Circulating Sphingolipids in Insulin Resistance, Diabetes and Associated Complications
Abstract
:1. Introduction
1.1. Overview of Sphingolipid Metabolism
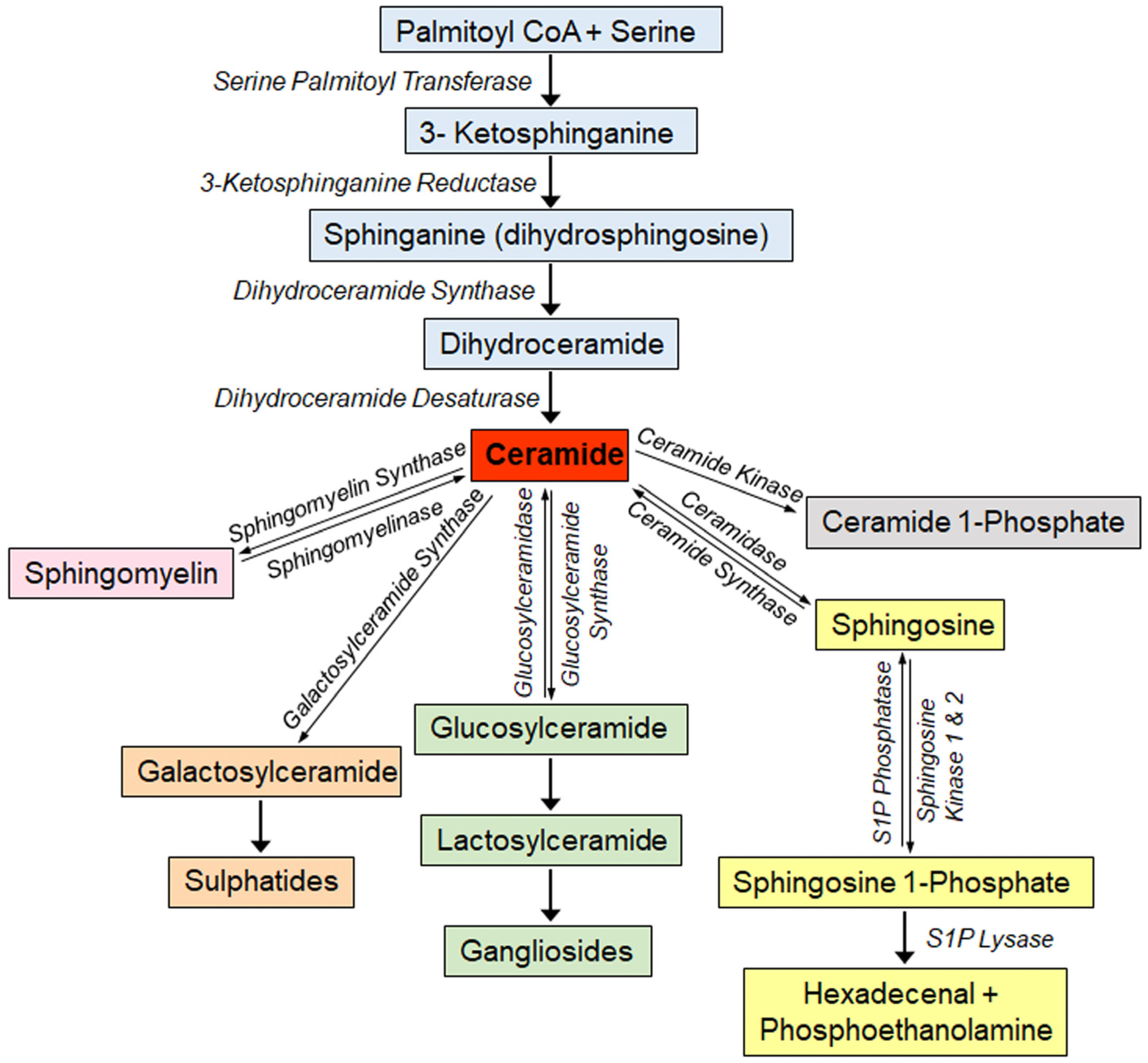
1.2. Sphingolipids as Biomarkers of Disease
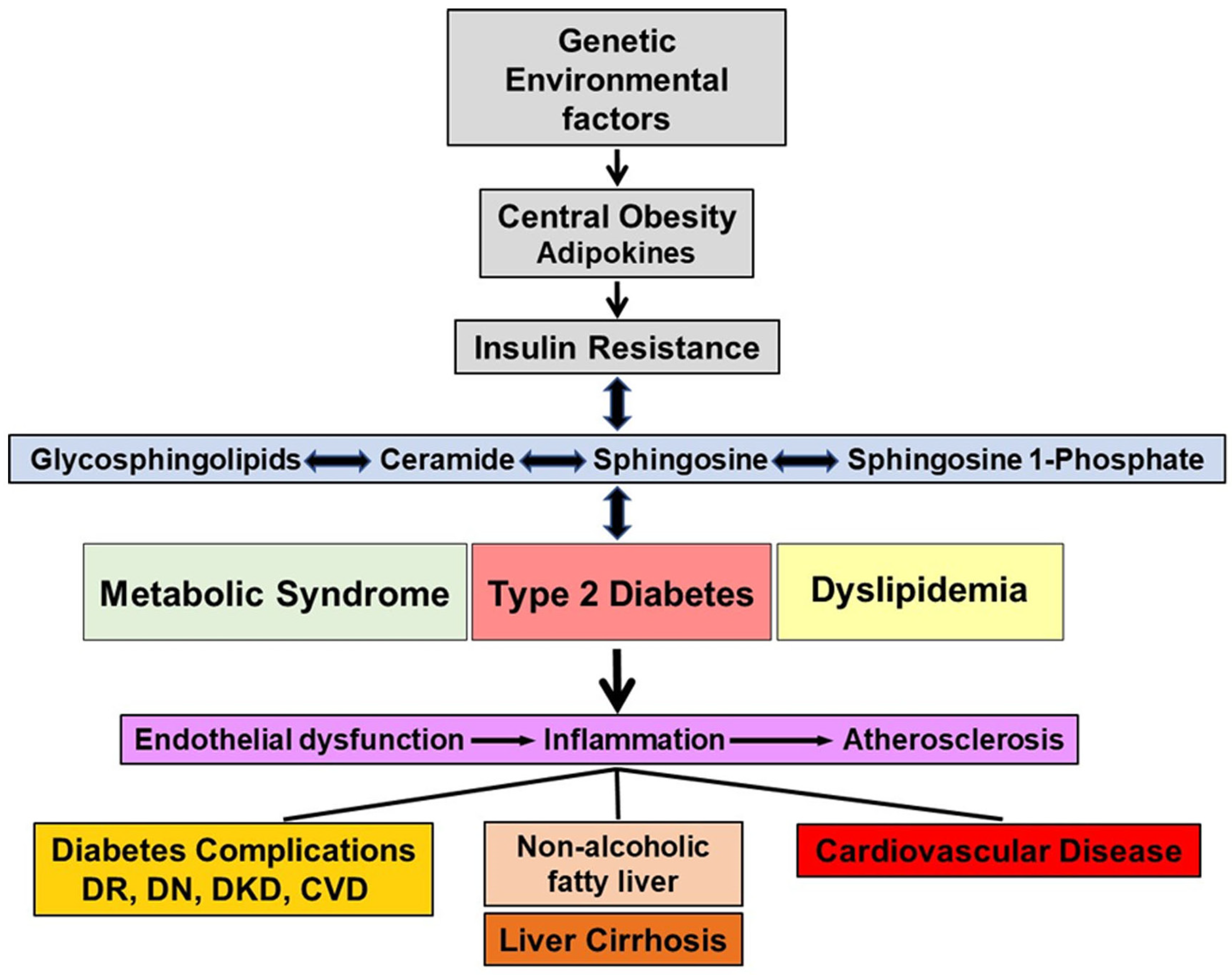
2. Sphingolipids, Obesity and Insulin Resistance
3. Sphingolipids and Diabetes
3.1. Type 1 Diabetes
3.1.1. In Vitro Cultured-Cell Studies
3.1.2. Animal Studies
3.1.3. Human Studies
3.2. Type 2 Diabetes
3.3. Plasma/Serum Sphingolipidomics and Features of Metabolic Syndrome
3.4. Advantage of Sphingolipidomics of Circulating Lipoproteins
3.5. Predictive Value of Plasma/Serum Sphingolipids
3.6. Effect of Environmental Factors
4. Cardiovascular Disease
4.1. Human Studies
4.1.1. Cardiovascular Disease without Diabetes
4.1.2. Cardiovascular Disease with Diabetes
4.2. In Vitro Cultured-Cell and Animal Studies
5. Diabetic Kidney Disease
5.1. In Vitro Cultured-Cell and Animal Studies
5.2. Type 1 Diabetes Human Studies
5.3. Type 2 Diabetes Human Studies
5.4. Conclusions
6. Diabetic Retinopathy
7. Diabetic Neuropathy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette family A protein1 |
| ABCC10 | ATP-binding cassette family C protein10 |
| AER | Albumin excretion rate |
| Akt/PKB | Serine/threonine kinase, also known as protein kinase B |
| Apo B | Apolipoprotein B |
| Apo M | Apolipoprotein M |
| BMI | Body mass index |
| C1P | Ceramide 1-phosphate |
| CerS | Ceramide synthase |
| CVD | Cardiovascular disease |
| DAG | Diacylglycerol |
| DCCT/EDIC | Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications |
| eGFR | Estimated glomerular filtration rate |
| ELOVL | Elongation of very long-chain fatty acids protein |
| ER | Endoplasmic reticulum |
| ESRD | End-stage renal disease |
| FFA | Free fatty acids |
| HbA1c | Hemoglobin A1C |
| HDL | High-density lipoproteins |
| HPLC-MS/MS | High performance liquid chromatography-Tandem mass spectrometry |
| IDL | Intermediate-density lipoproteins |
| IFN | Interferon |
| IL-1 | Interleukin-1 |
| IRS-1 | Insulin receptor substrate 1 |
| LC3B | Acetylated microtubule-associated protein 1 light chain 3B |
| LDL | Low-density lipoproteins |
| Mfsd2b | Major facilitator superfamily domain-containing protein 2B |
| MMP | Matrix metalloproteinase |
| MTP | Microsomal triglyceride transfer protein |
| NAFL | Non-alcoholic fatty liver |
| NASH | Non-alcoholic steatohepatitis |
| NOD | Non-obese diabetic |
| ORMDL3 | Orosomucoid like 3; ORMDL sphingolipid biosynthesis regulator 3 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIP2 | Phosphatidylinositol 4,5 bisphosphate |
| PIP3 | Phosphatidylinositol (3,4,5)-triphosphate |
| PKC | Protein kinase C |
| PP2A | Protein phosphatase A2 |
| ROS | Reactive oxygen species |
| S1P | Sphingosine 1-phosphate |
| SK | Sphingosine kinase |
| SLE | Systemic lupus erythematosus |
| SPL | Sphingosine phosphate lyase |
| SPP2 | Secreted phosphoprotein 2 |
| SPNS2 | Spinster homologue 2 |
| SPT | Serine palmitoyl transferase |
| STZ | Streptozotocin |
| TIMP | Tissue inhibitor of metalloproteinase |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
| VLDL | Very-low-density lipoproteins |
References
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A. Functions of Ceramide in Coordinating Cellular Responses to Stress. Science 1996, 274, 1855–1859. [Google Scholar] [CrossRef]
- Mathias, S.; Peña, L.A.; Kolesnick, R.N. Signal transduction of stress via ceramide. Biochem. J. 1998, 335, 465–480. [Google Scholar] [CrossRef]
- Spiegel, S.; Merrill, A.H., Jr. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996, 10, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.A.; Bourquin, F.; Hornemann, T. Serine Palmitoyltransferase Subunit 3 and Metabolic Diseases. Adv. Exp. Med. Biol. 2022, 1372, 47–56. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol. Metab. 2017, 28, 506–518. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Merrill, A.; Schmelz, E.-M.; Dillehay, D.; Spiegel, S.; Shayman, J.; Schroeder, J.; Riley, R.; Voss, K.; Wang, E. Sphingolipids—The Enigmatic Lipid Class: Biochemistry, Physiology, and Pathophysiology. Toxicol. Appl. Pharmacol. 1997, 142, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Ozbayraktar, F.B.K.; Ulgen, K.O. Molecular facets of sphingolipids: Mediators of diseases. Biotechnol. J. 2009, 4, 1028–1041. [Google Scholar] [CrossRef]
- Verheij, M.; Bose, R.; Lin, X.H.; Yao, B.; Jarvis, W.D.; Grant, S.; Birrer, M.J.; Szabo, E.; Zon, L.I.; Kyriakis, J.M.; et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 1996, 380, 75–79. [Google Scholar] [CrossRef]
- Augé, N.; Nikolova-Karakashian, M.; Carpentier, S.; Parthasarathy, S.; Nègre-Salvayre, A.; Salvayre, R.; Merrill, A.H.; Levade, T. Role of Sphingosine 1-Phosphate in the Mitogenesis Induced by Oxidized Low Density Lipoprotein in Smooth Muscle Cells via Activation of Sphingomyelinase, Ceramidase, and Sphingosine Kinase. J. Biol. Chem. 1999, 274, 21533–21538. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.; Schmelz, E.-M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H. Sphingolipids in Food and the Emerging Importance of Sphingolipids to Nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef]
- Hammad, S.M.; Pierce, J.S.; Soodavar, F.; Smith, K.J.; Al Gadban, M.M.; Rembiesa, B.; Klein, R.L.; Hannun, Y.A.; Bielawski, J.; Bielawska, A. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. J. Lipid Res. 2010, 51, 3074–3087. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M. Blood Sphingolipids in Homeostasis and Pathobiology. Adv. Exp. Med. Biol. 2011, 721, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M.; Al Gadban, M.M.; Semler, A.J.; Klein, R.L. Sphingosine 1-Phosphate Distribution in Human Plasma: Associations with Lipid Profiles. J. Lipids 2012, 2012, 180705. [Google Scholar] [CrossRef]
- Kumpula, L.S.; Kumpula, J.M.; Taskinen, M.-R.; Jauhiainen, M.; Kaski, K.; Ala-Korpela, M. Reconsideration of hydrophobic lipid distributions in lipoprotein particles. Chem. Phys. Lipids 2008, 155, 57–62. [Google Scholar] [CrossRef]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Tarugi, P.; Hegele, R.A.; Davidson, N.O.; Rader, D.J.; Klein, R.L.; Hussain, M.M. Microsomal Triglyceride Transfer Protein Transfers and Determines Plasma Concentrations of Ceramide and Sphingomyelin but Not Glycosylceramide. J. Biol. Chem. 2015, 290, 25863–25875. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Rader, D.J.; Hussain, M.M. ATP binding cassette family A protein 1 determines hexosylceramide and sphingomyelin levels in human and mouse plasma. J. Lipid Res. 2018, 59, 2084–2097. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hussain, M.M. ATP-Binding Cassette Transporter Family C Protein 10 Participates in the Synthesis and Efflux of Hexosylceramides in Liver Cells. Nutrients 2022, 14, 4401. [Google Scholar] [CrossRef]
- Therond, P.; Chapman, M.J. Sphingosine-1-phosphate: Metabolism, transport, atheroprotection and effect of statin treatment. Curr. Opin. Infect. Dis. 2022, 33, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, G.M.; Carey, A.L.; Selathurai, A.; Kingwell, B.A.; Bruce, C.R. Plasma Sphingosine-1-Phosphate Is Elevated in Obesity. PLoS ONE 2013, 8, e72449. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry Disease, an Under-Recognized Multisystemic Disorder: Expert Recommendations for Diagnosis, Management, and Enzyme Replacement Therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Mechtler, T.; Kasper, D.C.; Desnick, R.J. Correlation of Lyso-Gb3 levels in dried blood spots and sera from patients with classic and Later-Onset Fabry disease. Mol. Genet. Metab. 2017, 121, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Matanes, F.; Twal, W.O.; Hammad, S.M. Sphingolipids as Biomarkers of Disease. Adv. Exp. Med. Biol. 2019, 1159, 109–138. [Google Scholar] [CrossRef]
- Harden, O.C.; Hammad, S.M. Sphingolipids and Diagnosis, Prognosis, and Organ Damage in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 586737. [Google Scholar] [CrossRef]
- Gurgul-Convey, E. Sphingolipids in Type 1 Diabetes: Focus on Beta-Cells. Cells 2020, 9, 1835. [Google Scholar] [CrossRef]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Meikle, P.J.; Summers, S.A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Taskinen, M.-R.; Olofsson, S.-O.; Levin, M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013, 274, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Dhe-Paganon, S.; Melendez, P.A.; Lee, J.; Shoelson, S.E. Two New Substrates in Insulin Signaling, IRS5/DOK4 and IRS6/DOK5. J. Biol. Chem. 2003, 278, 25323–25330. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pilch, P.F. The insulin receptor: Structure, function, and signaling. Am. J. Physiol. Physiol. 1994, 266, C319–C334. [Google Scholar] [CrossRef]
- Sun, X.J.; Rothenberg, P.; Kahn, C.R.; Backer, J.M.; Araki, E.; Wilden, P.A.; Cahill, D.A.; Goldstein, B.J.; White, M.F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 1991, 352, 73–77. [Google Scholar] [CrossRef]
- Hajduch, E.; Litherland, G.J.; Hundal, H.S. Protein kinase B (PKB/Akt)—A key regulator of glucose transport? FEBS Lett. 2001, 492, 199–203. [Google Scholar] [CrossRef]
- Burke, J.E.; Vadas, O.; Berndt, A.; Finegan, T.; Perisic, O.; Williams, R.L. Dynamics of the Phosphoinositide 3-Kinase p110δ Interaction with p85α and Membranes Reveals Aspects of Regulation Distinct from p110α. Structure 2011, 19, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- McGlade, C.J.; Ellis, C.; Reedijk, M.; Anderson, D.; Mbamalu, G.; Reith, A.D.; Panayotou, G.; End, P.; Bernstein, A.; Kazlauskas, A. SH2 domains of the p85 alpha subunit of phosphatidylinositol 3-kinase regulate binding to growth factor receptors. Mol. Cell. Biol. 1992, 12, 991–997. [Google Scholar] [CrossRef]
- Salinas, M.; López-Valdaliso, R.; Martín, D.; Alvarez, A.; Cuadrado, A. Inhibition of PKB/Akt1 by C2-Ceramide Involves Activation of Ceramide-Activated Protein Phosphatase in PC12 Cells. Mol. Cell. Neurosci. 2000, 15, 156–169. [Google Scholar] [CrossRef]
- Schubert, K.M.; Scheid, M.P.; Duronio, V. Ceramide Inhibits Protein Kinase B/Akt by Promoting Dephosphorylation of Serine 473. Perspect. Surg. 2000, 275, 13330–13335. [Google Scholar] [CrossRef]
- Chavez, J.A.; Knotts, T.A.; Wang, L.-P.; Li, G.; Dobrowsky, R.T.; Florant, G.L.; Summers, S.A. A Role for Ceramide, but Not Diacylglycerol, in the Antagonism of Insulin Signal Transduction by Saturated Fatty Acids. J. Biol. Chem. 2003, 278, 10297–10303. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.J.; Hajduch, E.; Kular, G.; Hundal, H.S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 2003, 23, 7794–7808. [Google Scholar] [CrossRef] [PubMed]
- Hajduch, E.; Turban, S.; Le Liepvre, X.; Le Lay, S.; Lipina, C.; Dimopoulos, N.; Dugail, I.; Hundal, H.S. Targeting of PKCζ and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem. J. 2008, 410, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.; Khoury, R.; Blachnio-Zabielska, A.; Turban, S.; Loiseau, N.; Lipina, C.; Stretton, C.; Bourron, O.; Ferré, P.; Foufelle, F.; et al. Characterising the Inhibitory Actions of Ceramide upon Insulin Signaling in Different Skeletal Muscle Cell Models: A Mechanistic Insight. PLoS ONE 2014, 9, e101865. [Google Scholar] [CrossRef]
- Roszczyc-Owsiejczuk, K.; Zabielski, P. Sphingolipids as a Culprit of Mitochondrial Dysfunction in Insulin Resistance and Type 2 Diabetes. Front. Endocrinol. 2021, 12, 635175. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired Mitochondrial Activity in the Insulin-Resistant Offspring of Patients with Type 2 Diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef]
- Ritov, V.B.; Menshikova, E.V.; He, J.; Ferrell, R.E.; Goodpaster, B.H.; Kelley, D.E. Deficiency of Subsarcolemmal Mitochondria in Obesity and Type 2 Diabetes. Diabetes 2005, 54, 8–14. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of Mitochondria in Human Skeletal Muscle in Type 2 Diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef]
- Mogensen, M.; Sahlin, K.; Fernstrom, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Højlund, K. Mitochondrial Respiration Is Decreased in Skeletal Muscle of Patients with Type 2 Diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef]
- Holloway, G.P.; Han, X.X.; Jain, S.S.; Bonen, A.; Chabowski, A. Chronic muscle stimulation improves insulin sensitivity while increasing subcellular lipid droplets and reducing selected diacylglycerol and ceramide species in obese Zucker rats. Diabetologia 2014, 57, 832–840. [Google Scholar] [CrossRef]
- Wahwah, N.; Kras, K.A.; Roust, L.R.; Katsanos, C.S. Subpopulation-specific differences in skeletal muscle mitochondria in humans with obesity: Insights from studies employing acute nutritional and exercise stimuli. Am. J. Physiol. Metab. 2020, 318, E538–E553. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, P.; Ostkotte, D.; Nolte, H.; Gerl, M.J.; Jais, A.; Brunner, H.L.; Sprenger, H.-G.; Awazawa, M.; Nicholls, H.T.; Turpin-Nolan, S.M.; et al. CerS6-Derived Sphingolipids Interact with Mff and Promote Mitochondrial Fragmentation in Obesity. Cell 2019, 177, 1536–1552.e23. [Google Scholar] [CrossRef] [PubMed]
- Gudz, T.I.; Tserng, K.-Y.; Hoppel, C.L. Direct Inhibition of Mitochondrial Respiratory Chain Complex III by Cell-permeable Ceramide. Perspect. Surg. 1997, 272, 24154–24158. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Muoio, D.M.; Neufer, P.D. Lipid-Induced Mitochondrial Stress and Insulin Action in Muscle. Cell Metab. 2012, 15, 595–605. [Google Scholar] [CrossRef]
- Di Paola, M.; Cocco, T.; Lorusso, M. Ceramide Interaction with the Respiratory Chain of Heart Mitochondria. Biochemistry 2000, 39, 6660–6668. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Colell, A.; Marí, M.; Morales, A.; Fernández-Checa, J.C. Direct Effect of Ceramide on the Mitochondrial Electron Transport Chain Leads to Generation of Reactive Oxygen Species. J. Biol. Chem. 1997, 272, 11369–11377. [Google Scholar] [CrossRef]
- Boslem, E.; Meikle, P.J.; Biden, T.J. Roles of ceramide and sphingolipids in pancreatic β-cell function and dysfunction. Islets 2012, 4, 177–187. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Villate, O.; Turatsinze, J.-V.; Mascali, L.G.; Grieco, F.A.; Nogueira, T.C.; Cunha, D.A.; Nardelli, T.R.; Sammeth, M.; Salunkhe, V.A.; Esguerra, J.L.S.; et al. Nova1 is a master regulator of alternative splicing in pancreatic beta cells. Nucleic Acids Res. 2014, 42, 11818–11830. [Google Scholar] [CrossRef] [PubMed]
- Dooley, J.; Tian, L.; Schonefeldt, S.; Delghingaro-Augusto, V.; Garcia-Perez, J.E.; Pasciuto, E.; Di Marino, D.; Carr, E.J.; Oskolkov, N.; Lyssenko, V.; et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat. Genet. 2016, 48, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, K.T.; von Herrath, M.G. The Type 1 Diabetes Signature: Hardwired to Trigger Inflammation? Diabetes 2014, 63, 3581–3583. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Grieco, F.A. On the Immense Variety and Complexity of Circumstances Conditioning Pancreatic β-Cell Apoptosis in Type 1 Diabetes. Diabetes 2012, 61, 1661–1663. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Coppieters, K.T.; Von Herrath, M.G. Type 1 Diabetes: Etiology, Immunology, and Therapeutic Strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef]
- Roep, B.O.; Peakman, M. Diabetogenic T lymphocytes in human Type 1 diabetes. Curr. Opin. Immunol. 2011, 23, 746–753. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Mandrup-Poulsen, T. Beta Cell Death and Protection. Ann. N. Y. Acad. Sci. 2003, 1005, 32–42. [Google Scholar] [CrossRef]
- Nerup, J.; Mandrap-Poulsen, T.; Helqvist, S.; Andersen, H.U.; Pociot, F.; Reimers, J.I.; Cuartero, B.G.; Karlsen, A.E.; Bjerre, U.; Lorenzen, T. On the pathogenesis of IDDM. Diabetologia 1994, 37, S82–S89. [Google Scholar] [CrossRef]
- Horwitz, E.; Krogvold, L.; Zhitomirsky, S.; Swisa, A.; Fischman, M.; Lax, T.; Dahan, T.; Hurvitz, N.; Weinberg-Corem, N.; Klochendler, A.; et al. β-Cell DNA Damage Response Promotes Islet Inflammation in Type 1 Diabetes. Diabetes 2018, 67, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, A.; Suarez-Pinzon, W.L. Role of Cytokines in the Pathogenesis of Autoimmune Diabetes Mellitus. Rev. Endocr. Metab. Disord. 2003, 4, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Sammeth, M.; Bouckenooghe, T.; Bottu, G.; Sisino, G.; Igoillo-Esteve, M.; Ortis, F.; Santin, I.; Colli, M.L.; Barthson, J.; et al. The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines. PLOS Genet. 2012, 8, e1002552. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Muñoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.-G.; Trueba, M.; Ordoñez, M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef]
- Welsh, N. Interleukin-1β-induced Ceramide and Diacylglycerol Generation 5 Lead to Activation of the c-Jun NH2-terminal Kinase and the Transcription Factor ATF2 in the Insulin-producing Cell Line RINm5F. J. Biol. Chem. 1996, 271, 8307–8312. [Google Scholar] [CrossRef]
- Lei, X.; Bone, R.N.; Ali, T.; Zhang, S.; Bohrer, A.; Tse, H.M.; Bidasee, K.R.; Ramanadham, S. Evidence of Contribution of iPLA2β-Mediated Events During Islet β-Cell Apoptosis Due to Proinflammatory Cytokines Suggests a Role for iPLA2β in T1D Development. Endocrinology 2014, 155, 3352–3364. [Google Scholar] [CrossRef]
- Hahn, C.; Tyka, K.; Saba, J.D.; Lenzen, S.; Gurgul-Convey, E. Overexpression of sphingosine-1-phosphate lyase protects insulin-secreting cells against cytokine toxicity. J. Biol. Chem. 2017, 292, 20292–20304. [Google Scholar] [CrossRef]
- Lei, X.; Bone, R.N.; Ali, T.; Wohltmann, M.; Gai, Y.; Goodwin, K.J.; Bohrer, A.E.; Turk, J.; Ramanadham, S. Genetic modulation of islet β-cell iPLA2β expression provides evidence for its impact on β-cell apoptosis and autophagy. Islets 2013, 5, 29–44. [Google Scholar] [CrossRef]
- Oleinik, N.; Kim, J.; Roth, B.M.; Selvam, S.P.; Gooz, M.; Johnson, R.H.; Lemasters, J.J.; Ogretmen, B. Mitochondrial protein import is regulated by p17/PERMIT to mediate lipid metabolism and cellular stress. Sci. Adv. 2019, 5, eaax1978. [Google Scholar] [CrossRef]
- Fugio, L.B.; Coeli-Lacchini, F.B.; Leopoldino, A.M. Sphingolipids and Mitochondrial Dynamic. Cells 2020, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, C.; He, X.; Shang, W.; Bi, Y.; Wang, D. Long-term effect of FTY720 on lymphocyte count and islet allograft survival in mice. Microsurgery 2007, 27, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Jörns, A.; Akin, M.; Arndt, T.; Terbish, T.; zu Vilsendorf, A.M.; Wedekind, D.; Hedrich, H.-J.; Lenzen, S. Anti-TCR therapy combined with fingolimod for reversal of diabetic hyperglycemia by β cell regeneration in the LEW.1AR1-iddm rat model of type 1 diabetes. J. Mol. Med. 2014, 92, 743–755. [Google Scholar] [CrossRef]
- Jörns, A.; Akin, M.; Arndt, T.; Terbish, T.; zu Vilsendorf, A.M.; Wedekind, D.; Hedrich, H.-J.; Lenzen, S. Diabetes prevention by immunomodulatory FTY720 treatment in the LEW.1AR1-iddm rat despite immune cell activation. Endocrinology 2010, 151, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Penaranda, C.; Tang, Q.; Ruddle, N.H.; Bluestone, J.A. Prevention of Diabetes by FTY720-Mediated Stabilization of Peri-Islet Tertiary Lymphoid Organs. Diabetes 2010, 59, 1461–1468. [Google Scholar] [CrossRef]
- Fox, T.E.; Bewley, M.C.; Unrath, K.A.; Pedersen, M.M.; Anderson, R.E.; Jung, D.Y.; Jefferson, L.S.; Kim, J.K.; Bronson, S.K.; Flanagan, J.M.; et al. Circulating sphingolipid biomarkers in models of type 1 diabetes. J. Lipid Res. 2011, 52, 509–517. [Google Scholar] [CrossRef]
- Bleich, D.; Polak, M.; Chen, S.; Swiderek, K.M.; Lévy-Marchal, C. Sera from Children with Type 1 Diabetes mellitus React against a New Group of Antigens Composed of Lysophospholipids. Horm. Res. Paediatr. 1999, 52, 86–94. [Google Scholar] [CrossRef]
- Holm, L.J.; Krogvold, L.; Hasselby, J.P.; Kaur, S.; Claessens, L.A.; Russell, M.A.; Mathews, C.E.; Hanssen, K.F.; Morgan, N.G.; Koeleman, B.P.C.; et al. Abnormal islet sphingolipid metabolism in type 1 diabetes. Diabetologia 2018, 61, 1650–1661. [Google Scholar] [CrossRef]
- Oresic, M.; Simell, S.; Sysi-Aho, M.; Nanto-Salonen, K.; Seppänen-Laakso, T.; Parikka, V.; Katajamaa, M.; Hekkala, A.; Mattila, I.; Keskinen, P.; et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J. Exp. Med. 2008, 205, 2975–2984. [Google Scholar] [CrossRef]
- Wei, N.; Pan, J.; Pop-Busui, R.; Othman, A.; Alecu, I.; Hornemann, T.; Eichler, F.S. Altered sphingoid base profiles in type 1 compared to type 2 diabetes. Lipids Health Dis. 2014, 13, 161. [Google Scholar] [CrossRef]
- Sen, P.; Dickens, A.M.; López-Bascón, M.A.; Lindeman, T.; Kemppainen, E.; Lamichhane, S.; Rönkkö, T.; Ilonen, J.; Toppari, J.; Veijola, R.; et al. Metabolic alterations in immune cells associate with progression to type 1 diabetes. Diabetologia 2020, 63, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Kemppainen, E.; Trošt, K.; Siljander, H.; Hyöty, H.; Ilonen, J.; Toppari, J.; Veijola, R.; Hyötyläinen, T.; Knip, M.; et al. Circulating metabolites in progression to islet autoimmunity and type 1 diabetes. Diabetologia 2019, 62, 2287–2297. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Ahonen, L.; Dyrlund, T.S.; Dickens, A.M.; Siljander, H.; Hyöty, H.; Ilonen, J.; Toppari, J.; Veijola, R.; Hyötyläinen, T.; et al. Cord-Blood Lipidome in Progression to Islet Autoimmunity and Type 1 Diabetes. Biomolecules 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Rodriguez-Calvo, T.; Gerling, I.C.; Mathews, C.E.; Kaddis, J.S.; Russell, M.A.; Zeissler, M.; Leete, P.; Krogvold, L.; Dahl-Jørgensen, K.; et al. Islet cell hyperexpression of HLA class I antigens: A defining feature in type 1 diabetes. Diabetologia 2016, 59, 2448–2458. [Google Scholar] [CrossRef]
- Colli, M.L.; Nogueira, T.C.; Allagnat, F.; Cunha, D.A.; Gurzov, E.N.; Cardozo, A.K.; Roivainen, M.; de Beeck, A.O.; Eizirik, D.L. Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance. PLOS Pathog. 2011, 7, e1002267. [Google Scholar] [CrossRef]
- Liu, D.; Cardozo, A.K.; Darville, M.I.; Eizirik, D.L. Double-Stranded RNA Cooperates with Interferon-γ and IL-1β to Induce Both Chemokine Expression and Nuclear Factor-κB-Dependent Apoptosis in Pancreatic β-Cells: Potential Mechanisms for Viral-Induced Insulitis and β-Cell Death in Type 1 Diabetes Mellitus. Endocrinology 2002, 143, 1225–1234. [Google Scholar] [CrossRef]
- Kutlu, B.; Darville, M.I.; Cardozo, A.K.; Eizirik, D.L. Molecular Regulation of Monocyte Chemoattractant Protein-1 Expression in Pancreatic β-Cells. Diabetes 2003, 52, 348–355. [Google Scholar] [CrossRef]
- Lind, K.; Richardson, S.J.; Leete, P.; Morgan, N.G.; Korsgren, O.; Flodström-Tullberg, M. Induction of an Antiviral State and Attenuated Coxsackievirus Replication in Type III Interferon-Treated Primary Human Pancreatic Islets. J. Virol. 2013, 87, 7646–7654. [Google Scholar] [CrossRef]
- Richardson, S.J.; Leete, P.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia 2012, 56, 185–193. [Google Scholar] [CrossRef]
- Yeung, W.-C.G.; Al-Shabeeb, A.; Pang, C.N.I.; Wilkins, M.R.; Catteau, J.; Howard, N.J.; Rawlinson, W.D.; Craig, M.E. Children With Islet Autoimmunity and Enterovirus Infection Demonstrate a Distinct Cytokine Profile. Diabetes 2012, 61, 1500–1508. [Google Scholar] [CrossRef]
- Andersson, K.; Buschard, K.; Fredman, P.; Kaas, A.; Lidström, A.-M.; Madsbad, S.; Mortensen, H.; Månsson, J.-E. Patients with Insulin-dependent Diabetes but not those with Non-insulin-dependent Diabetes have Anti-sulfatide Antibodies as Determined with a New ELISA Assay. Autoimmunity 2002, 35, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, M.; Kaas, A.; Månsson, J.-E.; Formby, B.; Rynmark, B.-M.; Buschard, K.; Fredman, P. Developmental expression of the type I diabetes related antigen sulfatide and sulfated lactosylceramide in mammalian pancreas. J. Cell. Biochem. 2003, 89, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Buschard, K.; Blomqvist, M.; Osterbye, T.; Fredman, P. Involvement of sulfatide in beta cells and type 1 and type 2 diabetes. Diabetologia 2005, 48, 1957–1962. [Google Scholar] [CrossRef]
- Buschard, K.; Høy, M.; Bokvist, K.; Olsen, H.L.; Madsbad, S.; Fredman, P.; Gromada, J. Sulfatide Controls Insulin Secretion by Modulation of ATP-sensitive K+-Channel Activity and Ca2+-Dependent Exocytosis in Rat Pancreatic β-Cells. Diabetes 2002, 51, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Kavishwar, A.; Medarova, Z.; Moore, A. Unique sphingomyelin patches are targets of a beta-cell-specific antibody. J. Lipid Res. 2011, 52, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, D.J.; Berlivet, S.; Hunninghake, G.M.; Madore, A.-M.; Larivière, M.; Moussette, S.; Grundberg, E.; Kwan, T.; Ouimet, M.; Ge, B.; et al. Allele-Specific Chromatin Remodeling in the ZPBP2/GSDMB/ORMDL3 Locus Associated with the Risk of Asthma and Autoimmune Disease. Am. J. Hum. Genet. 2009, 85, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Galadari, A.; Thayyullathil, F. Role of ceramide in diabetes mellitus: Evidence and mechanisms. Lipids Health Dis. 2013, 12, 98. [Google Scholar] [CrossRef]
- Sysi-Aho, M.; Ermolov, A.; Gopalacharyulu, P.V.; Tripathi, A.; Seppänen-Laakso, T.; Maukonen, J.; Mattila, I.; Ruohonen, S.T.; Vähätalo, L.; Yetukuri, L.; et al. Metabolic Regulation in Progression to Autoimmune Diabetes. PLoS Comput. Biol. 2011, 7, e1002257. [Google Scholar] [CrossRef]
- Denimal, D.; de Barros, J.-P.P.; Petit, J.-M.; Bouillet, B.; Vergès, B.; Duvillard, L. Significant abnormalities of the HDL phosphosphingolipidome in type 1 diabetes despite normal HDL cholesterol concentration. Atherosclerosis 2015, 241, 752–760. [Google Scholar] [CrossRef]
- Hammad, S.M.; Hunt, K.J.; Baker, N.L.; Klein, R.L.; Lopes-Virella, M.F. Diabetes and kidney dysfunction markedly alter the content of sphingolipids carried by circulating lipoproteins. J. Clin. Lipidol. 2022, 16, 173–183. [Google Scholar] [CrossRef]
- Mandal, N.; Grambergs, R.; Mondal, K.; Basu, S.K.; Tahia, F.; Dagogo-Jack, S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J. Diabetes Its Complicat. 2021, 35, 107734. [Google Scholar] [CrossRef]
- Russo, S.B.; Ross, J.S.; Cowart, L.A. Sphingolipids in Obesity, Type 2 Diabetes, and Metabolic Disease. Handb. Exp. Pharmacol. 2013, 216, 373–401. [Google Scholar] [CrossRef]
- Kremer, G.J.; Atzpodien, W.; Schnellbacher, E. Plasma glycosphingolipids in diabetics and normals. J. Mol. Med. 1975, 53, 637–638. [Google Scholar] [CrossRef]
- Berkowitz, L.; Cabrera-Reyes, F.; Salazar, C.; Ryff, C.D.; Coe, C.; Rigotti, A. Sphingolipid Profiling: A Promising Tool for Stratifying the Metabolic Syndrome-Associated Risk. Front. Cardiovasc. Med. 2022, 8, 785124. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef]
- Berkowitz, L.; Salazar, C.; Ryff, C.D.; Coe, C.L.; Rigotti, A. Serum sphingolipid profiling as a novel biomarker for metabolic syndrome characterization. Front. Cardiovasc. Med. 2022, 9, 1092331. [Google Scholar] [CrossRef] [PubMed]
- Tonks, K.T.; Coster, A.C.; Christopher, M.J.; Chaudhuri, R.; Xu, A.; Gagnon-Bartsch, J.; Chisholm, D.J.; James, D.E.; Meikle, P.J.; Greenfield, J.R.; et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity 2016, 24, 908–916. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Weir, J.M.; Greeve, M.A.; MacIntosh, G.L.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Kowalczyk, A.; et al. Plasma Lipid Profiling Shows Similar Associations with Prediabetes and Type 2 Diabetes. PLoS ONE 2013, 8, e74341. [Google Scholar] [CrossRef]
- Sui, J.; He, M.; Wang, Y.; Zhao, X.; He, Y.; Shi, B. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Exp. Ther. Med. 2019, 18, 3603–3614. [Google Scholar] [CrossRef]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; DeFronzo, R.A.; Kirwan, J.P. Plasma Ceramides Are Elevated in Obese Subjects with Type 2 Diabetes and Correlate with the Severity of Insulin Resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef]
- Górska, M.; Dobrzyn, A.; Baranowski, M. Concentrations of sphingosine and sphinganine in plasma of patients with type 2 diabetes. Med. Sci. Monit. 2005, 11, 38. [Google Scholar]
- Zakiev, E.; Rached, F.; Lhomme, M.; Darabi-Amin, M.; Ponnaiah, M.; Becker, P.H.; Therond, P.; Serrano, C.V.; Santos, R.D.; Chapman, M.J.; et al. Distinct phospholipid and sphingolipid species are linked to altered HDL function in apolipoprotein A-I deficiency. J. Clin. Lipidol. 2019, 13, 468–480.e8. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Orsoni, A.; Tan, R.; Mellett, N.A.; Nguyen, A.; Robillard, P.; Giral, P.; Thérond, P.; Meikle, P.J. LDL subclass lipidomics in atherogenic dyslipidemia: Effect of statin therapy on bioactive lipids and dense LDL. J. Lipid Res. 2020, 61, 911–932. [Google Scholar] [CrossRef] [PubMed]
- Brinck, J.W.; Thomas, A.; Lauer, E.; Jornayvaz, F.R.; Brulhart-Meynet, M.-C.; Prost, J.-C.; Pataky, Z.; Löfgren, P.; Hoffstedt, J.; Eriksson, M.; et al. Diabetes Mellitus Is Associated with Reduced High-Density Lipoprotein Sphingosine-1-Phosphate Content and Impaired High-Density Lipoprotein Cardiac Cell Protection. Arter. Thromb. Vasc. Biol. 2016, 36, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Couzens, E.; Hwang, A.; Russell, M.; Barlow, C.E.; DeFina, L.F.; Hoofnagle, A.N.; Kim, F. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS ONE 2018, 13, e0192616. [Google Scholar] [CrossRef] [PubMed]
- Randriamboavonjy, V.; Badenhoop, K.; Schmidt, H.; Geisslinger, G.; Fisslthaler, B.; Fleming, I. The S1P2 receptor expressed in human platelets is linked to the RhoA-Rho kinase pathway and is down regulated in type 2 diabetes. Basic Res. Cardiol. 2009, 104, 333–340. [Google Scholar] [CrossRef]
- Tong, X.; Peng, H.; Liu, D.; Ji, L.; Niu, C.; Ren, J.; Pan, B.; Hu, J.; Zheng, L.; Huang, Y. High-density lipoprotein of patients with Type 2 Diabetes Mellitus upregulates cyclooxgenase-2 expression and prostacyclin I-2 release in endothelial cells: Relationship with HDL-associated sphingosine-1-phosphate. Cardiovasc. Diabetol. 2013, 12, 27. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; De Angelis, M.H.; Peters, A.; et al. Identification of Serum Metabolites Associated with Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Othman, A.; Rütti, M.F.; Ernst, D.; Saely, C.H.; Rein, P.; Drexel, H.; Porretta-Serapiglia, C.; Lauria, G.; Bianchi, R.; Von Eckardstein, A.; et al. Plasma deoxysphingolipids: A novel class of biomarkers for the metabolic syndrome? Diabetologia 2012, 55, 421–431. [Google Scholar] [CrossRef]
- Perreault, L.; Starling, A.P.; Glueck, D.; Brozinick, J.T.; Sanders, P.; Siddall, P.; Kuo, M.S.; Dabelea, D.; Bergman, B.C. Biomarkers of Ectopic Fat Deposition: The Next Frontier in Serum Lipidomics. J. Clin. Endocrinol. Metab. 2016, 101, 176–182. [Google Scholar] [CrossRef]
- Fretts, A.M.; Jensen, P.N.; Hoofnagle, A.N.; McKnight, B.; Howard, B.V.; Umans, J.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Djousse, L.; et al. Plasma ceramides containing saturated fatty acids are associated with risk of type 2 diabetes. J. Lipid Res. 2021, 62, 100119. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Howard, B.V.; Umans, J.; Yu, C.; Sitlani, C.; Siscovick, D.S.; King, I.B.; et al. Plasma Ceramide Species Are Associated with Diabetes Risk in Participants of the Strong Heart Study. J. Nutr. 2020, 150, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, M.; Kulkarni, H.; Wong, G.; Weir, J.M.; Barlow, C.K.; Dyer, T.D.; Almasy, L.; Mahaney, M.C.; Comuzzie, A.G.; Glahn, D.C.; et al. Lipidomic risk score independently and cost-effectively predicts risk of future type 2 diabetes: Results from diverse cohorts. Lipids Health Dis. 2016, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Kasumov, T.; Solomon, T.P.; Hwang, C.; Huang, H.; Haus, J.M.; Zhang, R.; Kirwan, J.P. Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity 2015, 23, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Manialawy, Y.; Obersterescu, A.; Cox, B.J.; Gunderson, E.P.; Wheeler, M.B. Diminished Sphingolipid Metabolism, a Hallmark of Future Type 2 Diabetes Pathogenesis, Is Linked to Pancreatic β Cell Dysfunction. iScience 2020, 23, 101566. [Google Scholar] [CrossRef]
- Serés-Noriega, T.; Ortega, E.; Perea, V.; Giménez, M.; Boswell, L.; Mariaca, K.; Font, C.; Mesa, A.; Viñals, C.; Blanco, J.; et al. Nuclear Magnetic Resonance-Based Lipidomics in the Assessment of Cardiometabolic Risk in Type 1 Diabetes: An Exploratory Analysis. Diabetes Ther. 2023, 14, 553–567. [Google Scholar] [CrossRef]
- Sasset, L.; Zhang, Y.; Dunn, T.M.; Di Lorenzo, A. Sphingolipid De Novo Biosynthesis: A Rheostat of Cardiovascular Homeostasis. Trends Endocrinol. Metab. 2016, 27, 807–819. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Paultre, F.; Pearson, T.A.; Reed, R.G.; Francis, C.K.; Lin, M.; Berglund, L.; Tall, A.R. Plasma Sphingomyelin Level as a Risk Factor for Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2000, 20, 2614–2618. [Google Scholar] [CrossRef]
- Guyton, J.R.; Klemp, K.F. Development of the Lipid-Rich Core in Human Atherosclerosis. Arter. Thromb. Vasc. Biol. 1996, 16, 4–11. [Google Scholar] [CrossRef]
- Schissel, S.L.; Tweedie-Hardman, J.; Rapp, J.H.; Graham, G.; Williams, K.J.; Tabas, I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Investig. 1996, 98, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Kauhanen, D.; Sysi-Aho, M.; Koistinen, K.M.; Laaksonen, R.; Sinisalo, J.; Ekroos, K. Development and validation of a high-throughput LC–MS/MS assay for routine measurement of molecular ceramides. Anal. Bioanal. Chem. 2016, 408, 3475–3483. [Google Scholar] [CrossRef]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2019, 41, 371–380. [Google Scholar] [CrossRef]
- Jensen, P.N.; Fretts, A.M.; Hoofnagle, A.N.; Sitlani, C.M.; McKnight, B.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma Ceramides and Sphingomyelins in Relation to Atrial Fibrillation Risk: The Cardiovascular Health Study. J. Am. Heart Assoc. 2020, 9, e012853. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Fretts, A.M.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma Ceramides and Sphingomyelins in Relation to Heart Failure Risk. Circ. Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef]
- Piek, A.; de Boer, R.A.; Silljé, H.H.W. The fibrosis-cell death axis in heart failure. Heart Fail. Rev. 2016, 21, 199–211. [Google Scholar] [CrossRef]
- Fretts, A.M.; Jensen, P.N.; Hoofnagle, A.N.; McKnight, B.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Psaty, B.M.; Sotoodehnia, N.; Lemaitre, R.N. Circulating Ceramides and Sphingomyelins and Risk of Mortality: The Cardiovascular Health Study. Clin. Chem. 2021, 67, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, S.; Lee, S.-H.; Lee, J.H. Association between Arterial Stiffness and Serum L-Octanoylcarnitine and Lactosylceramide in Overweight Middle-Aged Subjects: 3-Year Follow-Up Study. PLoS ONE 2015, 10, e0119519. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, M.; Gordillo, R.; Koliaki, C.; Gancheva, S.; Jelenik, T.; De Filippo, E.; Herder, C.; Markgraf, D.; Jankowiak, F.; Esposito, I.; et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Diabetes Care 2018, 41, 1235–1243. [Google Scholar] [CrossRef]
- Hammad, S.M.; Harden, O.C.; Wilson, D.A.; Twal, W.O.; Nietert, P.J.; Oates, J.C. Plasma Sphingolipid Profile Associated With Subclinical Atherosclerosis and Clinical Disease Markers of Systemic Lupus Erythematosus: Potential Predictive Value. Front. Immunol. 2021, 12, 694318. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Virella, M.F.; Baker, N.L.; Hunt, K.J.; Hammad, S.M.; Arthur, J.; Virella, G.; Klein, R.L. Glycosylated sphingolipids and progression to kidney dysfunction in type 1 diabetes. J. Clin. Lipidol. 2019, 13, 481–491.e1. [Google Scholar] [CrossRef]
- Hammad, S.M.; Hardin, J.R.; Wilson, D.A.; Twal, W.O.; Nietert, P.J.; Oates, J.C. Race disparity in blood sphingolipidomics associated with lupus cardiovascular comorbidity. PLoS ONE 2019, 14, e0224496. [Google Scholar] [CrossRef]
- Knapp, M.; Żendzian-Piotrowska, M.; Błachnio-Zabielska, A.; Zabielski, P.; Kurek, K.; Górski, J. Myocardial infarction differentially alters sphingolipid levels in plasma, erythrocytes and platelets of the rat. Basic Res. Cardiol. 2012, 107, 294. [Google Scholar] [CrossRef]
- Jeong, T.; Schissel, S.L.; Tabas, I.; Pownall, H.J.; Tall, A.R.; Jiang, X. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J. Clin. Investig. 1998, 101, 905–912. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Dong, J.-B.; Li, Y.; Wu, M.-P. Sphingomyelin synthase 2 over-expression induces expression of aortic inflammatory biomarkers and decreases circulating EPCs in ApoE KO mice. Life Sci. 2012, 90, 867–873. [Google Scholar] [CrossRef]
- Kasumov, T.; Li, L.; Li, M.; Gulshan, K.; Kirwan, J.P.; Liu, X.; Previs, S.; Willard, B.; Smith, J.D.; McCullough, A. Ceramide as a Mediator of Non-Alcoholic Fatty Liver Disease and Associated Atherosclerosis. PLoS ONE 2015, 10, e0126910. [Google Scholar] [CrossRef]
- Cordis, G.; Yoshida, T.; Das, D. HPTLC analysis of sphingomylein, ceramide and sphingosine in ischemic/reperfused rat heart. J. Pharm. Biomed. Anal. 1998, 16, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-C.; Kim, B.-R.; Lee, S.-Y.; Park, T.-S. Sphingolipid Metabolism and Obesity-Induced Inflammation. Front. Endocrinol. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Lisowska, A.; Zabielski, P.; Musiał, W.; Baranowski, M. Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat. 2013, 106, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Egom, E.E.; Mamas, M.A.; Chacko, S.; Stringer, S.E.; Charlton-Menys, V.; El-Omar, M.; Chirico, D.; Clarke, B.; Neyses, L.; Cruickshank, J.K.; et al. Serum sphingolipids level as a novel potential marker for early detection of human myocardial ischaemic injury. Front. Physiol. 2013, 4, 130. [Google Scholar] [CrossRef]
- Sattler, K.; Gräler, M.; Keul, P.; Weske, S.; Reimann, C.-M.; Jindrová, H.; Kleinbongard, P.; Sabbadini, R.; Bröcker-Preuss, M.; Erbel, R.; et al. Defects of High-Density Lipoproteins in Coronary Artery Disease Caused by Low Sphingosine-1-Phosphate Content. J. Am. Coll. Cardiol. 2015, 66, 1470–1485. [Google Scholar] [CrossRef]
- Varre, J.V.; Holland, W.L.; Summers, S.A. You aren't IMMUNE to the ceramides that accumulate in cardiometabolic disease. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159125. [Google Scholar] [CrossRef]
- Rohrbach, T.; Maceyka, M.; Spiegel, S. Sphingosine kinase and sphingosine-1-phosphate in liver pathobiology. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 543–553. [Google Scholar] [CrossRef]
- González-Fernández, B.; Sánchez, D.I.; González-Gallego, J.; Tuñón, M.J. Sphingosine 1-Phosphate Signaling as a Target in Hepatic Fibrosis Therapy. Front. Pharmacol. 2017, 8, 579. [Google Scholar] [CrossRef]
- Simon, J.; Ouro, A.; Ala-Ibanibo, L.; Presa, N.; Delgado, T.C.; Martínez-Chantar, M.L. Sphingolipids in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma: Ceramide Turnover. Int. J. Mol. Sci. 2019, 21, 40. [Google Scholar] [CrossRef]
- Wang, E.; He, X.; Zeng, M. The Role of S1P and the Related Signaling Pathway in the Development of Tissue Fibrosis. Front. Pharmacol. 2019, 9, 1504. [Google Scholar] [CrossRef]
- Li, C.; Zheng, S.; You, H.; Liu, X.; Lin, M.; Yang, L.; Li, L. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J. Hepatol. 2010, 54, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yue, S.; Li, C.; Yang, L.; You, H.; Li, L. Essential roles of sphingosine 1-phosphate receptor types 1 and 3 in human hepatic stellate cells motility and activation. J. Cell. Physiol. 2010, 226, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef] [PubMed]
- von Frankenberg, A.D.; Reis, A.F.; Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arq. Bras. de Endocrinol. Metabol. 2017, 61, 614–622. [Google Scholar] [CrossRef]
- Holland, W.L.; Summers, S.A. Sphingolipids, Insulin Resistance, and Metabolic Disease: New Insights from in Vivo Manipulation of Sphingolipid Metabolism. Endocr. Rev. 2008, 29, 381–402. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Davis, C.N.; Tabarean, I.; Gaidarova, S.; Behrens, M.M.; Bartfai, T. IL-1β induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J. Neurochem. 2006, 98, 1379–1389. [Google Scholar] [CrossRef]
- Breckenridge, W.C.; Halloran, J.L.; Kovacs, K.; Silver, M.D. Increase of gangliosides in atherosclerotic human aortas. Lipids 1975, 10, 256–259. [Google Scholar] [CrossRef]
- Garner, B.; Priestman, D.A.; Stocker, R.; Harvey, D.J.; Butters, T.D.; Platt, F.M. Increased glycosphingolipid levels in serum and aortae of apolipoprotein E gene knockout mice. J. Lipid Res. 2002, 43, 205–214. [Google Scholar] [CrossRef]
- Margalit, M.; Shalev, Z.; Pappo, O.; Sklair-Levy, M.; Alper, R.; Gomori, M.; Engelhardt, D.; Rabbani, E.; Ilan, Y. Glucocerebroside Ameliorates the Metabolic Syndrome in OB/OB Mice. Experiment 2006, 319, 105–110. [Google Scholar] [CrossRef]
- Zigmond, E.; Zangen, S.W.; Pappo, O.; Sklair-Levy, M.; Lalazar, G.; Zolotaryova, L.; Raz, I.; Ilan, Y. β-Glycosphingolipids improve glucose intolerance and hepatic steatosis of the Cohen diabetic rat. Am. J. Physiol. Metab. 2009, 296, E72–E78. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bedja, D.; Mishra, S.; Amuzie, C.; Avolio, A.; Kass, D.A.; Berkowitz, D.; Renehan, M. Inhibition of Glycosphingolipid Synthesis Ameliorates Atherosclerosis and Arterial Stiffness in Apolipoprotein E −/− Mice and Rabbits Fed a High-Fat and -Cholesterol Diet. Circulation 2014, 129, 2403–2413. [Google Scholar] [CrossRef]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef] [PubMed]
- Balram, A.; Thapa, S.; Chatterjee, S. Glycosphingolipids in Diabetes, Oxidative Stress, and Cardiovascular Disease: Prevention in Experimental Animal Models. Int. J. Mol. Sci. 2022, 23, 15442. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Lee, E.-J.; Shin, K.-O.; Kim, M.H.; Pewzner-Jung, Y.; Lee, Y.-M.; Park, J.-W.; Futerman, A.H.; Park, W.-J. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Bilous, R. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Mann, J.F.E.; Yi, Q.; Zinman, B.; Dinneen, S.F.; Hoogwerf, B.; Hallé, J.P.; Young, J.; Rashkow, A.; Joyce, C.; et al. Albuminuria and Risk of Cardiovascular Events, Death, and Heart Failure in Diabetic and Nondiabetic Individuals. JAMA 2001, 286, 421–426. [Google Scholar] [CrossRef]
- Fox, T.E.; Kester, M. Therapeutic Strategies for Diabetes and Complications: A Role for Sphingolipids? Adv. Exp. Med. Biol. 2010, 688, 206–216. [Google Scholar] [CrossRef]
- Klein, R.L.; Hammad, S.M.; Baker, N.L.; Hunt, K.J.; Al Gadban, M.M.; Cleary, P.A.; Virella, G.; Lopes-Virella, M.F. Decreased plasma levels of select very long chain ceramide species Are associated with the development of nephropathy in type 1 diabetes. Metabolism 2014, 63, 1287–1295. [Google Scholar] [CrossRef]
- Kady, N.M.; Liu, X.; Lydic, T.A.; Syed, M.H.; Navitskaya, S.; Wang, Q.; Hammer, S.S.; O’reilly, S.; Huang, C.; Seregin, S.S.; et al. ELOVL4-Mediated Production of Very Long-Chain Ceramides Stabilizes Tight Junctions and Prevents Diabetes-Induced Retinal Vascular Permeability. Diabetes 2018, 67, 769–781. [Google Scholar] [CrossRef]
- United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2022. Available online: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds (accessed on 13 March 2023).
- Mather, A.R.; Siskind, L.J. Glycosphingolipids and Kidney Disease. Sphingolipids Metab. Dis. 2011, 721, 121–138. [Google Scholar] [CrossRef]
- Zador, I.Z.; Deshmukh, G.D.; Kunkel, R.; Johnson, K.; Radin, N.S.; Shayman, J.A. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J. Clin. Investig. 1993, 91, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Subathra, M.; Korrapati, M.; Howell, L.A.; Arthur, J.M.; Shayman, J.A.; Schnellmann, R.G.; Siskind, L.J. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am. J. Physiol. Physiol. 2015, 309, F204–F215. [Google Scholar] [CrossRef] [PubMed]
- Nowling, T.K.; Mather, A.R.; Thiyagarajan, T.; Hernández-Corbacho, M.J.; Powers, T.W.; Jones, E.E.; Snider, A.J.; Oates, J.C.; Drake, R.R.; Siskind, L.J. Renal Glycosphingolipid Metabolism Is Dysfunctional in Lupus Nephritis. J. Am. Soc. Nephrol. 2015, 26, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Rutkute, K.; Asmis, R.H.; Nikolova-Karakashian, M.N. Regulation of neutral sphingomyelinase-2 by GSH: A new insight to the role of oxidative stress in aging-associated inflammation. J. Lipid Res. 2007, 48, 2443–2452. [Google Scholar] [CrossRef]
- Alessenko, A.; Bugrova, A.; Dudnik, L. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer’s disease. Biochem. Soc. Trans. 2004, 32, 144–146. [Google Scholar] [CrossRef]
- Ogiso, M.; Hoshi, M.; Nishigori, H. Neutral and Acidic Glycosphingolipids in Glucocorticoid-induced Cataract in Chick Lens. Exp. Eye Res. 1999, 68, 229–236. [Google Scholar] [CrossRef]
- Zager, R.A.; Conrad, D.S.; Burkhart, K. Ceramide accumulation during oxidant renal tubular injury. J. Am. Soc. Nephrol. 1998, 9, 1670–1680. [Google Scholar] [CrossRef]
- Nikolova-Karakashian, M.; Karakashian, A.; Rutkute, K. Role of Neutral Sphingomyelinases in Aging and Inflammation. Subcell Biochem. 2008, 49, 469–486. [Google Scholar] [CrossRef]
- Sacket, S.J.; Chung, H.-Y.; Okajima, F.; Im, D.-S. Increase in sphingolipid catabolic enzyme activity during aging. Acta Pharmacol. Sin. 2009, 30, 1454–1461. [Google Scholar] [CrossRef]
- Mäkinen, V.-P.; Tynkkynen, T.; Soininen, P.; Forsblom, C.; Peltola, T.; Kangas, A.J.; Groop, P.-H.; Ala-Korpela, M. Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study). Metabolomics 2012, 8, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Barlovic, D.P.; Harjutsalo, V.; Sandholm, N.; Forsblom, C.; Groop, P.-H.; on behalf of the FinnDiane Study Group. Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia 2020, 63, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Tofte, N.; Suvitaival, T.; Ahonen, L.; Winther, S.A.; Theilade, S.; Frimodt-Møller, M.; Ahluwalia, T.S.; Rossing, P. Lipidomic analysis reveals sphingomyelin and phosphatidylcholine species associated with renal impairment and all-cause mortality in type 1 diabetes. Sci. Rep. 2019, 9, 16398. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A. Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef]
- Uchida, Y. The role of fatty acid elongation in epidermal structure and function. Dermato-Endocrinology 2011, 3, 65–69. [Google Scholar] [CrossRef]
- Han, L.-D.; Xia, J.-F.; Liang, Q.-L.; Wang, Y.; Wang, Y.-M.; Hu, P.; Li, P.; Luo, G.-A. Plasma esterified and non-esterified fatty acids metabolic profiling using gas chromatography–mass spectrometry and its application in the study of diabetic mellitus and diabetic nephropathy. Anal. Chim. Acta 2011, 689, 85–91. [Google Scholar] [CrossRef]
- Zhu, C.; Liang, Q.-L.; Hu, P.; Wang, Y.-M.; Luo, G.-A. Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta 2011, 85, 1711–1720. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ghosh, S.; Kovalik, J.-P.; Ching, J.; Choi, H.W.; Tavintharan, S.; Ong, C.N.; Sum, C.F.; Summers, S.A.; Tai, E.S.; et al. Profiling of Plasma Metabolites Suggests Altered Mitochondrial Fuel Usage and Remodeling of Sphingolipid Metabolism in Individuals With Type 2 Diabetes and Kidney Disease. Kidney Int. Rep. 2016, 2, 470–480. [Google Scholar] [CrossRef]
- Afshinnia, F.; Nair, V.; Lin, J.; Rajendiran, T.M.; Soni, T.; Byun, J.; Sharma, K.; Fort, P.E.; Gardner, T.W.; Looker, H.C.; et al. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 2019, 4, e130317. [Google Scholar] [CrossRef]
- Shayman, J.A. Sphingolipids: Their role in intracellular signaling and renal growth. J. Am. Soc. Nephrol. 1996, 7, 171–182. [Google Scholar] [CrossRef]
- Weinberg, J. Lipotoxicity. Kidney Int. 2006, 70, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.J.; Pezzolesi, M.G.; Summers, S.A. Rotten to the Cortex: Ceramide-Mediated Lipotoxicity in Diabetic Kidney Disease. Front. Endocrinol. 2021, 11, 622692. [Google Scholar] [CrossRef]
- Hammad, S.M.; Twal, W.O.; Arif, E.; Semler, A.J.; Klein, R.L.; Nihalani, D. Transcriptomics Reveal Altered Metabolic and Signaling Pathways in Podocytes Exposed to C16 Ceramide-Enriched Lipoproteins. Genes 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Busik, J.V.; Esselman, W.J.; E Reid, G. Examining the role of lipid mediators in diabetic retinopathy. Clin. Lipidol. 2012, 7, 661–675. [Google Scholar] [CrossRef]
- Rajab, H.A.; Baker, N.L.; Hunt, K.J.; Klein, R.; Cleary, P.A.; Lachin, J.; Virella, G.; Lopes-Virella, M.F. The predictive role of markers of Inflammation and endothelial dysfunction on the course of diabetic retinopathy in type 1 diabetes. J. Diabetes Its Complicat. 2015, 29, 108–114. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Fu, D.; Azar, M.; Stoner, J.A.; Kaufman, D.G.; Zhang, S.; Klein, R.L.; Lopes-Virella, M.F.; Ma, J.-X.; Lyons, T.J. Clinical correlates of serum pigment epithelium-derived factor in type 2 diabetes patients. J. Diabetes Its Complicat. 2014, 28, 353–359. [Google Scholar] [CrossRef]
- Lopes-Virella, M.F.; Baker, N.L.; Hunt, K.J.; Lyons, T.J.; Jenkins, A.J.; Virella, G.; DCCT/EDIC Study Group. High Concentrations of AGE-LDL and Oxidized LDL in Circulating Immune Complexes Are Associated With Progression of Retinopathy in Type 1 Diabetes. Diabetes Care 2012, 35, 1333–1340. [Google Scholar] [CrossRef]
- Fu, D.; Yu, J.Y.; Yang, S.; Wu, M.; Hammad, S.M.; Connell, A.R.; Du, M.; Chen, J.; Lyons, T.J. Survival or death: A dual role for autophagy in stress-induced pericyte loss in diabetic retinopathy. Diabetologia 2016, 59, 2251–2261. [Google Scholar] [CrossRef]
- Fu, D.; Wu, M.; Zhang, J.; Du, M.; Yang, S.; Hammad, S.M.; Wilson, K.; Chen, J.; Lyons, T.J. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia 2012, 55, 3128–3140. [Google Scholar] [CrossRef]
- Diffley, J.M.; Wu, M.; Sohn, M.; Song, W.; Hammad, S.M.; Lyons, T.J. Apoptosis induction by oxidized glycated LDL in hu-man retinal capillary pericytes is independent of activation of MAPK signaling pathways. Mol. Vis. 2009, 15, 135–145. [Google Scholar] [PubMed]
- Fu, D.; Yu, J.Y.; Wu, M.; Du, M.; Chen, Y.; Abdelsamie, S.A.; Li, Y.; Chen, J.; Boulton, M.E.; Ma, J.-X.; et al. Immune complex formation in human diabetic retina enhances toxicity of oxidized LDL towards retinal capillary pericytes. J. Lipid Res. 2014, 55, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Ru, J.H.; Lopes-Virella, M.F.; Lyons, T.J.; Huang, Y. Interaction of palmitate and LPS regulates cytokine expression and apoptosis through sphingolipids in human retinal microvascular endothelial cells. Exp. Eye Res. 2018, 178, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Kaneko, H. Lipid Signaling in Ocular Neovascularization. Int. J. Mol. Sci. 2020, 21, 4758. [Google Scholar] [CrossRef]
- Alshaikh, R.A.; Ryan, K.B.; Waeber, C. Sphingosine 1-phosphate, a potential target in neovascular retinal disease. Br. J. Ophthalmol. 2021, 106, 1187–1195. [Google Scholar] [CrossRef]
- Sinha, T.; Ikelle, L.; Naash, M.I.; Al-Ubaidi, M.R. The Intersection of Serine Metabolism and Cellular Dysfunction in Retinal Degeneration. Cells 2020, 9, 674. [Google Scholar] [CrossRef]
- Holm, L.J.; Buschard, K. L-serine: A neglected amino acid with a potential therapeutic role in diabetes. APMIS 2019, 127, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.J.; Haupt-Jorgensen, M.; Larsen, J.; Giacobini, J.D.; Bilgin, M.; Buschard, K. L-serine supplementation lowers diabetes incidence and improves blood glucose homeostasis in NOD mice. PLoS ONE 2018, 13, e0194414. [Google Scholar] [CrossRef]
- Arianti, R.; Vinnai, B.; Tóth, B.B.; Shaw, A.; Csősz, É.; Vámos, A.; Győry, F.; Fischer-Posovszky, P.; Wabitsch, M.; Kristóf, E.; et al. ASC-1 transporter-dependent amino acid uptake is required for the efficient thermogenic response of human adipocytes to adrenergic stimulation. FEBS Lett. 2021, 595, 2085–2098. [Google Scholar] [CrossRef]
- Jersin, R.; Tallapragada, D.S.P.; Madsen, A.; Skartveit, L.; Fjære, E.; McCann, A.; Lawrence-Archer, L.; Willems, A.; Bjune, J.-I.; Bjune, M.S.; et al. Role of the Neutral Amino Acid Transporter SLC7A10 in Adipocyte Lipid Storage, Obesity, and Insulin Resistance. Diabetes 2021, 70, 680–695. [Google Scholar] [CrossRef]
- Jersin, R.; Jonassen, L.R.; Dankel, S.N. The neutral amino acid transporter SLC7A10 in adipose tissue, obesity and insulin resistance. Front. Cell Dev. Biol. 2022, 10, 974338. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Russo, S.B.; Chavis, G.C.; Cowart, L.A. Sphingolipid regulators of cellular dysfunction in Type 2 diabetes mellitus: A systems overview. Clin. Lipidol. 2014, 9, 553–569. [Google Scholar] [CrossRef]
- Bertea, M.; Rütti, M.F.; Othman, A.; Marti-Jaun, J.; Hersberger, M.; von Eckardstein, A.; Hornemann, T. Deoxysphingoid bases as plasma markers in Diabetes mellitus. Lipids Health Dis. 2010, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, R.; Sandhoff, K. Neuronal Ganglioside and Glycosphingolipid (GSL) Metabolism and Disease. Adv. Neurobiol. 2022, 29, 333–390. [Google Scholar] [CrossRef]
- Hla, T.; Kolesnick, R. C16:0-Ceramide Signals Insulin Resistance. Cell Metab. 2014, 20, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Hundal, H.S. Ganglioside GM3 as a gatekeeper of obesity-associated insulin resistance: Evidence and mechanisms. FEBS Lett. 2015, 589, 3221–3227. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Yu, K.; Rapport, M.M.; Swanki, K. (Eds.) Ganglioside Structure, Function and Biomedical Potential; Part of the book series: Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 1984; Volume 174. [Google Scholar] [CrossRef]
- Fedele, D.; Giugliano, D. Peripheral Diabetic Neuropathy. Drugs 1997, 54, 414–421. [Google Scholar] [CrossRef]
- Pfeifer, M.A.; Schumer, M.P. Clinical Trials of Diabetic Neuropathy: Past, Present, and Future. Diabetes 1995, 44, 1355–1361. [Google Scholar] [CrossRef]
- Penno, A.; Reilly, M.M.; Houlden, H.; Laurá, M.; Rentsch, K.; Niederkofler, V.; Stoeckli, E.T.; Nicholson, G.; Eichler, F.; Brown, R.H.; et al. Hereditary Sensory Neuropathy Type 1 Is Caused by the Accumulation of Two Neurotoxic Sphingolipids. J. Biol. Chem. 2010, 285, 11178–11187. [Google Scholar] [CrossRef]
- Esaki, K.; Sayano, T.; Sonoda, C.; Akagi, T.; Suzuki, T.; Ogawa, T.; Okamoto, M.; Yoshikawa, T.; Hirabayashi, Y.; Furuya, S. l-Serine Deficiency Elicits Intracellular Accumulation of Cytotoxic Deoxysphingolipids and Lipid Body Formation. J. Biol. Chem. 2015, 290, 14595–14609. [Google Scholar] [CrossRef]
- Alecu, I.; Tedeschi, A.; Behler, N.; Wunderling, K.; Lamberz, C.; Lauterbach, M.A.R.; Gaebler, A.; Ernst, D.; Van Veldhoven, P.P.; Al-Amoudi, A.; et al. Localization of 1-deoxysphingolipids to mitochondria induces mitochondrial dysfunction. J. Lipid Res. 2017, 58, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Semler, A.; Hammad, S.; Lopes-Virella, M.F.; Klein, R.L.; Huang, Y. Deoxysphingolipids Upregulate MMP-1, Downregulate TIMP-1, and Induce Cytotoxicity in Human Schwann Cells. NeuroMolecular Med. 2021, 24, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M.; DCCT/EDIC Group of Investigators; Baker, N.L.; El Abiad, J.M.; Spassieva, S.D.; Pierce, J.S.; Rembiesa, B.; Bielawski, J.; Lopes-Virella, M.F.; Klein, R.L. Increased Plasma Levels of Select Deoxy-ceramide and Ceramide Species are Associated with Increased Odds of Diabetic Neuropathy in Type 1 Diabetes: A Pilot Study. NeuroMolecular Med. 2016, 19, 46–56. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, S.M.; Lopes-Virella, M.F. Circulating Sphingolipids in Insulin Resistance, Diabetes and Associated Complications. Int. J. Mol. Sci. 2023, 24, 14015. https://doi.org/10.3390/ijms241814015
Hammad SM, Lopes-Virella MF. Circulating Sphingolipids in Insulin Resistance, Diabetes and Associated Complications. International Journal of Molecular Sciences. 2023; 24(18):14015. https://doi.org/10.3390/ijms241814015
Chicago/Turabian StyleHammad, Samar M., and Maria F. Lopes-Virella. 2023. "Circulating Sphingolipids in Insulin Resistance, Diabetes and Associated Complications" International Journal of Molecular Sciences 24, no. 18: 14015. https://doi.org/10.3390/ijms241814015
APA StyleHammad, S. M., & Lopes-Virella, M. F. (2023). Circulating Sphingolipids in Insulin Resistance, Diabetes and Associated Complications. International Journal of Molecular Sciences, 24(18), 14015. https://doi.org/10.3390/ijms241814015





