Static Cold Storage with Mitochondria-Targeted Hydrogen Sulfide Donor Improves Renal Graft Function in an Ex Vivo Porcine Model of Controlled Donation-after-Cardiac-Death Kidney Transplantation
Abstract
:1. Introduction
2. Results
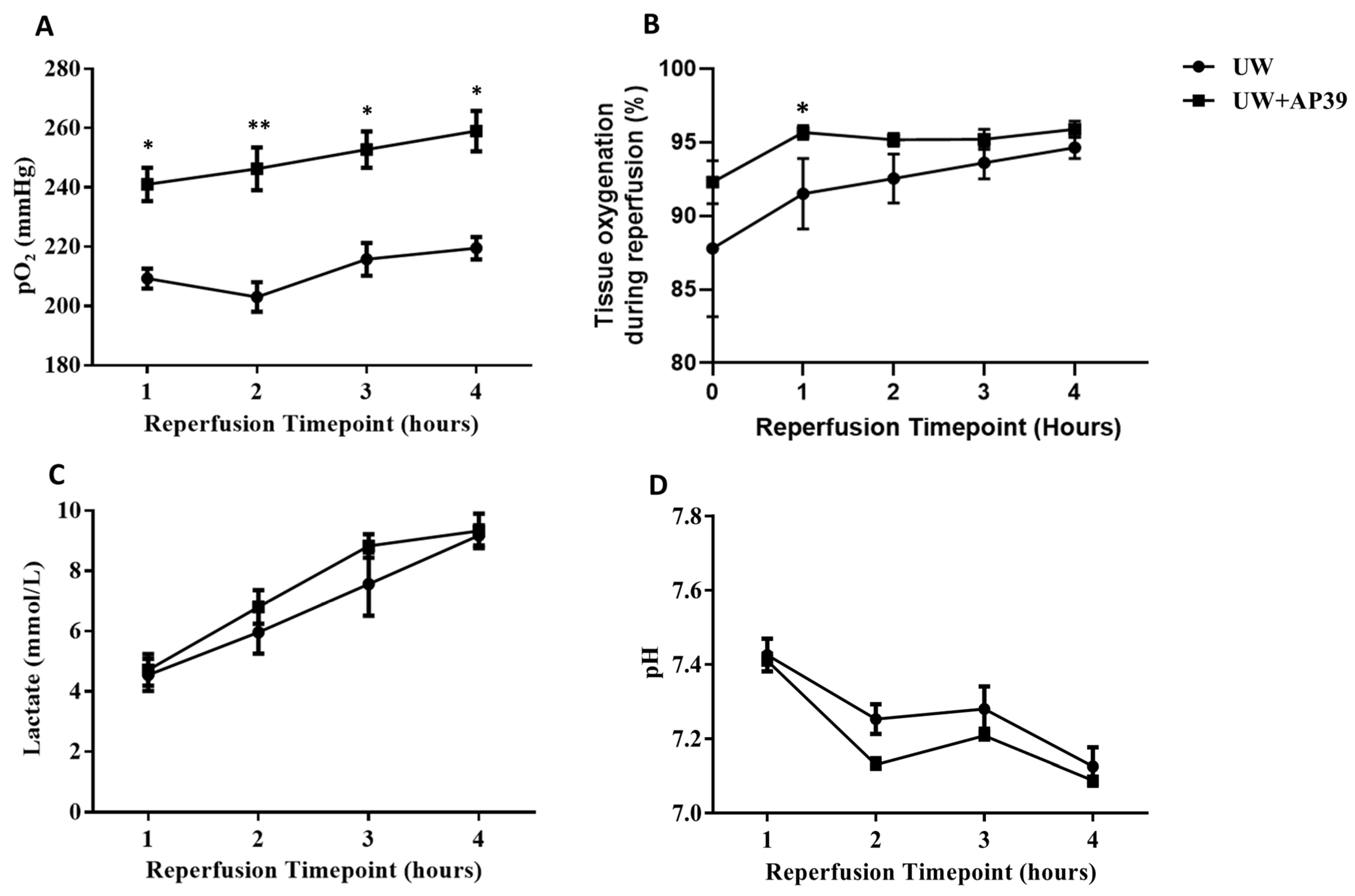
2.1. Addition of AP39 to UW Solution Improves Arterial pO2 and Tissue Oxygenation during Reperfusion
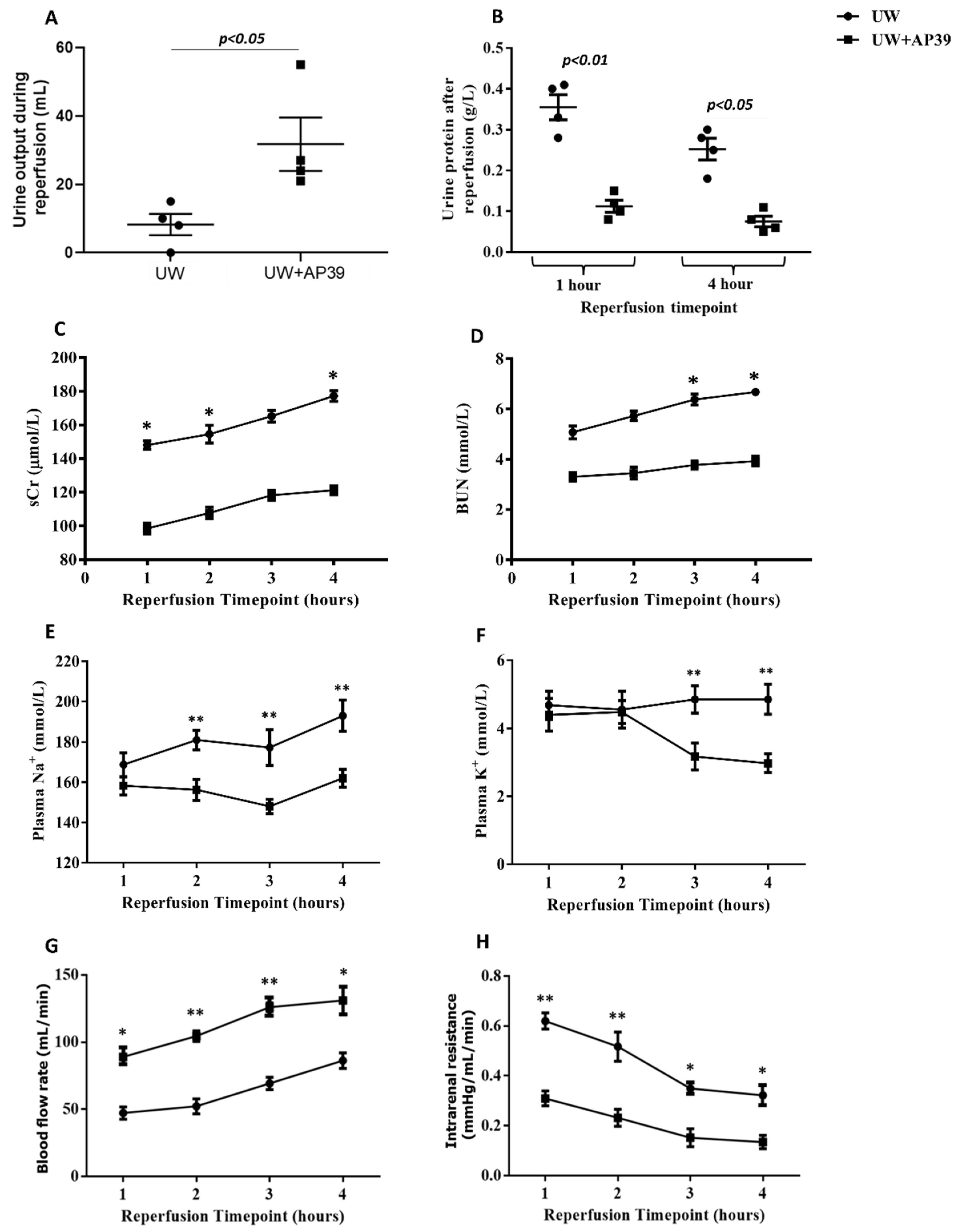
2.2. Supplementation of UW Solution with AP39 Improves Renal Graft Function during Reperfusion
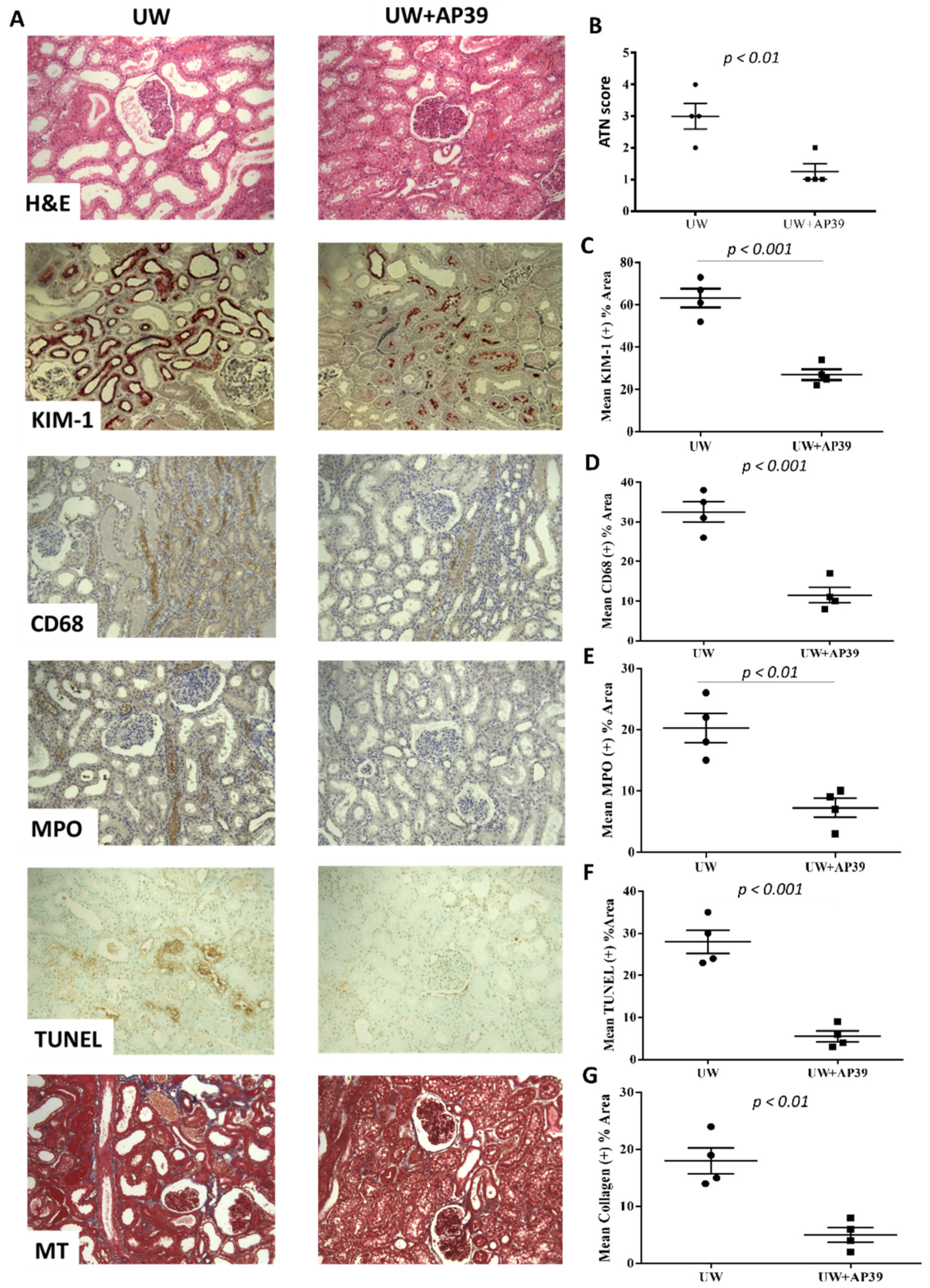
2.3. Storage of Renal Grafts in AP39-Supplemented UW Solution Preserves Graft Structure after Reperfusion
3. Discussion
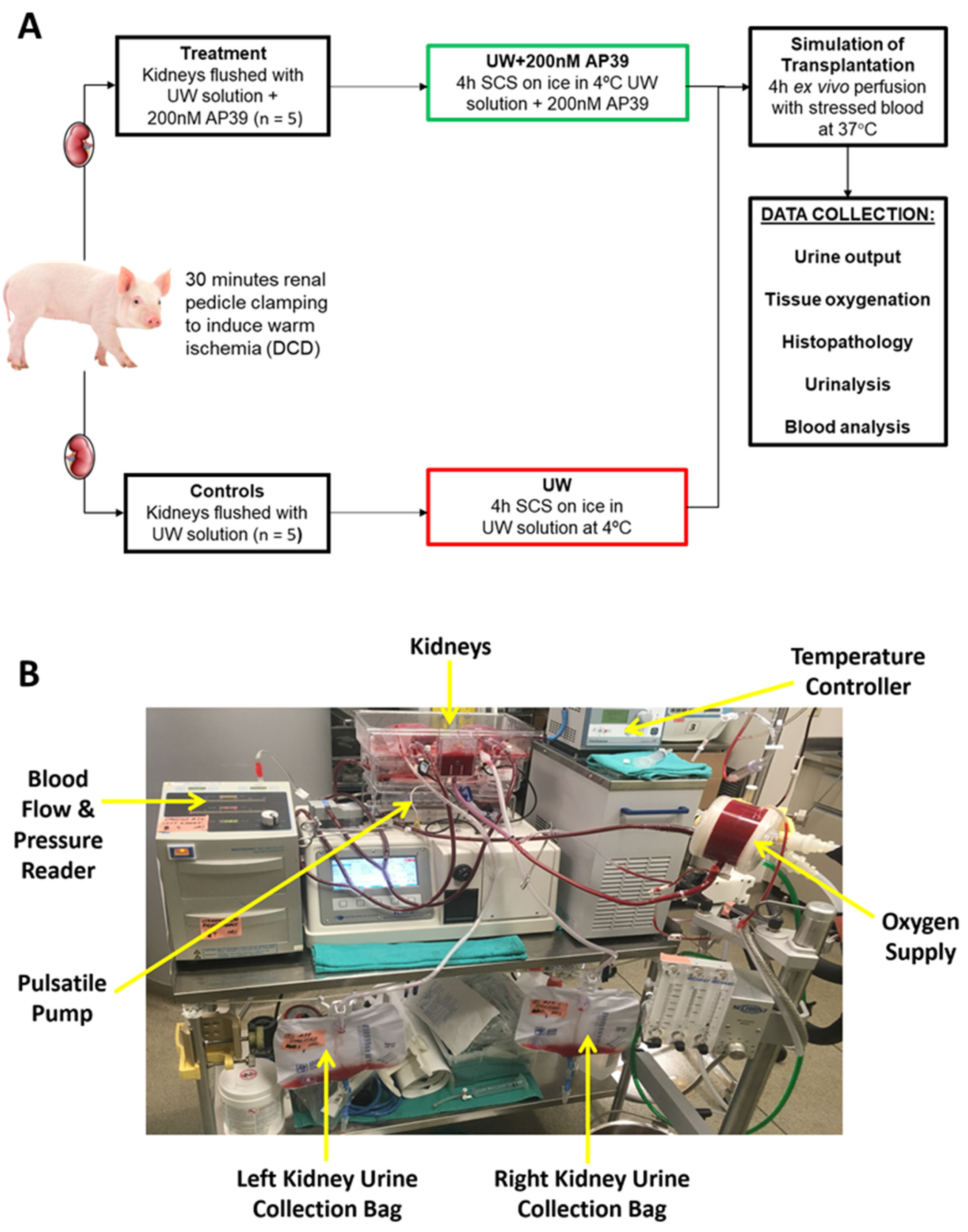
4. Materials and Methods
4.1. Induction of Warm Ischemia to Mimic-Controlled DCD
4.2. Preservation of Renal Grafts by Static Cold Storage
4.3. Ex Vivo Perfusion with Stressed Autologous Blood
4.4. Analysis of Renal Function
4.5. Histopathological Examination of Renal Grafts
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, K.D.; Kim, C.T.; Kim, M.H.; Noh, J.; Kim, G.; Kim, H.; An, J.N.; Park, J.Y.; Cho, H.; Kim, K.H.; et al. Superior outcomes of kidney transplantation compared with dialysis: An optimal matched analysis of a national population-based cohort study between 2005 and 2008 in Korea. Medicine 2016, 95, e4352. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.C.; Kainz, A.; Baer, H.; Oberbauer, R. Dialysis Vintage and Outcomes after Kidney Transplantation: A Retrospective Cohort Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.S.; Hu, L.K.; Wang, Y.; Liu, H.Y.; Chen, J.; Meng, X.W.; Pu, J.X.; Huang, Y.H.; Hou, J.Q.; Feng, X.M.; et al. Effect of Perioperative Dexmedetomidine on Delayed Graft Function Following a Donation-After-Cardiac-Death Kidney Transplant: A Randomized Clinical Trial. JAMA Netw. Open. 2022, 5, e2215217. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, J.; Yu, X.; Wang, L.; Peng, W.; Chen, J.; Wu, J. Comparison of Graft Outcome Between Donation After Circulatory Death and Living-Donor Kidney Transplantation. Transplant. Proc. 2020, 52, 111–118. [Google Scholar] [CrossRef]
- Cron, D.C.; Murakami, N.M.; Xiang, L.; Markmann, J.F.; Yeh, H.; Adler, J.T. Anastomosis Time and Outcomes after Donation after Circulatory Death Kidney Transplantation. J. Am. Coll. Surg. 2022, 234, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, M.; Kubota, Y.; Takahashi, H.; Sasaki, H.; Kawai, A.; Takenaka, M.; Fukami, N.; Kenmochi, T.; Shiroki, R.; Hoshinaga, K.; et al. Warm ischemic time as a critical risk factor of graft failure from donors after cardiac death: A single-center experience over three decades in the Kidney Donor Profile Index/Kidney Donor Risk Index era in Japan. Int. J. Urol. 2019, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, M.; Jia, J.; Zhang, Z.; Gaut, J.P.; Upadhya, G.A.; Manning, P.T.; Lin, Y.; Chapman, W.C. CD47 blockade reduces ischemia/reperfusion injury in donation after cardiac death rat kidney transplantation. Am. J. Transplant. 2018, 18, 843–854. [Google Scholar] [CrossRef]
- Kusaka, M.; Kawai, A.; Takahara, K.; Sasaki, H.; Ito, T.; Kenmochi, T.; Shiroki, R. Total Cell-Free DNA as a Noninvasive Biomarker of a Delayed Graft Function After Kidney Transplantation from Donors After Cardiac Death. Transplant. Proc. 2023, 55, 733–736. [Google Scholar] [CrossRef]
- Maassen, H.; Said, M.Y.; Frenay, A.-R.S.; Koning, A.; Post, A.; Riphagen, I.J.; Heiner-Fokkema, M.R.; Drabert, K.; Fernandez, B.O.; Gans, R.O.; et al. Nitric oxide and long-term outcomes after kidney transplantation: Results of the Transplant Lines cohort study. Nitric Oxide 2022, 125–126, 1–11. [Google Scholar] [CrossRef]
- Abe, T.; Yazawa, K.; Fujino, M.; Imamura, R.; Hatayama, N.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Ichimaru, N.; Kaimori, J.-Y.; et al. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab. Investig. 2017, 97, 468–477. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Alornyo, K.K.; Luke, P.P.; Sener, A. Application of carbon monoxide in kidney and heart transplantation: A novel pharmacological strategy for a broader use of suboptimal renal and cardiac grafts. Pharmacol. Res. 2021, 173, 105883. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, Q.; Wu, F.; Sun, F.; Meng, Y.; Zhang, Y.; Zhao, M. Bibliometric evaluation of 2011–2021 publications on hydrogen sulfide in heart preservation research. Front. Cardiovasc. Med. 2023, 9, 941374. [Google Scholar] [CrossRef] [PubMed]
- Juriasingani, S.; Jackson, A.; Zhang, M.Y.; Ruthirakanthan, A.; Dugbartey, G.J.; Sogutdelen, E.; Levine, M.; Mandurah, M.; Whiteman, M.; Luke, P.; et al. Evaluating the Effects of Subnormothermic Perfusion with AP39 in a Novel Blood-Free Model of Ex Vivo Kidney Preservation and Reperfusion. Int. J. Mol. Sci. 2021, 22, 7180. [Google Scholar] [CrossRef] [PubMed]
- Giraud, S.; Favreau, F.; Chatauret, N.; Thuillier, R.; Maiga, S.; Hauet, T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: The preclinical model. J. Biomed. Biotechnol. 2011, 2011, 532127. [Google Scholar] [CrossRef]
- Dehoux, J.-P.; Gianello, P. The importance of large animal models in transplantation. Front. Biosci. 2007, 12, 4864–4880. [Google Scholar] [CrossRef]
- Lobb, I.; Jiang, J.; Lian, D.; Liu, W.; Haig, A.; Saha, M.N.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Sener, A. Hydrogen Sulfide Protects Renal Grafts Against Prolonged Cold Ischemia-Reperfusion Injury via Specific Mitochondrial Actions. Am. J. Transplant. 2017, 17, 341–352. [Google Scholar] [CrossRef]
- Nishime, K.; Miyagi-Shiohira, C.; Kuwae, K.; Tamaki, Y.; Yonaha, T.; Sakai-Yonaha, M.; Saitoh, I.; Watanabe, M.; Noguchi, H. Preservation of pancreas in the University of Wisconsin solution supplemented with AP39 reduces reactive oxygen species production and improves islet graft function. Am. J. Transplant. 2021, 21, 2698–2708. [Google Scholar] [CrossRef]
- Wu, J.; Wei, J.; You, X.; Chen, X.; Zhu, H.; Zhu, X.; Liu, Y.; Xu, M. Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J. Surg. Res. 2013, 182, e25–e33. [Google Scholar] [CrossRef]
- Dragun, D.; Hoff, U.; Park, J.-K.; Qun, Y.; Schneider, W.; Luft, F.C.; Haller, H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001, 60, 1173–1181. [Google Scholar] [CrossRef]
- Salahudeen, A.K.; Haider, N.; May, W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004, 65, 713–718. [Google Scholar] [CrossRef]
- Quiroga, I.; McShane, P.; Koo, D.D.H.; Gray, D.; Friend, P.J.; Fuggle, S.; Darby, C. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol. Dial. Transplant. 2006, 21, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Kayler, L.K.; Magliocca, J.; Zendejas, I.; Srinivas, T.R.; Schold, J.D. Impact of cold ischemia time on graft survival among ECD transplant recipients: A paired kidney analysis. Am. J. Transplant. 2011, 11, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Rouslin, W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am. J. Physiol. 1983, 244, H743–H748. [Google Scholar] [CrossRef] [PubMed]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Goto, Y.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Hu, L.F.; Lu, M.; Wu, Z.Y.; Wong, P.T.; Bian, J.S. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 2009, 75, 27–34. [Google Scholar] [CrossRef]
- Jaeschke, H.; Mitchell, J.R. Mitochondrial and xanthine oxidase both generate reactive oxygen species in isolated perfused rat liver after hypoxic injury. Biochem. Biophys. Res. Commun. 1989, 160, 140. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Castilho, R.F.; Grijalba, M.T.; Bechara, E.J.; Vercesi, A.E. Effect of inorganic phosphate concentration on nature of inner mitochondrial membrane alterations mediated by Ca2+ ions. J. Biol. Chem. 1996, 271, 2929. [Google Scholar] [CrossRef]
- Qian, T.; Nieminen, A.L.; Herman, B.; Lemasters, J.J. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am. J. Physiol. 1997, 273, C1783–C1792. [Google Scholar] [CrossRef]
- van den Eijnden, M.M.; Leuvenink, H.G.; Ottens, P.J.; ‘t Hart, N.A.; van Oeveren, W.; Morariu, A.M.; van Goor, H.; Ploeg, R.J. Effect of brain death and non-heart-beating kidney donation on renal function and injury: An assessment in the isolated perfused rat kidney. Exp. Clin. Transplant. 2003, 1, 85–95. [Google Scholar]
- Kurokawa, T.; Kobayashi, H.; Nonami, T.; Harada, A.; Nakao, A.; Takagi, H. Mitochondrial glutathione redox and energy producing function during liver ischemia and reperfusion. J. Surg. Res. 1996, 66, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jassem, W.; Ciarimboli, C.; Cerioni, P.N.; Saba, V.; Norton, S.J.; Principato, G. Glyoxalase II and glutathione levels in rat liver mitochondria during cold storage in Euro-Collins and University of Wisconsin solutions. Transplantation 1996, 61, 1416. [Google Scholar] [CrossRef] [PubMed]
- Zwacka, R.M.; Zhou, W.; Zhang, Y.; Darby, C.J.; Dudus, L.; Halldorson, J.; Oberley, L.; Engelhardt, J.F. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-kB activation. Nat. Med. 1998, 4, 698. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br. J. Surg. 2010, 97, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Maassen, H.; Hendriks, K.D.W.; Venema, L.H.; Henning, R.H.; Hofker, S.H.; van Goor, H.; Leuvenink, H.G.D.; Coester, A.M. Hydrogen sulfide-induced hypometabolism in human-sized porcine kidneys. PLoS ONE 2019, 14, e0225152. [Google Scholar] [CrossRef]
- Ertugrul, I.A.; van Suylen, V.; Damman, K.; de Koning, M.L.Y.; van Goor, H.; Erasmus, M.E. Donor Heart Preservation with Hydrogen Sulfide: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 5737. [Google Scholar] [CrossRef]
- Meng, G.; Wang, J.; Xiao, Y.; Bai, W.; Xie, L.; Shan, L.; Moore, P.K.; Ji, Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J. Biomed. Res. 2015, 29, 203–213. [Google Scholar]
- Dugbartey, G.J.; Talaei, F.; Houwertjes, M.C.; Goris, M.; Epema, A.H.; Bouma, H.R.; Henning, R.H. Dopamine treatment attenuates acute kidney injury in a rat model of deep hypothermia and rewarming—The role of renal H2S-producing enzymes. Eur. J. Pharmacol. 2015, 769, 225–233. [Google Scholar] [CrossRef]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016, 113 Pt A, 186–198. [Google Scholar] [CrossRef]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341, 233. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Papu John, A.S.; Kundu, S.; Pushpakumar, S.; Amin, M.; Tyagi, S.C.; Sen, U. Hydrogen sulfide inhibits Ca2+-induced mitochondrial permeability transition pore opening in type-1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E269–E283. [Google Scholar] [CrossRef] [PubMed]
- Strutyns’ka, N.A.; Dorofeieva, N.O.; Vavilova, H.L.; Sahach, V.F. Hydrogen sulfide inhibits Ca(2+)-induced mitochondrial permeability transition pore opening in spontaneously hypertensive rats. Fiziol. Zhurnal 2013, 59, 3–10. [Google Scholar] [CrossRef]
- Strutyns’ka, N.A.; Semenykhina, O.M.; Chorna, S.V.; Vavilova, H.L.; Sahach, V.F. Hydrogen sulfide inhibits Ca(2+)-induced mitochondrial permeability transition pore opening in adult and old rat heart. Fiziol. Zhurnal 2011, 57, 3–14. [Google Scholar] [CrossRef]
- Brinkkoetter, P.-T.; Song, H.; Lösel, R.; Schnetzke, U.; Gottmann, U.; Feng, Y.; Hanusch, C.; Beck, G.; Schnuelle, P.; Wehling, M.; et al. Hypothermic injury: The mitochondrial calcium, ATP and ROS love-hate triangle out of balance. Cell. Physiol. Biochem. 2008, 22, 195–204. [Google Scholar] [CrossRef]
- McAnulty, J.F. The effect of calcium on hypothermia-facilitated resuscitation of warm ischemic kidney tissue slices: A role for the mitochondrial permeability transition pore? Cryobiology 1998, 36, 12–19. [Google Scholar] [CrossRef]
- Zhu, C.; Su, Y.; Juriasingani, S.; Zheng, H.; Veramkovich, V.; Jiang, J.; Sener, A.; Whiteman, M.; Lacefield, J.; Nagpal, D.; et al. Supplementing preservation solution with mitochondria-targeted H2S donor AP39 protects cardiac grafts from prolonged cold ischemia-reperfusion injury in heart transplantation. Am. J. Transplant. 2019, 19, 3139–3148. [Google Scholar] [CrossRef]
- Hayes, W.; Longley, C.; Scanlon, N.; Bryant, W.; Stojanovic, J.; Kessaris, N.; Van’t Hoff, W.; Bockenhauer, D.; Marks, S.D. Plasma electrolyte imbalance in pediatric kidney transplant recipients. Pediatr. Transplant. 2019, 23, e13411. [Google Scholar] [CrossRef]
- Xia, M.; Chen, L.; Muh, R.W.; Li, P.-L.; Li, N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J. Pharmacol. Exp. Ther. 2009, 329, 1056–1062. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.H.; Islam, M.T.; Prieto, M.C.; Majid, D.S. Interdependency of cystathione γ-lyase and cystathione β-synthase in hydrogen sulfide-induced blood pressure regulation in rats. Am. J. Hypertens. 2012, 25, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.N.; Zhao, M.M.; Wu, D.D.; Chen, Y.; Wang, Y.; Zhu, J.H.; Cai, W.J.; Zhu, Y.Z.; Zhu, Y.C. Hydrogen sulfide targets EGFR Cys797/Cys798 residues to induce Na(+)/K(+)-ATPase endocytosis and inhibition in renal tubular epithelial cells and increase sodium excretion in chronic salt-loaded rats. Antioxid. Redox Signal. 2014, 21, 2061–2082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, S.; Liu, H.; Zhang, B.; Zhao, Y.; Ma, K.; Zhao, D.; Wang, Q.; Ma, H.; Zhang, Z. Hydrogen sulfide prevents hydrogen peroxide-induced activation of epithelial sodium channel through a PTEN/PI(3,4,5)P3 dependent pathway. PLoS ONE 2013, 8, e64304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Akbari, M.; Liu, W.; Haig, A.; McLeod, P.; Arp, J.; Sener, A. Sodium thiosulfate-supplemented UW solution protects renal grafts against prolonged cold ischemia-reperfusion injury in a murine model of syngeneic kidney transplantation. Biomed. Pharmacother. 2022, 145, 112435. [Google Scholar] [CrossRef] [PubMed]
- Juriasingani, S.; Ruthirakanthan, A.; Richard-Mohamed, M.; Akbari, M.; Aquil, S.; Patel, S.; Al-Ogaili, R.; Whiteman, M.; Luke, P.; Sener, A. Subnormothermic Perfusion with H2S Donor AP39 Improves DCD Porcine Renal Graft Outcomes in an Ex Vivo Model of Kidney Preservation and Reperfusion. Biomolecules 2021, 11, 446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugbartey, G.J.; Juriasingani, S.; Richard-Mohamed, M.; Rasmussen, A.; Levine, M.; Liu, W.; Haig, A.; Whiteman, M.; Arp, J.; Luke, P.P.W.; et al. Static Cold Storage with Mitochondria-Targeted Hydrogen Sulfide Donor Improves Renal Graft Function in an Ex Vivo Porcine Model of Controlled Donation-after-Cardiac-Death Kidney Transplantation. Int. J. Mol. Sci. 2023, 24, 14017. https://doi.org/10.3390/ijms241814017
Dugbartey GJ, Juriasingani S, Richard-Mohamed M, Rasmussen A, Levine M, Liu W, Haig A, Whiteman M, Arp J, Luke PPW, et al. Static Cold Storage with Mitochondria-Targeted Hydrogen Sulfide Donor Improves Renal Graft Function in an Ex Vivo Porcine Model of Controlled Donation-after-Cardiac-Death Kidney Transplantation. International Journal of Molecular Sciences. 2023; 24(18):14017. https://doi.org/10.3390/ijms241814017
Chicago/Turabian StyleDugbartey, George J., Smriti Juriasingani, Mahms Richard-Mohamed, Andrew Rasmussen, Max Levine, Winnie Liu, Aaron Haig, Matthew Whiteman, Jacqueline Arp, Patrick P.W. Luke, and et al. 2023. "Static Cold Storage with Mitochondria-Targeted Hydrogen Sulfide Donor Improves Renal Graft Function in an Ex Vivo Porcine Model of Controlled Donation-after-Cardiac-Death Kidney Transplantation" International Journal of Molecular Sciences 24, no. 18: 14017. https://doi.org/10.3390/ijms241814017




