activity relationship (QSAR) model for disinfection byproduct (DBP) research: A review of methods and resources. J. Hazard. Mater. 2015, 299, 260–279. [Google Scholar] [CrossRef]
- Lill, M.A. Multi-dimensional QSAR in drug discovery. Drug Discov. Today 2007, 12, 1013–1017. [Google Scholar] [CrossRef]
- Ghafourian, T.; Zandasrar, P.; Hamishekar, H.; Nokhodchi, A. The effect of penetration enhancers on drug delivery through skin: A QSAR study. J. Control. Release 2004, 99, 113–125. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Devillers, J. Neural Networks in QSAR and Drug Design; Harcourt Brace: San Diego, CA, USA, 1996. [Google Scholar]
- Low, Y.; Uehara, T.; Minowa, Y.; Yamada, H.; Ohno, Y.; Urushidani, T.; Sedykh, A.; Murato, E.; Kuz’min, V.; Fourches, D.; et al. Predicting Drug-induced Hepatotoxicity Using QSAR and Toxicogenomics Approaches. Chem. Res. Toxicol. 2011, 24, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, F.; Topliss, J.G. QSAR model for drug human oral bioavailability. J. Med. Chem. 2000, 43, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Hajalsiddig, T.T.H.; Osman, A.B.M.; Saeed, A.E.M. 2D-QSAR modeling and molecular docking studies on 1H-Pyrazole-1-carbothioamide derivatives as EGFR kinase inhibitors. ACS Omega 2020, 5, 18662–18674. [Google Scholar] [CrossRef] [PubMed]
- Sobhi, W.; Attoui, A.; Lemaoui, T.; Erto, A.; Benguerba, Y. In silico drug discovery of Acetylcholinesterase and Butyrylcholinesterase enzymes inhibitors based on Quantitative Structure-Activity Relationship (QSAR) and drug-likeness evaluation. J. Mol. Struct. 2021, 1229, 129845. [Google Scholar]
- Viskupicova, J.; Danihelova, M.; Majekova, M.; Liptaj, T.; Sturdik, E. Polyphenol fatty acid esters as serine protease inhibitors: A quantum-chemical QSAR analysis. J. Enzym. Inhib. Med. Chem. 2012, 27, 800–809. [Google Scholar] [CrossRef]
- Nongonierma, A.; Fitzgerald, D. Learnings from quantitative structure activity relationship (QSAR) studies with respect to food protein-derived bioactive peptides: A review. RSC Adv. 2016, 6, 75400–75413. [Google Scholar] [CrossRef]
- Mccoy, E.F.; Sykes, M.J. Quantum-mechanical QSAR/QSPR descriptors from momentum-space wave functions. J. Chem. Inf. Comput. Sci. 2003, 43, 545–553. [Google Scholar] [CrossRef]
- Gozalbes, R.; Doucet, J.P.; Derouin, F. Application of topological descriptors in QSAR and drug design: History and new trends. Curr. Drug Targets Infect. Disord. 2002, 2, 93–102. [Google Scholar] [CrossRef]
- Karelson, M.; Lobanov, V.S.; Katritzky, A.R. Quantum-Chemical Descriptors in QSAR/QSPR Studies. Chem. Rev. 1996, 96, 1027–1044. [Google Scholar] [CrossRef]
- Borota, A.; Mracec, M.; Gruia, A.; Rad, R.C.; Ostopovici, L.; Mracec, M. A QSAR study using MTD method and Dragon descriptors for a series of selective ligands of αC adrenoceptor. Eur. J. Med. Chem. 2011, 46, 877–884. [Google Scholar] [CrossRef]
- Davood, A.; Nematollahi, A.; Iman, M.; Shafiee, A. Computational studies of new 1,4-dihydropyridines containing 4-(5)-chloro-2-ethyl-5-(4)-imidazolyl substituent: QSAR and docking. Med. Chem. Res. 2010, 19, 58–70. [Google Scholar] [CrossRef]
- Milan, M.; Mirjana, M.; Desanka, B.; Sanja, M.; Nede, N.; Vladimir, M.; Nenad, V.; Slobodan, S.; Slavica, S. In Vitro Antioxidant Activity of Selected 4-Hydroxy-chromene-2-one Derivatives-SAR, QSAR and DFT Studies. Int. J. Mol. Sci. 2011, 12, 2822–2841. [Google Scholar]
- Zhang, L.; Wan, J.; Yang, G. A DFT-based QSARs study of protoporphyrinogen oxidase inhibitors: Phenyl triazolinones. Bioorg. Med. Chem. 2004, 12, 6183–6191. [Google Scholar] [CrossRef]
- Morales-Bayuelo, A.; Matute, R.A.; Caballero, J. Understanding the comparative molecular field analysis (CoMFA) in terms of molecular quantum similarity and DFT-based reactivity descriptors. J. Mol. Model. 2015, 21, 156. [Google Scholar] [CrossRef]
- Ran, T.; Lu, T.; Yuan, H.; Liu, H.C.; Wang, J.; Zhang, W.W.; Leng, Y.; Lin, G.W.; Zhuang, S.L.; Chen, Y.D. A selectivity study on mTOR/PI3Kα inhibitors by homology modeling and 3D-QSAR. J. Mol. Model. 2012, 18, 171–186. [Google Scholar] [CrossRef]
- Bharate, S.B.; Singh, B.; Bharate, J.B.; Jain, S.K.; Meena, S.; Vishwakarma, R.A. QSAR and Pharmacophore Modeling of N-Acetyl-2-aminobenzothiazole Class of Phosphoinositide-3-kinase-α Inhibitors. Med. Chem. Res. 2012, 22, 890–899. [Google Scholar] [CrossRef]
- Wrobel, T.M.; Kiełbus, M.; Kaczor, A.A.; Kryštof, V.; Karczmarzyk, Z.; Wysocki, W.; Fruziński, A.; Król, S.K.; Grabarska, A.; Stepulak, A.; et al. Discovery of nitroaryl urea derivatives with antiproliferative properties. J. Enzym. Inhib. Med. Chem. 2016, 31, 608–618. [Google Scholar] [CrossRef]
- Yang, S.Y. Pharmacophore modeling and applications in drug discovery: Challenges and recent advances. Drug Discov. Today 2010, 15, 444–450. [Google Scholar] [CrossRef]
- Schaller, D.; Šribar, D.; Noonan, T.; Deng, L.H.; Nguyen, T.N.; Pach, S.; Machalz, D.; Bermudez, M.; Wolber, G. Next generation 3D pharmacophore modeling. Wiley Interdiscip. Rev. 2020, 10, e1468. [Google Scholar] [CrossRef]
- Giordano, D.; Biancaniello, C.; Argenio, M.A.; Facchiano, A. Drug Design by Pharmacophore and Virtual Screening Approach. Pharmaceuticals 2022, 15, 646. [Google Scholar] [CrossRef]
- Seidel, T.; Wieder, O.; Garon, A.; Langer, T. Applications of the Pharmacophore Concept in Natural Product inspired Drug Design. Mol. Informat. 2020, 39, 2000059. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Wang, X.; Qiang, S.J.; Zhao, Z.N.; Wu, Z.X.; Ashby, C.R.; Li, J.Z.; Chen, Z.S. The discovery of novel BCR-ABL tyrosine kinase inhibitors using a pharmacophore modeling and virtual screening approach. Front. Cell Dev. Biol. 2021, 9, 649434. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.T.; Fang, S.W.; Li, Z.S.; Xiao, D.X.; Cheng, J.L.; Ying, H.Z.; Du, Y.J.; Zhao, J.H.; Dong, X.W. Discovery of novel succinate dehydrogenase inhibitors by the integration of in silico library design and pharmacophore mapping. J. Agric. Food Chem. 2017, 65, 3204–3211. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Wu, J.W.; Liu, H.L.; Zhao, J.H.; Liu, K.T.; Chuang, C.K.; Lin, H.Y.; Tsai, W.B.; Ho, Y. The discovery of potential acetylcholinesterase inhibitors: A combination of pharmacophore modeling, virtual screening, and molecular docking studies. J. Biomed. Sci. 2011, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.T.; Akl-Yalcin, E. Pharmacophore Modeling in Drug Discovery: Methodology and Current Status. J. Turk. Chem. Soc. Sec. A Chem. 2021, 8, 748–762. [Google Scholar] [CrossRef]
- Seidel, T.; Schuetz, D.A.; Garon, A.; Langer, T. The Pharmacophore Concept and Its Applications in Computer-Aided Drug Design. Prog. Chem. Org. Nat. Prod. 2019, 110, 99–141. [Google Scholar]
- Levinson, A.D.; Oppermann, H.; Levintow, L.; Varmus, H.E.; Bishop, J.M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell 1978, 15, 561–572. [Google Scholar] [CrossRef]
- Lerea, K.M.; Tonks, N.K.; Krebs, E.G.; Fischer, E.H.; Glomset, J.A. Vanadate and molybdate increase tyrosine phosphorylation in a 50-kilodalton protein and stimulate secretion in electropermeabilized platelets. Biochemistry 1989, 28, 9286–9292. [Google Scholar] [CrossRef]
- Erickson, A.K.; Payne, D.M.; Martino, P.A.; Rossomando, A.J.; Shabanowitz, J.; Weber, M.; Hunt, D.F.; Sturgill, T.W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J. Biol. Chem. 1990, 265, 19728–19735. [Google Scholar] [CrossRef]
- Endicott, J.A.; Johnson, L.N. Protein Kinase Inhibitors: Insights into Drug Design from Structure. Science 2004, 303, 1800–1805. [Google Scholar]
- Hiles, I.D.; Otsu, M.; Volinia, S.; Gout, I.; Dhand, R.; Panyaotou, G.; Ruiz-Larrea, F.; Thompson, A.; Totty, N.F. Phosphatidylinositol 3-kinase: Structure and expression of the 110 kd catalytic subunit. Cell 1992, 70, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.P.; Pirola, L. Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta 1998, 1436, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gray, N.S.; Liu, Y.; Gray, N.S. Rational design of inhibitors that bind to inactive kinase conformations. Nat. Chem. Biol. 2006, 2, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Tengholm, A.; Meyer, T. A PI3-Kinase Signaling Code for Insulin-Triggered Insertion of Glucose Transporters into the Plasma Membrane. Curr. Biol. 2002, 12, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Ung, P.M.; Schlessinger, A. DFGmodel: Predicting protein kinase structures in inactive states for structure-based discovery of type-II inhibitors. ACS Chem. Biol. 2015, 10, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Mk, A.; Jd, V. Small molecule inhibitors of phosphoinositide 3-kinase (PI3K) delta and gamma. Curr. Top. Med. Chem. 2009, 9, 738–753. [Google Scholar]
- Johnson, L.N.; Lewis, R.J. Structural basis for control by phosphorylation. Chem. Rev. 2001, 101, 2209. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2023, 187, 106552. [Google Scholar] [CrossRef]
- Peng, W.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar]
- Knight, Z.A.; Shokat, K.M. Features of selective kinase inhibitors. Chem. Bio. 2005, 12, 621–637. [Google Scholar] [CrossRef]
- Norman, R.A.; Toader, D.; Ferguson, A.D. Structural approaches to obtain kinase selectivity. Trends Pharmacol. Sci. 2012, 33, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.J.; Shomin, C.D.; Ghosh, I. Tinkering outside the kinase ATP box: Allosteric (type IV) and bivalent (type V) inhibitors of protein kinases. Future Med. Chem. 2011, 3, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Lamba, V.; Ghosh, I. New directions in targeting protein kinases: Focusing upon true allosteric and bivalent inhibitors. Curr. Pharm. Des. 2012, 18, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Gazit, A.; Yaish, P.; Gilon, C.; Levitzki, A. Tyrphostins I: Synthesis and biological activity of protein tyrosine kinase inhibitors. J. Med. Chem. 1989, 32, 2344–2352. [Google Scholar] [CrossRef]
- Yaish, P.; Gazit, A.; Gilon, C.; Levitzki, A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science 1988, 242, 933–935. [Google Scholar] [CrossRef]
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002, 1, 493–502. [Google Scholar] [CrossRef]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. Small-molecule kinase inhibitors: An analysis of FDA-approved drugs. Drug Discov. Today 2016, 21, 5–10. [Google Scholar] [CrossRef]
- Dorsch, D.; Schadt, O.; Stieber, F.; Meyring, M.; Grädler, U.; Bladt, F.; Friese-Hamim, M.; Knühl, C.; Pehl, U.; Blaukat, A. Identification and optimization of pyridazinones as potent and selective c-Met kinase inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1597–1602. [Google Scholar] [CrossRef]
- Lanman, B.A.; Allen, J.R.; Allen, J.G.; Amegadzie, A.K.; Ashton, K.S.; Booker, K.S.; Chen, J.J.; Chen, N.; Frohn, M.J.; Goodman, J.; et al. Discovery of a covalent inhibitor of KRASG12C (AMG 510) for the treatment of solid tumors. J. Med. Chem. 2020, 63, 52–65. [Google Scholar] [CrossRef]
- Schoepfer, J.; Jahnke, W.; Berellini, G.; Buonamici, S.; Cotesta, S.; Cowan-Jacob, S.W.; Dodd, S.; Drueckes, P.; Fabbro, D.; Gabriel, T.; et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J. Med. Chem. 2018, 61, 8120–8135. [Google Scholar] [CrossRef]
- William, A.D.; Lee, A.C.H.; Blanchard, S.; Poulsen, A.; Teo, E.L.; Nagaraj, H.; Tan, E.; Chen, D.Z.; Williams, M.; Sun, E.T.; et al. Discovery of the Macrocycle 11-(2-Pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a Potent Janus Kinase 2/Fms-Like Tyrosine Kinase-3 (JAK2/FLT3) Inhibitor for the Treatment of Myelofibrosis and Lymphoma. J. Med. Chem. 2011, 54, 4638–4658. [Google Scholar] [PubMed]
- Wrobleski, S.T.; Moslin, R.; Lin, S.; Zhang, Y.L.; Spergel, S.; Kempson, J.; Tokarski, J.S.; Strnad, J.; Zupa-Fernandez, A.; Cheng, L.H.; et al. Highly Selective Inhibition of Tyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery of the Allosteric Inhibitor BMS-986165. J. Med. Chem. 2019, 62, 8973–8995. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.B.; Fischer, J.P.; Baer, B.R.; Blake, J.F.; Bouhana, K.; Briere, D.M.; Brown, K.D.; Burgess, L.E.; Burns, A.C.; Burkard, M.R.; et al. Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer. J. Med. Chem. 2020, 63, 6679–6693. [Google Scholar] [CrossRef] [PubMed]
- Nivolas, G. Compounds Useful as Kinase Inhibitors. WO2017103611, 22 June 2017. [Google Scholar]






















| Software | Scoring Function | Charge | Website | Ref. |
|---|---|---|---|---|
| Amber | Mainly for biological system | AmberTools Free | http://ambermd.org/, accessed on 16 July 2023). | [87] |
| CPMD | Biological and chemical systems | Free | http://www.cpmd.org/, accessed on 16 July 2023). | [88] |
| NAMD | Biological and chemical systems | Free | http://www.ks.uiuc.edu/Research/namd, accessed on 16 July 2023). | [89] |
| Lammps | Material and solid-state physical systems | Free | https://www.lammps.org/, accessed on 16 July 2023). | [90] |
| Gromacs | Mainly for biological system | Free | https://www.gromacs.org/, accessed on 16 July 2023). | [91] |
| Charmm | Mainly for biological system | Free | https://www.charmm.org/, accessed on 16 July 2023). | [92] |
| Tinker | Mainly for biological system | Free | http://dasher.wustl.edu/tinker/, accessed on 16 July 2023). | [93] |
| Definition | Name |
|---|---|
| Charges | |
| QA | net atomic charge on atom A |
| Qmin, Qmax | net charges of the most negative and most positive atoms |
| QAB | net group charge on atoms A and B |
| QT, QA | sum of absolute values of the charges of all the atoms in a given molecule |
| QT2, QA2 | sum of squares of the charges of all the atoms in a given molecule or functional group |
| Qm | mean absolute atomic charge (i.e., the average of the absolute values of the charges on all atoms) |
| HOMO and LUMO Energies | |
| EHOMO, ELUMO | energies of the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) |
| ∆ELUMO-HOMO | HOMO and LUMO orbital energy difference |
| η = (ELUMO − EHOMO)/2 | hardness |
| S = 1/(ELUMO − EHOMO). | softness |
| ∆η = ηR − ηT | activation hardness. R and T stand for reactant and transition states |
| Molecular Polarizabilities | |
| α | molecular polarizability |
| α = (αxx + αyy + αzz)/3 | mean polarizability of the molecule |
| β2 = [(αxx − αyy)2 + (αyy − αzz)2 + (αzz − αxx)2] | anisotropy of the polarizability |
| Dipole Moments and Polarity Indices | |
| µ | molecular dipole moment |
| µchar, µ | charge and hybridization components of the dipole moment |
| µ2 | square of the molecular dipole moment |
| DX, DY, DZ | components of dipole moment along inertia axes |
| ∆ | submolecular polarity parameter (largest difference in electron charges between two atoms) |
| τ | quadrupole moment tensor |
| Energies | |
| E | total energy |
| H | Enthalpy |
| G | Gibbs free energy |
| S | entropy |
| IP | ionization potential |
| EA | electron affinity, difference in total energy between the neutral and anion radical species |
| Orbital Electron Densities | |
| qA, σ, qA, π | σ- and π-electron densities of atom A |
| QA,H, QA,L | HOMO/LUMO electron densities of atom A |
| FrE = frE/EHOMO | electrophilic atomic frontier electron densities |
| FrN = frN/ELUMO | |
| Atom–Atom Polarizabilities | |
| πAA, πAB | self–atom polarizabilities and atom–atom polarizabilities |
| Superdelocalizabilities | |
| SE, A, SN, A | electrophilic and nucleophilic superdelocalizabilities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, S.; Wang, B.; Liu, F.; Xu, S.; Li, P.; Chen, Y. An Updated Review on Developing Small Molecule Kinase Inhibitors Using Computer-Aided Drug Design Approaches. Int. J. Mol. Sci. 2023, 24, 13953. https://doi.org/10.3390/ijms241813953
Li L, Liu S, Wang B, Liu F, Xu S, Li P, Chen Y. An Updated Review on Developing Small Molecule Kinase Inhibitors Using Computer-Aided Drug Design Approaches. International Journal of Molecular Sciences. 2023; 24(18):13953. https://doi.org/10.3390/ijms241813953
Chicago/Turabian StyleLi, Linwei, Songtao Liu, Bi Wang, Fei Liu, Shu Xu, Pirui Li, and Yu Chen. 2023. "An Updated Review on Developing Small Molecule Kinase Inhibitors Using Computer-Aided Drug Design Approaches" International Journal of Molecular Sciences 24, no. 18: 13953. https://doi.org/10.3390/ijms241813953
APA StyleLi, L., Liu, S., Wang, B., Liu, F., Xu, S., Li, P., & Chen, Y. (2023). An Updated Review on Developing Small Molecule Kinase Inhibitors Using Computer-Aided Drug Design Approaches. International Journal of Molecular Sciences, 24(18), 13953. https://doi.org/10.3390/ijms241813953





