Application of Kombucha Fermentation Broth for Antibacterial, Antioxidant, and Anti-Inflammatory Processes
Abstract
:1. Introduction
2. Results
2.1. Bacteriostatic Activity of Tea Culture Broth on E. coli and S. aureus
2.2. Antioxidant Activity of Kombucha Fermentation Broth
2.3. Cytotoxicity of Kombucha Fermentation Broth to L929 and RAW264.7 Cells
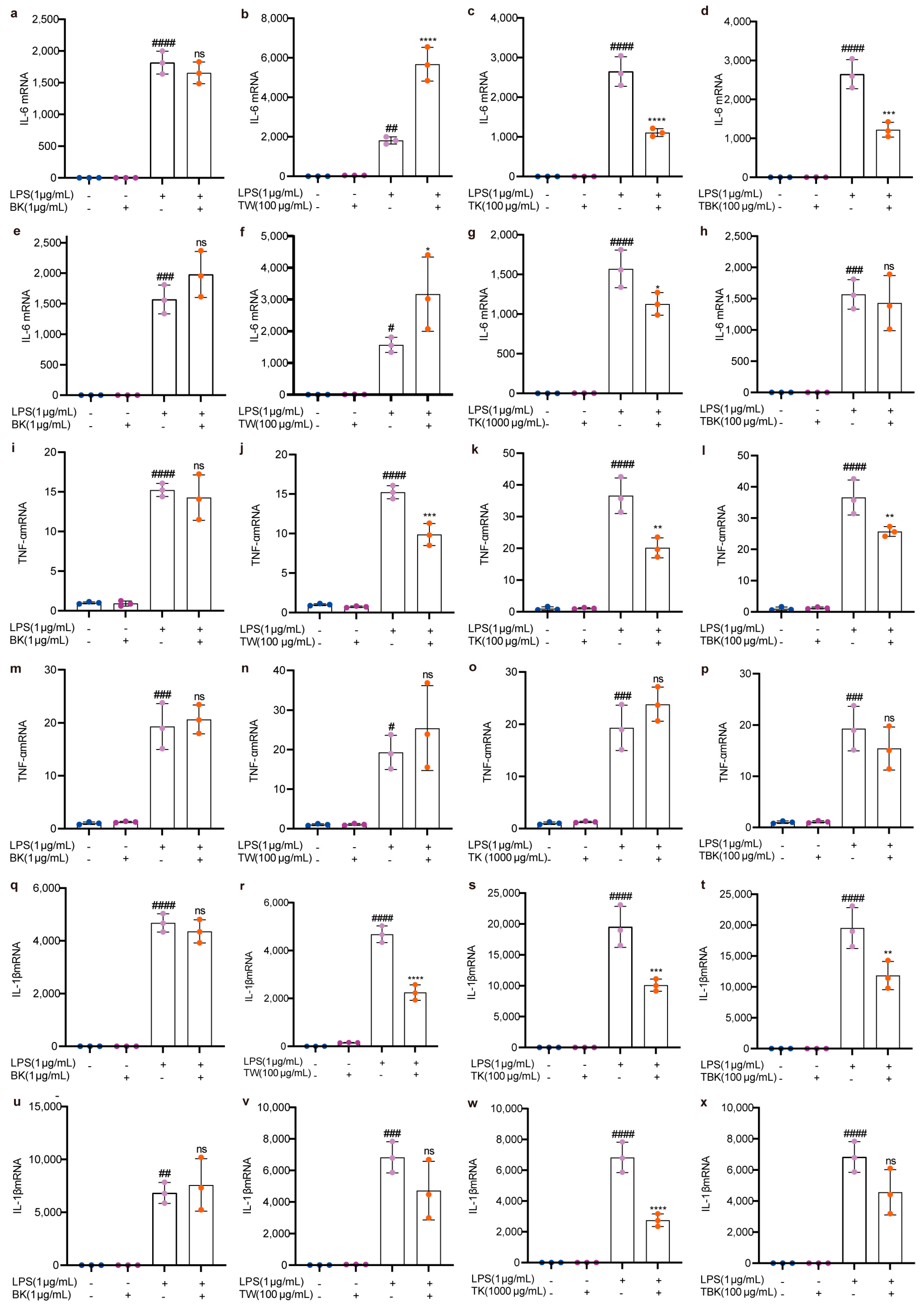
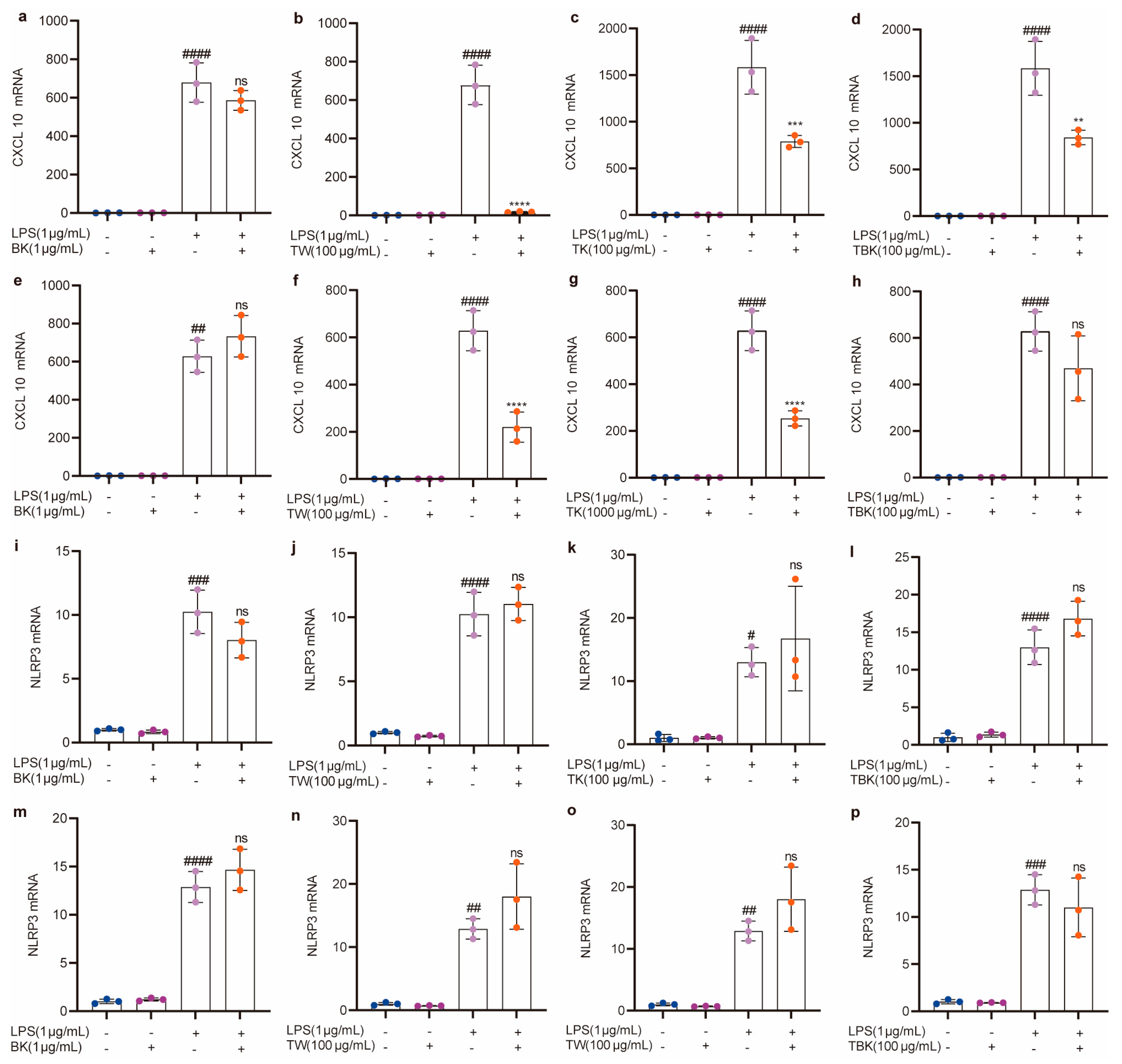
2.4. Effects of Lyophilized Powder in Turmeric Groups on the Expression Level of Cellular Inflammatory Factors Caused by LPS
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Strains
4.3. Domestication and Culture of Black Tea Bacteria in Different Media Preparations of Fermentation Broth
4.4. Preparation of Fermentation Broth
4.5. Preparation of Freeze-Dried Powder Samples
4.6. Bacterial Culture and Preparation of Bacterial Liquid
4.7. Antibacterial Activity Test (MIC)
4.8. Bacteriostatic Rate Determination
4.9. Antioxidation Experiment
4.10. Cell Line
4.11. CCK-8 Detection
4.12. Cell Grouping Treatment
4.13. Real-Time Quantitative PCR
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Cao, C.; Yu, M.; Chai, Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cai, S.; Su, J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhu, M.L.; Wu, Q.; Huang, Y.; Xu, X.L.; Chen, W. The Potential of Drug Delivery Nanosystems for Sepsis Treatment. J. Inflamm. Res. 2021, 14, 7065–7077. [Google Scholar] [CrossRef]
- Mann, F.M.; Dickmann, M.; Schneider, R.; Armando, S.; Seehusen, K.; Hager, P.; Strauss, M.J. Analysis of the role of acidity and tea substrate on the inhibition of α-amylase by Kombucha. J. Nutr. Food Res. Technol. 2017, 1–5. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Wang, J.; Geng, W. Kombucha reduces hyperglycemia in Type 2 diabetes of mice by regulating gut microbiota and its metabolites. Foods 2022, 11, 754. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Enhancement of the antioxidant and starch hydrolase inhibitory activities of king coconut water (Cocos nucifera var. aurantiaca) by fermentation with kombucha ‘tea fungus’. Int. J. Food Sci. Technol. 2016, 51, 490–498. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Bhattacharya, S.; Patra, M.M.; Chakravorty, S.; Sarkar, S.; Chakraborty, W.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of kombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, H.; Hashemi Gahruie, H.; Golmakani, M.T.; Eskandari, M.H.; Movahedi, M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of kombucha. Food Sci. Nutr. 2018, 6, 2568–2577. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, Y.; Chen, J. Changes in functional components and biological activity of Lycium barbarum after fermentation with Kombucha SCOBY. J. Food Process. Preserv. 2022, 46, e16758. [Google Scholar] [CrossRef]
- Wang, P.; Feng, Z.; Sang, X.; Chen, W.; Zhang, X.; Xiao, J.; Chen, Y.; Chen, Q.; Yang, M.; Su, J. Kombucha ameliorates LPS-induced sepsis in a mouse model. Food Funct. 2021, 12, 10263–10280. [Google Scholar] [CrossRef]
- Vázquez-Cabral, B.D.; Larrosa-Pérez, M.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Rutiaga-Quiñones, J.G.; Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E. Oak kombucha protects against oxidative stress and inflammatory processes. Chem. Biol. Interact. 2017, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A pharmacological review of bioactive constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother. Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The application of fermentation technology in traditional Chinese medicine: A review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Kan, H.; Zhang, D.; Chen, W.; Wang, S.; He, Z.; Pang, S.; Qu, S.; Wang, Y. Identification of anti-inflammatory components in panax ginseng of Sijunzi Decoction based on spectrum-effect relationship. Chin. Herb. Med. 2023, 15, 123–131. [Google Scholar] [CrossRef]
- Han, J.M.; Lee, E.K.; Gong, S.Y.; Sohng, J.K.; Kang, Y.J.; Jung, H.J. Sparassis crispa exerts anti-inflammatory activity via suppression of TLR-mediated NF-κB and MAPK signaling pathways in LPS-induced RAW264.7 macrophage cells. J. Ethnopharmacol. 2019, 231, 10–18. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Sun, T.Y.; Li, J.S.; Chen, C. Effects of blending wheatgrass juice on enhancing phenolic compounds and antioxidant activities of traditional kombucha beverage. J. Food Drug Anal. 2015, 23, 709–718. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Wang, F.; Xu, J.; Tang, X.; Li, N. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Akter, J.; Hossain, M.A.; Takara, K.; Islam, M.Z.; Hou, D.X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp): Isolation of active compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 215, 9–17. [Google Scholar] [CrossRef]
- Shakya, S.; Danshiitsoodol, N.; Sugimoto, S.; Noda, M.; Sugiyama, M. Anti-oxidant and anti-inflammatory substance generated newly in paeoniae radix alba extract fermented with plant-derived Lactobacillus brevis 174A. Antioxidants 2021, 10, 1071. [Google Scholar] [CrossRef]
- Yong, C.C.; Yoon, Y.; Yoo, H.S.; Oh, S. Effect of lactobacillus fermentation on the anti-inflammatory potential of turmeric. J. Microbiol. Biotechnol. 2019, 29, 1561–1569. [Google Scholar] [CrossRef]
- Lim, J.; Nguyen, T.T.H.; Pal, K.; Gil Kang, C.; Park, C.; Kim, S.W.; Kim, D. Phytochemical properties and functional characteristics of wild turmeric (Curcuma aromatica) fermented with Rhizopus oligosporus. Food Chem. X 2022, 13, 100198. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Moreira, L.P.D.; de Campos Costa, M.A.; Toledo, R.C.L.; Grancieri, M.; Nascimento, T.P.D.; Ferreira, M.S.L.; da Matta, S.L.P.; Eller, M.R.; Duarte Martino, H.S.; et al. Kombuchas from green and black teas reduce oxidative stress, liver steatosis and inflammation, and improve glucose metabolism in Wistar rats fed a high-fat high-fructose diet. Food Funct. 2021, 12, 10813–10827. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; Ben Abid, S.; Hamdi, M. Development of a beverage from red grape juice fermented with the Kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of snake fruit (Salacca zalacca (Gaerth.) Voss) for the development of a beverage through fermentation with the Kombucha consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef]
- Zubaidah, E.; Nisak, Y.K.; Wijayanti, S.A.; Christianty, R.A. Characteristic of microbiological, chemical, and antibacterial activity of turmeric (Curcuma longa) kombucha. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012080. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Jeong, S.A.; Park, C.W.; Kim, D.H.; Lim, B.O. The anti-inflammatory activities of fermented curcuma that contains butyrate mitigate DSS-induced colitis in mice. Molecules 2022, 27, 4745. [Google Scholar] [CrossRef] [PubMed]
- Pianpumepong, P.; Anal, A.K.; Doungchawee, G.; Noomhorm, A. Study on enhanced absorption of phenolic compounds of Lactobacillus-fermented turmeric (Curcuma longa Linn.) beverages in rats. Int. J. Food Sci. Technol. 2012, 47, 2380–2387. [Google Scholar] [CrossRef]




| Fermentation Time/d | BK | TE | TK | TBK |
|---|---|---|---|---|
| 7 | 2.5 ± 0 | - | 5 ± 0 | 2.5 ± 0 |
| 14 | 1.25 ± 0 | - | 2.5 ± 0 | 2.5 ± 0 |
| Fermentation Time/d | BK | TE | TK | TBK |
|---|---|---|---|---|
| 7 | 1.25 ± 0 | - | 5 ± 0 | 1.25 ± 0 |
| 14 | 0.625 ± 0 | - | 1.25 ± 0 | 1.25 ± 0 |
| Fermentation Time/d | BK | PE | PK | TPK |
|---|---|---|---|---|
| 7 | 2.5 ± 0 | - | 1.25 ± 0 | 1.25 ± 0 |
| 14 | 0.625 ± 0 | 2.5 ± 0 | 0.625 ± 0 | 0.3125 ± 0 |
| Fermentation Time/d | BK | PE | PK | TPK |
|---|---|---|---|---|
| 7 | 1.25 ± 0 | 0.3125 ± 0 | 0.625 ± 0 | 1.25 ± 0 |
| 14 | 0.3125 ± 0 | 0.3125 ± 0 | 0.3125 ± 0 | 0.3125 ± 0 |
| Name and Specification of Drugs | Production Company |
|---|---|
| Black tea (Zhengshan race) | Haomingtian Tea Co., Ltd., Wuyishan, China. |
| Tryptone | Oxoid; Lenexa, KS, USA. |
| Yeast extract | Guangdong Huankai Biotechnology Co., Ltd., Guangzhou City, China. |
| NaCl | National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China. |
| 1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) | Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China. |
| vitamin C | Sigma-Aldrich; Saint Louis, MO, USA. |
| DMEM/HIGH GLUCOSE | Cytiva, Logan, UT, USA. |
| Penicillin–streptomycin double antibody | Beijing Soleibao Technology Co., Ltd., Beijing, China. |
| Gibco™ FBS | Samufei Invitrogen, Shanghai, China. |
| Trypan blue dye | Beijing Soleibao Technology Co., Ltd., Beijing, China. |
| Trypsin | HyClone Company of USA, Logan, UT, USA. |
| Lipopolysaccharide (LPS) | Cell Signaling Technology, Danvers, MA, USA. |
| CCK-8 Kit | Beijing Biyuntian Institute of Biotechnology, Beijing, China. |
| Trizol | Life Technologies Corporation, Carlsbad, CA, USA. |
| Trichloromethane | National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China. |
| Isopropyl alcohol propan-2-ol | Sigma-Aldrich. St. Louis, MO, USA. |
| ethanol | Xilong chemical co., Ltd., Chengdu, China. |
| RNA reverse transcription kit | Mona Biotechnology Co., Ltd., Suzhou, China. |
| qPCR SYBR Green Master Mix | Yisheng Biotechnology Co., Ltd., Shanghai, China. |
| Cell Culture Plate | Wuxi NEST Biotechnology Co., Ltd., Wuxi, China. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Tan, Q.; Wu, S.; Abbas, B.; Yang, M. Application of Kombucha Fermentation Broth for Antibacterial, Antioxidant, and Anti-Inflammatory Processes. Int. J. Mol. Sci. 2023, 24, 13984. https://doi.org/10.3390/ijms241813984
Su J, Tan Q, Wu S, Abbas B, Yang M. Application of Kombucha Fermentation Broth for Antibacterial, Antioxidant, and Anti-Inflammatory Processes. International Journal of Molecular Sciences. 2023; 24(18):13984. https://doi.org/10.3390/ijms241813984
Chicago/Turabian StyleSu, Jingqian, Qingqing Tan, Shun Wu, Bilal Abbas, and Minhe Yang. 2023. "Application of Kombucha Fermentation Broth for Antibacterial, Antioxidant, and Anti-Inflammatory Processes" International Journal of Molecular Sciences 24, no. 18: 13984. https://doi.org/10.3390/ijms241813984
APA StyleSu, J., Tan, Q., Wu, S., Abbas, B., & Yang, M. (2023). Application of Kombucha Fermentation Broth for Antibacterial, Antioxidant, and Anti-Inflammatory Processes. International Journal of Molecular Sciences, 24(18), 13984. https://doi.org/10.3390/ijms241813984






