Recent Advances in the Aggregation Behavior of Nanoplastics in Aquatic Systems
Abstract
:1. Introduction
- Summarize the recent research on MNPs pollution in aquatic environments.
- Discuss and clarify the supramolecular approaches and methods to promote the removal of NPs from aquatic systems.
- Summarize and discuss the recent approaches and methods applied in WTPs to minimize the release of NPs into the water cycle.
- Summarize the latest advances and future perspectives on NPs removal from aquatic systems.
2. Methodology
2.1. Aggregation Behavior of Nanoplastics: The Effect of Surface Chemistry and Physical Chemistry of the Surrounding Media
2.2. Proposed Supramolecular Solutions to Remove NPs from the Aquatic Systems
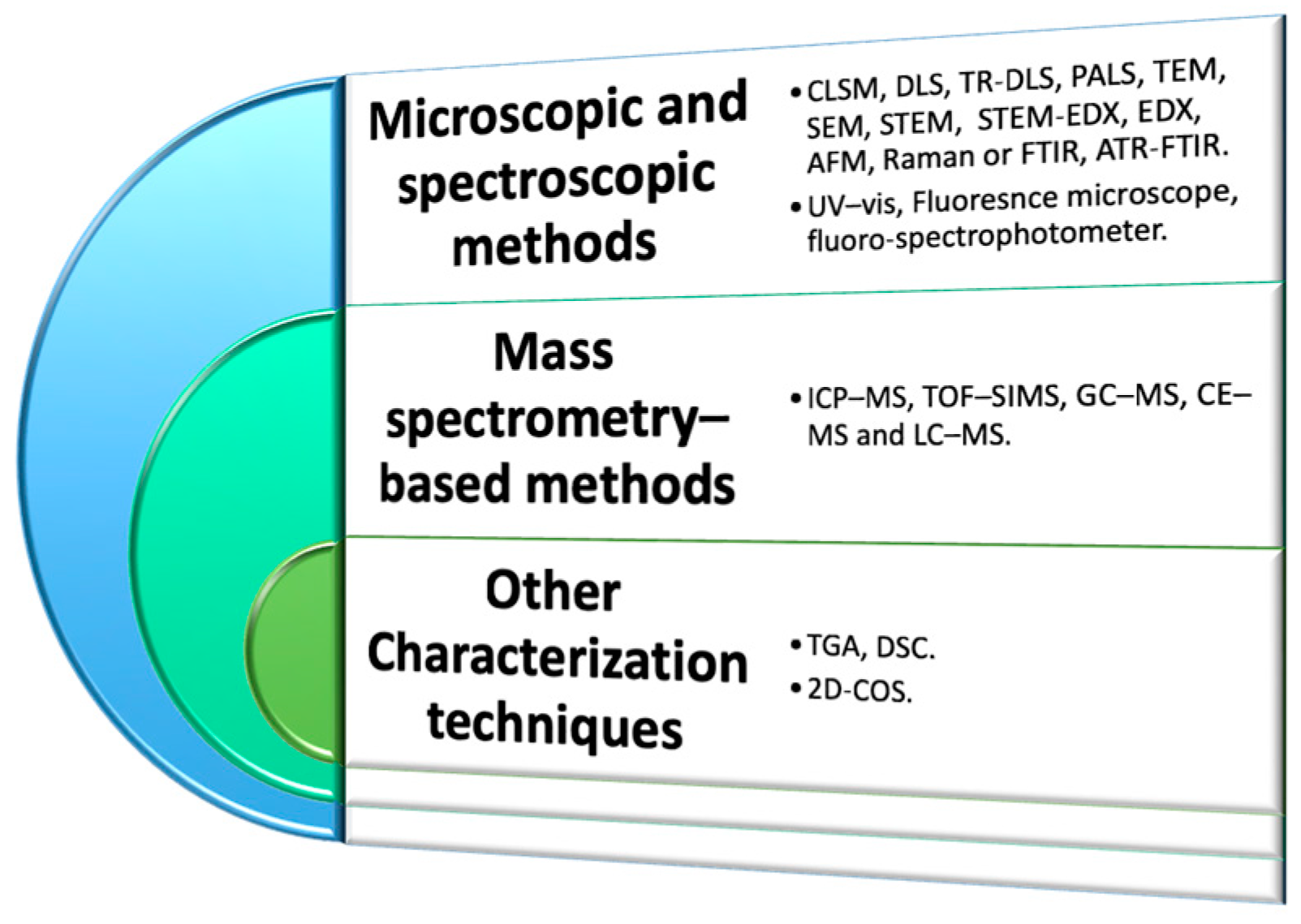
Characterization Methods
2.3. Current NPs’ Removal Efficiency
3. Discussion
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D-COS | Two-dimensional correlation spectroscopy |
| 3D-EEM | Three-dimensional fluorescence excitation–emission matrix |
| AOT | Sodium di-2-Ethylhexyl sulfosuccinate |
| aPLNPs | Amine-modified polystyrene latex NPs |
| ATR-FTIR | Attenuated total reflectance Fourier transform infrared spectroscopy |
| BET | Brunauer–Emmett–Teller |
| BPA | Bisphenol A |
| CCC | The critical coagulation concentration |
| CE–MS | Capillary electrophoresis–mass spectrometry |
| CFS | Coagulation/flocculation combined with sedimentation |
| CLSM | Confocal laser scanning microscopy |
| cPLNPs | Carboxyl-modified polystyrene latex NPs |
| DH | Hydrodynamic diamter |
| DLS | Dynamic Light Scattering |
| DLVO | Derjaguin–Landau–Verwey–Overbeek |
| DOC | Dissolved organic carbon |
| DSC | Differential scanning calorimetry |
| EDTA | Ethylenediaminetetraacetate |
| EDX | Energy dispersive X-ray |
| EPS | Extracellular polymeric substances |
| FTIR | Fourier Transform infrared spectroscopy |
| GC–MS | Gas chromatography–mass spectrometry |
| HA | Humic acid |
| HCH | Hexachlorocyclohexane |
| ICP-MS | Inductively coupled plasma–mass spectrometry |
| ICP–MS | Inductively coupled plasma–mass spectrometry () |
| LC–MS | Liquid chromatography–mass spectrometry |
| LDH | Layered double hydroxide |
| MNPs | Micro- and nano-plastics |
| MPs | Microplastics |
| n-PS–NH2 | Negatively charged amino-modified Polystyrene nanoplastics |
| NOM | Natural organic matter |
| NPDs | Nano-scale plastic debris |
| NPs | Nanoplastics |
| p-PS–NH2 | Positively charged amino-modified Polystyrene nanoplastics |
| PAHs | Polyaromatic hydrocarbons |
| PALS | Phase angle light scattering |
| PBTs | Persistent bioaccumulative and toxic compounds |
| PCBs | Polychlorinated biphenyls |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| pHpzc | Isoelectric point or point zero charge |
| PLNPs | Polystyrene latex NPs |
| PolyDADMAC | Poly-Diallyldimethylammonium Chloride |
| POM | Particulate organic matter |
| POPs | Persistent Organic Pollutants |
| PP | Polypropylene |
| PS | Polystyrene |
| PS-COOH | Carboxyl-modified polystyrene |
| PU | Polyurethane |
| PVAc | Poly(vinyl acetate) |
| PVC | Polyvinyl chloride |
| PXRD | Powder X-ray diffraction |
| PZCs | Point of zero charges |
| SDS | Sodium dodecyl sulfate |
| SEM | Scanning electron microscope |
| SRFA | Suwannee River fulvic acid |
| SRHA | Suwannee River humic acid |
| SRNOM | Suwannee River natural organic matter |
| STEM | Scanning transmission electron microscope |
| STEM-EDX | Scanning transmission electron microscope -Energy dispersive X-ray |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric analysis |
| TGDSC | Thermogravimetric-Differential scanning calorimetry |
| TOC analyzer | Total organic carbon analyzers |
| TOF–SIMS | Time-of-flight secondary ion mass spectrometry |
| uPLNPs | Unmodified polystyrene latex NPs |
| UV-Vis | Ultraviolet-Visible spectrophotometer |
| WTP | Water treatment plant |
| WWTP | Wastewater treatment plant |
| XEDS | X-ray energy-dispersive spectrometer |
| XPS | X-ray photoelectron spectroscopy |
| ζpot | Zeta potential |
References
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental Occurrences, Fate, and Impacts of Microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Plastics Europe’s Market Research and Statistics Group; Conversio Market Strategy GmbH. Plastics—The Facts; PlasticsEurope AISBL: Brussels, Belgium, 2019; Volume 2019. [Google Scholar]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Bashir, S.M.; Kimiko, S.; Mak, C.-W.; Fang, J.K.-H.; Gonçalves, D. Personal Care and Cosmetic Products as a Potential Source of Environmental Contamination by Microplastics in a Densely Populated Asian City. Front. Mar. Sci. 2021, 8, 683482. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Nathan, A.J.; Scobell, A. How China Sees America. Foreign Aff. 2012, 91, 32. [Google Scholar]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic Waste in the Marine Environment: A Review of Sources, Occurrence and Effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Year UNEP Year Book 2013 Emerging Issues in Our Global Environment; United Nations Environment Programme: Nairobi, Kenya, 2013; ISBN 9789280732849. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence That the Great Pacific Garbage Patch Is Rapidly Accumulating Plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in Marine Organism: Environmental and Toxicological Effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Cárdenas-Alcaide, M.F.; Godínez-Alemán, J.A.; González-González, R.B.; Iqbal, H.M.N.; Parra-Saldívar, R. Environmental Impact and Mitigation of Micro(Nano)Plastics Pollution Using Green Catalytic Tools and Green Analytical Methods. Green Anal. Chem. 2022, 3, 100031. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent Advances in Toxicological Research of Nanoplastics in the Environment: A Review. Environ. Pollut. 2019, 252, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Gambinossi, F.; Mylon, S.E.; Ferri, J.K. Aggregation Kinetics and Colloidal Stability of Functionalized Nanoparticles. Adv. Colloid Interface Sci. 2015, 222, 332–349. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic Behavior and Toxicity of Polystyrene Nanoplastic Particles with Different Functional Groups: Complex Roles of PH, Dissolved Organic Carbon and Divalent Cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef]
- Trompette, J.L.; Lahitte, J.F. Influence of the Counterion Nature on the Stability Sequence of Hydrophobic Latex Particles. J. Phys. Chem. B 2019, 123, 3859–3865. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Déniel, M.; Nicolai, T.; Chassenieux, C.; Lagarde, F. Towards More Realistic Reference Microplastics and Nanoplastics: Preparation of Polyethylene Micro/Nanoparticles with a Biosurfactant. Environ. Sci. Nano 2019, 6, 315–324. [Google Scholar] [CrossRef]
- Cai, L.; Hu, L.; Shi, H.; Ye, J.; Zhang, Y.; Kim, H. Effects of Inorganic Ions and Natural Organic Matter on the Aggregation of Nanoplastics. Chemosphere 2018, 197, 142–151. [Google Scholar] [CrossRef]
- Tallec, K.; Blard, O.; González-Fernández, C.; Brotons, G.; Berchel, M.; Soudant, P.; Huvet, A.; Paul-Pont, I. Surface Functionalization Determines Behavior of Nanoplastic Solutions in Model Aquatic Environments. Chemosphere 2019, 225, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shen, M.; Li, S.; Fu, Y.; Zhang, D.; Liu, H.; Liu, J. Aggregation Kinetics of Different Surface-Modified Polystyrene Nanoparticles in Monovalent and Divalent Electrolytes. Environ. Pollut. 2019, 255, 113302. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, R.; Lin, W.; Ouyang, G. Effect of Salinity and Humic Acid on the Aggregation and Toxicity of Polystyrene Nanoplastics with Different Functional Groups and Charges. Environ. Pollut. 2019, 245, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.F.; Vazquez, C.I.; Tsai, Y.Y.; Torres, G.V.; Chen, C.S.; Santschi, P.H.; Quigg, A.; Chin, W.C. Nano-Plastics Induce Aquatic Particulate Organic Matter (Microgels) Formation. Sci. Total Environ. 2020, 706, 135681. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, H.; Huangfu, X.; Liu, Y.; He, Q. Nanoplastics Display Strong Stability in Aqueous Environments: Insights from Aggregation Behaviour and Theoretical Calculations. Environ. Pollut. 2020, 258, 113760. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Charabi, S.; Hamadi, S.; Carbonnier, B. Latex Nanoparticles Surface Modified via the Layer-by-Layer Technique for Two Drugs Loading. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 28–34. [Google Scholar] [CrossRef]
- Cao, H.; An, B.; Wang, Y.; Zhou, K.; Lu, N. Investigation of Surfactant AOT Mediated Charging of PS Particles Dispersed in Aqueous Solutions. Coatings 2019, 9, 471. [Google Scholar] [CrossRef]
- Cid, A.; Acuña, A.; Alonso-Ferrer, M.; Astray, G.; García-Río, L.; Simal-Gándara, J.C.; Mejuto, J. Pseudophase model in microemulsions. In Microemulsion—A Chemical Nanoreactor [Working Title]; IntechOpen: London, UK, 2019. [Google Scholar]
- Moldes, Ó.A.; Cid, A.; Montoya, I.A.; Mejuto, J.C. Linear Polyethers as Additives for AOT-Based Microemulsions: Prediction of Percolation Temperature Changes Using Artificial Neural Networks. Tenside Surfactants Deterg. 2015, 52, 264–270. [Google Scholar] [CrossRef]
- Cid, A.; Gómez-Díaz, D.; Mejuto, J.C.; Navaza, J.M. Viscosity and Percolative Phenomena in AOT Based Microemulsions. Tenside Surfactants Deterg. 2011, 48, 165–169. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Beltzung, A.; Frehland, S.; Schmiedgruber, M.; Cingolani, A.; Schmidt, F. Synthesis of Metal-Doped Nanoplastics and Their Utility to Investigate Fate and Behaviour in Complex Environmental Systems. Nat. Nanotechnol. 2019, 14, 362–368. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Y.; Guo, X.; Xia, T.; Wang, T.; Jia, H.; Zhu, L. Charge Mediated Interaction of Polystyrene Nanoplastic (PSNP) with Minerals in Aqueous Phase. Water Res. 2020, 178, 115861. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Wu, A.; Tang, Z.; Niu, L.; Wu, F.; Wang, F.; Zhao, T.; Fu, Z. Aggregation and Stability of Sulfate-Modified Polystyrene Nanoplastics in Synthetic and Natural Waters. Environ. Pollut. 2020, 268, 114240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal Efficiency of Micro- and Nanoplastics (180 Nm–125 Μm) during Drinking Water Treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, E.; Singh, N.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Application of Zn/Al Layered Double Hydroxides for the Removal of Nano-Scale Plastic Debris from Aqueous Systems. J. Hazard. Mater. 2020, 397, 122769. [Google Scholar] [CrossRef]
- Astray, G.; Soria-Lopez, A.; Barreiro, E.; Mejuto, J.C.; Cid-Samamed, A. Machine Learning to Predict the Adsorption Capacity of Microplastics. Nanomaterials 2023, 13, 1061. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Wang, L.; Zhao, X.; Yan, F.; Yang, Y.; Li, G.; Gao, P.; Ji, P. Removal of Polystyrene Nanoplastics from Aqueous Solutions Using a Novel Magnetic Material: Adsorbability, Mechanism, and Reusability. Chem. Eng. J. 2022, 430, 133122. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of Microplastics from Water by Magnetic Nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Bradu, P.; Patil, M.; Biswas, A.; Murali, R.; Renu, K.; Dey, A.; Vellingiri, B.; Raja, G.; et al. Elimination of Microplastics from the Aquatic Milieu: A Dream to Achieve. Chemosphere 2022, 303, 135232. [Google Scholar] [CrossRef]
- Zhou, G.; Huang, X.; Xu, H.; Wang, Q.; Wang, M.; Wang, Y.; Li, Q.; Zhang, Y.; Ye, Q.; Zhang, J. Removal of Polystyrene Nanoplastics from Water by CuNi Carbon Material: The Role of Adsorption. Sci. Total Environ. 2022, 820, 153190. [Google Scholar] [CrossRef]



| NPs’ Nature and Size | NPs’ Modification | Dispersion Medium | Characterization Methods and Other Experiments | Size of Aggregates/Method Efficiency | Ref. |
|---|---|---|---|---|---|
| polystyrene latex nanoparticles (PLNPs) |
|
| DLS, TEM, UV-vis. |
| [16] |
| PS, 468 nm with a 0.08 PDI | - | Ions and co-ions. The ranking of CCC was IO3− ≤F− < Cl− < NO3− < I− < SCN−. | DLS, SEM, UV-vis. | Competitive behavior of the implied co-ions and counterions within the inner layer at the hydrophobic particle–aqueous solution interface. | [20] |
| Polyethylene particles with radii between 200 and 800 nm | - | The surfactants used to synthesize nanoplastics were Tween 60, Tween 80, and a biosurfactant. Emulsification of PE in toluene solutions in water. | CLSM, TGA, DSC, Bioavailability of PE particles in the presence of Daphnia magna. | - | [21] |
| Yellow-green, fluorescent Polystyrene nanoparticles with a diameter of 0.1 μm. | - |
| DLS, fluorescence microscope, Cryo-SEM. |
| [22] |
| Polystyrene beads (50 nm). |
|
| DLS. |
| [23] |
| 92.8 ± 6.9 nm, 97.3 ± 7.8 nm, 109.2 ± 8.5 nm, 60.2 ± 26.3 nm - data obtained by SEM-. | PS-Bare, PS-COOH PS-NH2 PS-Laser | mono- and divalent cations with and without SRNOM. | DLS, SEM, aggregation kinetics. | Attachment efficiency. | [24] |
| different functional groups and charges (PS, PS-COOH, n-PS-NH2, p-PS-NH2) | Humic acid (HA) and salinity. | TOC analyzer, TEM, DLS, for ζpot used PALS, elemental analyzer; fluorescence quenching of PS was carried by fluoro-spectrophotometer, a 48 h acute toxicity test on Daphnia magna was performed following the OECD guideline 202 (OECD, 2004). | The observations indicated that the stability, transport, and toxicity of NPs in aquatic systems were highly dependent on environmental factors (effect of HA content and salinity). | [25] |
| 25 nm polystyrene nanoparticles | - | Lake and river water can promote POM (microgel) formation and accelerate the DOM–POM transition. Authors adjusted various salinities of water samples to simulate plastic transport scenarios in waters flowing from rivers to seas. The sea salt (NaCl, MgSO4, MgCl2, CaCl2, KCl, and NaHCO3) To further examine the hydrophobic interactions and possible intermolecular cross-linking between DOM and polystyrene, EDTA was added to DOM samples (with/without polystyrene). | DLS, no subsequent micron-scale aggregates present (135.8 ± 79.66 nm). | Polystyrene-induced microgel formation appears to involve the hydrophobic interactions between plastics and DOM. The results indicate that polystyrene nanoparticles can interact with organic matter to form large organic particles. DOM polymers from both sources exhibited a similar assembly curve, reaching microgels of roughly 4–6 μm within 90–120 h. | [26] |
| Polystyrene nanoplastics (nano-PS). The nominal bead size of PS was 100 nm. | Nano-PS was artificially aged in an aging chamber equipped with two symmetrical UV lamps (UVC-254 nm, 15 w) |
| After the aging process, DLS, SEM, and ATR-FTIR changes were evaluated using X-ray photoelectron spectroscopy, ATR-FTIR spectra, and thermogravimetric-differential scanning calorimetry. Time-resolved DLS followed aggregation kinetics. | The hydrodynamic size of nano-PS was 113.8 nm with a range of 58.8–190.0 nm, close to the average diameter observed from the SEM images (104.9 ± 0.76 nm). At the end of the testing period, the hydrodynamic sizes of the nano-PS (more than 600, 1100, and 1200 nm in 400 mM, 500 mM, and 600 mM NaCl, respectively) in solutions without EPS were significantly larger than the hydrodynamic sizes of the nano-PS (less than 400, 800, and 1000 nm in 400 mM, 500 mM, and 600 mM NaCl, respectively) in solutions with EPS, which could be related to steric effects. | [27] |
| Poly(vinyl acetate) (PVAc) latex particles. The size was found to be lower than 200 nm. | NPs were coated with natural polyelectrolytes (alginate and chitosan) via layer-by-layer deposition. | The SDS was used as an emulsifier during the miniemulsion polymerization process. | DLS and TEM highlighted a spherical shape and a size lower than 200 nm. UV–vis. | The implemented strategy offers the advantage of ease and efficiency of nanocarrier elaboration via a scalable process, and the versatility of their preparation makes them adaptable to a range of applications. The process’s efficiency prevents any residual monomer from remaining in the colloids, avoiding toxicity issues. | [28] |
| Polystyrene (PS) particles. Three different sizes: 141, 311, and 535 nm. | NPs were dispersed in aqueous solutions of the surfactant sodium di-2-Ethylhexyl sulfosuccinate (AOT). | DLS; using PALS. | At different AOT concentrations (0.1–10 mM), the size of the PSNPs was 535 nm. | [29] | |
| The particles synthesized were polyacrylonitrile (PAN) core material, which contained the metal tracer, followed by the addition of a cross-linked polystyrene (PS) shell. | The reasoning for this multi-step process was to impart a few key benefits over a single batch emulsion, including acrylonitrile (AN), which is capable of chemically complexing the Pd in the water phase; the core/shell structure would ensure there was minimal leaching of metal from the particle, and the shell could be changed independently of the core. | Batch studies were performed to simulate a municipal WWTP’s activated sludge process using a continuously running pilot-scale system on-site using actual municipal wastewater. |
| Three independent objectives were: (1) evaluating the evenness of particle distribution in well-mixed activated sludge (and thereby optimizing sampling protocol for nanoplastics); (2) determining the affinity for heteroaggregation of nanoplastics with the suspended solids in the sludge (i.e., surface affinity and how quickly nanoplastics adhere to sludge flocs); and (3) assessing total nanoplastic retention in WWTP. | [33] |
| Polystyrene nanoplastic (PSNP) | Incubation of PSNP with other three metal oxides with different surface charges, MnO2, Al2O3, and SiO2 | Four typical minerals, including goethite, magnetite, kaolinite, and montmorillonite, in the aqueous phase were investigated. | TEM, SEM, FTIR, 2D-COS; UV-Vis Absorbance. The ζpot of the three particles as a function of the pH suspension and the PZCs (point of zero charges). |
| [34] |
| Polystyrene (PS) NPs | The PS NPs DH remained relatively constant (90–100 nm) from pH 2.3 to 11.1. | Different hydrochemical conditions such as pH, salt type (NaCl, CaCl2, Na2SO4), ionic strength (IS), and natural organic matter (NOM: SRHA, SRFA) were studied. | TEM, DLS, and TR-DLS, UV-Vis spectroscopy. The PS NPs’ surface composition was determined by X-ray photoelectron spectroscopy. The TOC contents of NOM and natural waters were quantified using the TOC analyzer. | The isoelectric point (pHzpc) of PS NP was <2.3. The ζ-potential of PS NPs was for pH 2.5 to 5.4 (from −15.4 ± 165 0.9 mV to −38.6 ± 0.4 mV) and for pH 5.4 to 11.1 (from −38.6 ± 0.4 mV to −49.6 ± 1.2 mV) | [35] |
| Microplastics and nanoplastics (80 nm, 1.2 μm, 10–20 μm, 45–53 μm, and 106–125 μm)). One was polyethylene (PE) (10–20 μm, 45–53 μm, and 106–125 μm), and the other was polystyrene (PS) (180 nm and 1.2 μm). | - | Coagulant Aid PolyDADMAC, biofilm. | CFS with Coagulant Aid PolyDADMAC. Filtration. Interactions of biofilms and microplastics. | The authors characterized the removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Future research should focus on removing small-sized plastics (10–20 μm) in water treatment processes because these particles are more difficult to remove yet could cause more significant health concerns if ingested. | [36] |
| Polystyrene (PS) nanoparticles (d = diameter of the particles is 55 nm and hydrophilic nature as coated with an anionic surfactant to make them uniformly disperse in water). | The synthesized Zn-Al layered double hydroxide (LDH) was confirmed by pH titration of Zn-Al LDH against NPDs at varying mass ratios (50:1 to 50:7). | ZnCl2, Al(NO3)3.9H2O, NaOH, NaNO3, Na2SO4, NaHPO4, NaHCO3, and NaCl. Synthesis of Zn-Al LDH | FTIR analysis for both before and after 2 h of contact time. | Sips isotherm observed fast removal in deionized and synthetic freshwater with a maximum sorption capacity (Qmax) of 164.49 mg/g and 162.62 mg/g, respectively. Removal was least in synthetic hard water, with a Qmax value of 53 mg/g. For 2 mM concentrations of SO42−, and PO43−, the adsorption capacity significantly decreased to 2%. The removal efficiency was found to be 100% at pH 4. | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cid-Samamed, A.; Diniz, M.S. Recent Advances in the Aggregation Behavior of Nanoplastics in Aquatic Systems. Int. J. Mol. Sci. 2023, 24, 13995. https://doi.org/10.3390/ijms241813995
Cid-Samamed A, Diniz MS. Recent Advances in the Aggregation Behavior of Nanoplastics in Aquatic Systems. International Journal of Molecular Sciences. 2023; 24(18):13995. https://doi.org/10.3390/ijms241813995
Chicago/Turabian StyleCid-Samamed, Antonio, and M. S. Diniz. 2023. "Recent Advances in the Aggregation Behavior of Nanoplastics in Aquatic Systems" International Journal of Molecular Sciences 24, no. 18: 13995. https://doi.org/10.3390/ijms241813995






