Effects of Low-Level Laser Therapy and Purified Natural Latex (Hevea brasiliensis) Protein on Injured Sciatic Nerve in Rodents: Morpho-Functional Analysis
Abstract
:1. Introduction
2. Results
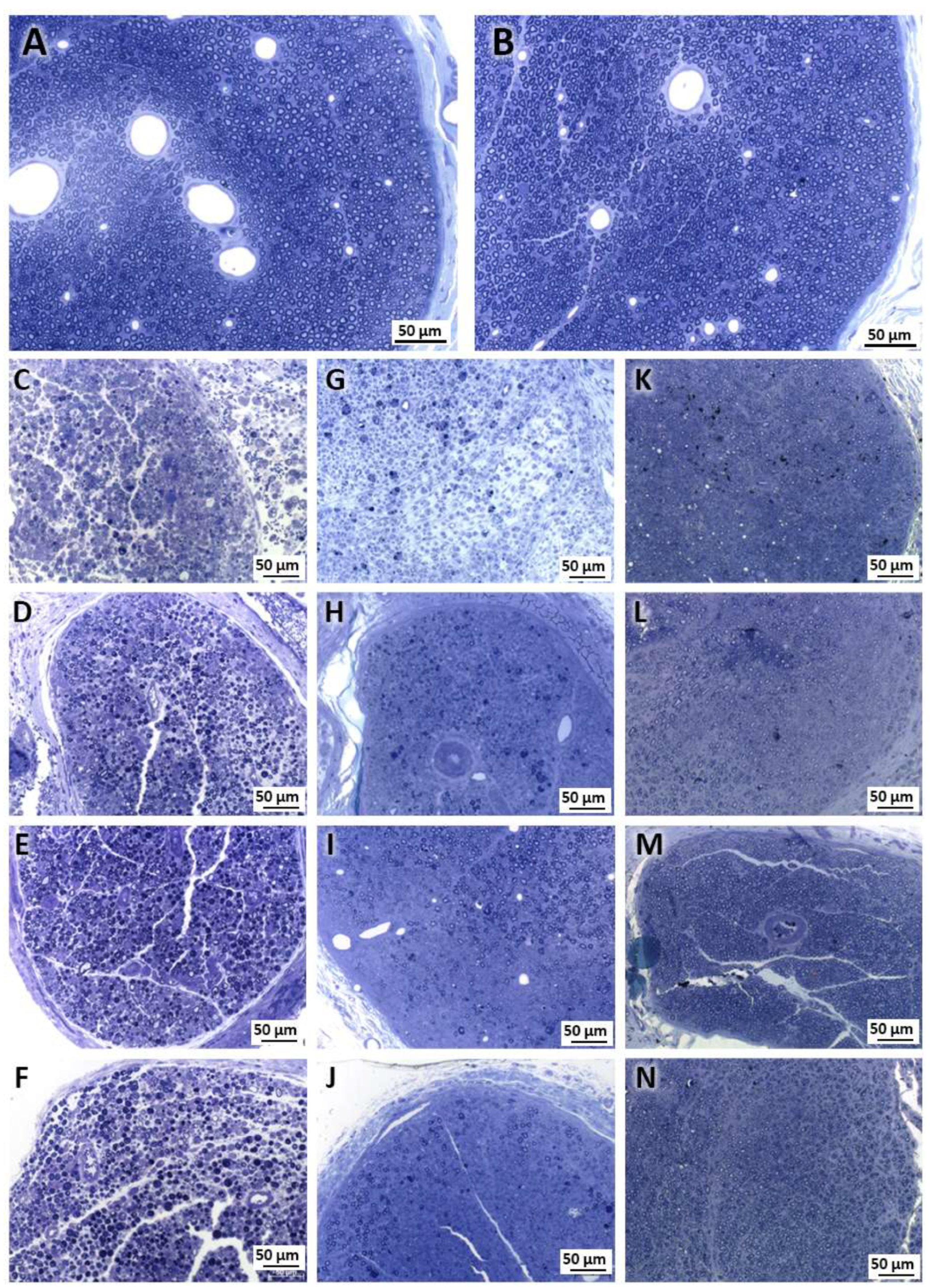
2.1. Morphological Analysis
2.2. Quantitative and Morphometric Analysis
2.2.1. Nerve Fiber Density
2.2.2. Minimum Diameter of Nerve Fibers
2.2.3. Blood Capillary Density
2.3. Ultrastructural Analysis—Transmission Electron Microscopy
2.4. Sensory Function Evaluation
2.4.1. Mechanical Allodynia
2.4.2. Mechanical Hyperalgesia
2.5. Motor Function Evaluation
2.5.1. Grip Strength Test
2.5.2. Gait Assessment (Sciatic Nerve Functional Index)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure and Nerve Injury
4.3. Low-Level Laser Therapy (LLLT)
4.4. Application of the Purified Protein of Natural Latex (Hevea brasiliensis)
4.5. Experimental Groups
4.6. Sample Processing—Histological and Ultrastructural Analysis
4.7. Morphometric and Quantitative Histological Analysis
4.8. Sensory Function Assessment
4.9. Motor Function Assessment
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raimondo, S.; Fornaro, M.; Tos, P.; Battiston, B.; Giacobini-Robecchi, M.G.; Geuna, S. Perspectives in regeneration and tissue engineering of peripheral nerves. Ann. Anat. 2011, 193, 334–340. [Google Scholar] [CrossRef]
- Shen, C.C.; Yang, Y.C.; Liu, B.S. Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury 2011, 42, 803–813. [Google Scholar] [CrossRef]
- Dubový, P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann. Anat. 2011, 193, 267–275. [Google Scholar] [CrossRef]
- Seddon, H.J. A Classification of Nerve Injuries. Br. Med. J. 1942, 2, 237–239. [Google Scholar] [CrossRef]
- Pachioni, C.A.S.; Mazzer, N.; Barbieri, C.H.; Fazan, V.P.S.; Padovani, C.R.; Moro, C.A.; Silva, C.A.A.D. Rats’ ischiatic nerve injury caused by smashing: A vascularization study. Acta Ortop. Bras. 2006, 14, 203–207. [Google Scholar] [CrossRef]
- Rovak, J.M.; Mungara, A.K.; Aydin, M.A.; Cederna, P.S. Effects of vascular endothelial growth factor on nerve regeneration in acellular nerve grafts. J. Reconstr. Microsurg. 2004, 20, 53–58. [Google Scholar] [CrossRef]
- Binan, L.; Ajji, A.; De Crescenzo, G.; Jolicoeur, M. Approaches for neural tissue regeneration. Stem Cell Rev. Rep. 2014, 10, 44–59. [Google Scholar] [CrossRef]
- Hashmi, J.T.; Huang, Y.Y.; Osmani, B.Z.; Sharma, S.K.; Naeser, M.A.; Hamblin, M.R. Role of low-level laser therapy in neurorehabilitation. Phys. Med. Rehabil. 2010, 2, S292–S305. [Google Scholar] [CrossRef]
- Gomes, L.E.; Dalmarco, E.M.; André, E.S. The brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, and induced nitric oxide synthase expressions after low-level laser therapy in an axonotmesis experimental model. Photomed. Laser Surg. 2012, 30, 642–647. [Google Scholar] [CrossRef]
- Rochkind, S. Phototherapy in peripheral nerve regeneration: From basic science to clinical study. Neurosurg. Focus. 2009, 26, E8. [Google Scholar] [CrossRef]
- Mrué, F.; Coutinho-Netto, J.; Ceneviva, R.; Lachat, J.J.; Thomazini, J.A.; Tambelini, H. Evaluation of The Biocompatibility of a New Biomembrane. Mater. Res. 2004, 7, 277–283. [Google Scholar] [CrossRef]
- Balabanian, C.A.; Coutinho-Netto, J.; Lamano-Carvalho, T.L.; Lacerda, S.A.; Brentegani, L.G. Biocompatibility of natural latex implanted into dental alveolus of rats. J. Oral Sci. 2006, 48, 201–205. [Google Scholar] [CrossRef]
- Domingos, A.L.; Tucci, S.J.; Garcia, S.B.; de Bessa, J.J.; Cologna, A.J.; Martins, A.C. Use of a latex biomembrane for bladder augmentation in a rabbit model: Biocompatibility, clinical and histological outcomes. Int. Braz. J. Urol. 2009, 35, 217–224. [Google Scholar] [CrossRef]
- Mendonça, R.J.; Maurício, V.B.; Teixeira, L.B.; Lachat, J.J.; Coutinho-Netto, J. Increased vascular permeability, angiogenesis and wound healing induced by the serum of natural latex of the rubber tree Hevea brasiliensis. Phytother. Res. 2010, 24, 764–768. [Google Scholar] [CrossRef]
- Andrade, T.A.; Iyer, A.; Das, P.K.; Foss, N.T.; Garcia, S.B.; Coutinho-Netto, J.; Jordão, A.A.J.; Frade, M.A. The inflammatory stimulus of a natural latex biomembrane improves healing in mice. Braz. J. Med. Biol. Res. 2011, 44, 1036–1047. [Google Scholar] [CrossRef]
- Frade, M.A.; Assis, R.V.; Coutinho Netto, J.; Andrade, T.A.; Foss, N.T. The vegetal biomembrane in the healing of chronic venous ulcers. An. Bras. Dermatol. 2012, 87, 45–51. [Google Scholar] [CrossRef]
- Ijkema-Paassen, J.; Jansen, K.; Gramsbergen, A.; Meek, M.F. Transection of peripheral nerves, bridging strategies and effect evaluation. Biomaterials 2004, 25, 1583–1592. [Google Scholar] [CrossRef]
- Dias, F.J.; Issa, J.P.; Iyomasa, M.M.; Coutinho-Netto, J.; Calzzani, R.A.; Iyomasa, D.M.; Sousa, L.G.; de Almeida, S.R.; Cury, D.P.; Watanabe, I.S. Application of a low-level laser therapy and the purified protein from natural latex (Hevea brasiliensis) in the controlled crush injury of the sciatic nerve of rats: A morphological, quantitative, and ultrastructural study. Biomed. Res. Int. 2013, 2013, 597863. [Google Scholar] [CrossRef]
- Barreiros, V.C.; Dias, F.J.; Iyomasa, M.M.; Coutinho-Netto, J.; de Sousa, L.G.; Fazan, V.P.; Antunes, R.S.; Watanabe, I.S.; Issa, J.P. Morphological and morphometric analyses of crushed sciatic nerves after application of a purified protein from natural latex and hyaluronic acid hydrogel. Growth Factors 2014, 32, 164–170. [Google Scholar] [CrossRef]
- Muniz, K.L.; Dias, F.J.; Coutinho-Netto, J.; Calzzani, R.A.; Iyomasa, M.M.; Sousa, L.G.; Santos, T.T.; Teles, V.O.; Watanabe, I.S.; Fazan, V.P.; et al. Properties of the tibialis anterior muscle after treatment with laser therapy and natural latex protein following sciatic nerve crush. Muscle Nerve 2015, 52, 869–875. [Google Scholar] [CrossRef]
- Dias, F.J.; Issa, J.P.; Coutinho-Netto, J.; Fazan, V.P.; Sousa, L.G.; Iyomasa, M.M.; Papa, P.C.; Watanabe, I.S. Morphometric and high resolution scanning electron microscopy analysis of low-level laser therapy and latex protein (Hevea brasiliensis) administration following a crush injury of the sciatic nerve in rats. J. Neurol. Sci. 2015, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.J.; Fazan, V.P.S.; Cury, D.P.; de Almeida, S.R.Y.; Borie, E.; Fuentes, R.; Coutinho-Netto, J.; Watanabe, I.S. Growth factors expression and ultrastructural morphology after application of low-level laser and natural latex protein on a sciatic nerve crush-type injury. PLoS ONE 2019, 14, e0210211. [Google Scholar] [CrossRef] [PubMed]
- Coradini, J.G.; Mattjie, T.F.; Bernardino, G.R.; Peretti, A.L.; Kakihata, C.M.; Errero, T.K.; Escher, A.R.; Bertolini, G.R. Comparison of low level laser, ultrasonic therapy and association in joint pain in Wistar rats. Rev. Bras. Reumatol. 2014, 54, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Schiaveto de Souza, A.; da Silva, C.A.; Del Bel, E.A. Methodological evaluation to analyze functional recovery after sciatic nerve injury. J. Neurotrauma 2004, 21, 627–635. [Google Scholar] [CrossRef]
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Ann. Anat. 2011, 193, 321–333. [Google Scholar] [CrossRef]
- Muratori, L.; Ronchi, G.; Raimondo, S.; Giacobini-Robecchi, M.G.; Fornaro, M.; Geuna, S. Can regenerated nerve fibers return to normal size? A long-term post-traumatic study of the rat median nerve crush injury model. Microsurgery 2012, 32, 383–387. [Google Scholar] [CrossRef]
- Hashimoto, T.; Suzuki, Y.; Kitada, M.; Kataoka, K.; Wu, S.; Suzuki, K.; Endo, K.; Nishimura, Y.; Ide, C. Peripheral nerve regeneration through alginate gel: Analysis of early outgrowth and late increase in diameter of regenerating axons. Exp. Brain Res. 2002, 146, 356–368. [Google Scholar] [CrossRef]
- Ishikawa, N.; Suzuki, Y.; Ohta, M.; Cho, H.; Suzuki, S.; Dezawa, M.; Ide, C. Peripheral nerve regeneration through the space formed by a chitosan gel sponge. J. Biomed. Mater. Res. A 2007, 83, 33–40. [Google Scholar] [CrossRef]
- Gordon, T.; Tyreman, N.; Raji, M.A. The basis for diminished functional recovery after delayed peripheral nerve repair. J. Neurosci. 2011, 31, 5325–5334. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- de Oliveira Martins, D.; Martinez dos Santos, F.; Evany de Oliveira, M.; de Britto, L.R.; Benedito Dias Lemos, J.; Chacur, M. Laser therapy and pain-related behavior after injury of the inferior alveolar nerve: Possible involvement of neurotrophins. J. Neurotrauma 2013, 30, 480–486. [Google Scholar] [CrossRef]
- Pires de Sousa, M.V.; Ferraresi, C.; Kawakubo, M.; Kaippert, B.; Yoshimura, E.M.; Hamblin, M.R. Transcranial low-level laser therapy (810 nm) temporarily inhibits peripheral nociception: Photoneuromodulation of glutamate receptors, prostatic acid phophatase, and adenosine triphosphate. Neurophotonics 2016, 3, 015003. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.L.; Fan, Y.C.; Yang, C.C. Low-level laser therapy alleviates mechanical and cold allodynia induced by oxaliplatin administration in rats. Support. Care Cancer 2016, 24, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.R.C.; Ciena, A.P.; Rosa, A.S.; Martins, D.O.; Chacur, M. Photobiostimulation reverses allodynia and peripheral nerve damage in streptozotocin-induced type 1 diabetes. Lasers Med. Sci. 2017, 32, 495–501. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Fawcett, J.W. Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J. Peripher. Nerv. Syst. 2007, 12, 11–27. [Google Scholar] [CrossRef]
- Sharma, R.; Aggarwal, A.N.; Bhatt, S.; Kumar, S.; Bhargava, S.K. Outcome of low level lasers versus ultrasonic therapy in de Quervain’s tenosynovitis. Indian J. Orthop. 2015, 49, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, F.U.; Saygı, E.K.; Senol, S.; Kapcı, S.; Aydeniz, B.; Aktaş, İ.; Gozke, E. New treatment alternatives in the ulnar neuropathy at the elbow: Ultrasound and low-level laser therapy. Acta Neurol. Belg. 2015, 115, 355–360. [Google Scholar] [CrossRef]
- Paolillo, A.R.; Paolillo, F.R.; João, J.P.; João, H.A.; Bagnato, V.S. Synergic effects of ultrasound and laser on the pain relief in women with hand osteoarthritis. Lasers Med. Sci. 2015, 30, 279–286. [Google Scholar] [CrossRef]
- Belchior, A.C.; dos Reis, F.A.; Nicolau, R.A.; Silva, I.S.; Perreira, D.M.; de Carvalho, P.T. Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med. Sci. 2009, 24, 893–899. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Marcolino, A.M.; de Jesus Guirro, R.R.; Mazzer, N.; Barbieri, C.H.; de Cássia Registro Fonseca, M. Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med. Sci. 2010, 25, 423–430. [Google Scholar] [CrossRef]
- Marcolino, A.M.; Barbosa, R.I.; das Neves, L.M.; Mazzer, N.; de Jesus Guirro, R.R.; de Cássia Registro Fonseca, M. Assessment of functional recovery of sciatic nerve in rats submitted to low-level laser therapy with different fluences. An experimental study: Laser in functional recovery in rats. J. Hand Microsurg. 2013, 5, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Takhtfooladi, M.A.; Jahanbakhsh, F.; Takhtfooladi, H.A.; Yousefi, K.; Allahverdi, A. Effect of low-level laser therapy (685 nm, 3 J/cm(2)) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med. Sci. 2015, 30, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Andraus, R.A.C.; Maia, L.P.; de Souza Lino, A.D.; Fernandes, K.B.P.; de Matos Gomes, M.V.; de Jesus Guirro, R.R.; Barbieri, C.H. LLLT actives MMP-2 and increases muscle mechanical resistance after nerve sciatic rat regeneration. Lasers Med. Sci. 2017, 32, 771–778. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.G.; Marcolino, A.M.; Kuriki, H.U.; Gonçalves, E.C.D.; Fonseca, M.C.R.; Barbosa, R.I. Comparative effect of photobiomodulation associated with dexamethasone after sciatic nerve injury model. Lasers Med. Sci. 2018, 33, 1341–1349. [Google Scholar] [CrossRef]
- Alayat, M.S.M.; Basalamah, M.A.; Elbarrany, W.G.E.A.; El-Sawy, N.A.M.; Abdel-Kafy, E.M.; El-Fiky, A.A. Dose-dependent effect of the pulsed Nd:YAG laser in the treatment of crushed sciatic nerve in Wister rats: An experimental model. Lasers Med. Sci. 2020, 35, 1989–1998. [Google Scholar] [CrossRef]
- de Almeida Melo Maciel Mangueira, M.; Caparelli-Dáquer, E.; Filho, O.P.G.; de Assis, D.S.F.R.; Sousa, J.K.C.; Lima, W.L.; Pinheiro, A.L.B.; Silveira, L.J.; Mangueira, N.M. Raman spectroscopy and sciatic functional index (SFI) after low-level laser therapy (LLLT) in a rat sciatic nerve crush injury model. Lasers Med. Sci. 2022, 37, 2957–2971. [Google Scholar] [CrossRef]
- Ganga, M.V.; Coutinho-Netto, J.; Colli, B.O.; Marques Junior, W.; Catalão, C.H.; Santana, R.T.; Oltramari, M.R.; Carraro, K.T.; Lachat, J.J.; Lopes, L.D.A.S. Sciatic nerve regeneration in rats by a nerve conduit engineering with a membrane derived from natural latex. Acta Cir. Bras. 2012, 27, 885–891. [Google Scholar] [CrossRef]
- Park, J.; Lim, E.; Back, S.; Na, H.; Park, Y.; Sun, K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J. Biomed. Mater. Res. A 2010, 93, 1091–1099. [Google Scholar] [CrossRef]
- Fazan, V.P.; Júnior, R.F.; Salgado, H.C.; Barreira, A.A. Morphology of aortic depressor nerve myelinated fibers in normotensive Wistar-Kyoto and spontaneously hypertensive rats. J. Auton. Nerv. Syst. 1999, 77, 133–139. [Google Scholar] [CrossRef]
- Fazan, V.P.; Salgado, H.C.; dos Reis, G.C.; Barreira, A.A. Relation between myelin area and axon diameter in the aortic depressor nerve of spontaneously hypertensive rats. J. Neurosci. Methods 2005, 148, 130–136. [Google Scholar] [CrossRef]
- Fazan, V.P.; Salgado, H.C.; Barreira, A.A. Aortic depressor nerve myelinated fibers in acute and chronic experimental diabetes. Am. J. Hypertens. 2006, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, A.; Jeronimo, C.A.; Rodrigues Filho, O.A.; Sanada, L.S.; Fazan, V.P. Microscopic anatomy of the sural nerve in the postnatal developing rat: A longitudinal and lateral symmetry study. J. Anat. 2005, 206, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, A.; Jeronimo, C.A.; Rodrigues Filho, O.A.; Sanada, L.S.; Fazan, V.P. A morphometric study on the longitudinal and lateral symmetry of the sural nerve in mature and aging female rats. Brain Res. 2008, 1222, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Filho, O.A.; Fazan, V.P. Streptozotocin induced diabetes as a model of phrenic nerve neuropathy in rats. J. Neurosci. Methods 2006, 151, 131–138. [Google Scholar] [CrossRef]
- Fazan, V.P.; Salgado, H.C.; Barreira, A.A. A descriptive and quantitative light and electron microscopy study of the aortic depressor nerve in normotensive rats. Hypertension 1997, 30, 693–698. [Google Scholar] [CrossRef]
- Fazan, V.P.; Salgado, H.C.; Barreira, A.A. Aortic depressor nerve unmyelinated fibers in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1560–H1564. [Google Scholar] [CrossRef]
- Fazan, V.P.; Ma, X.; Chapleau, M.W.; Barreira, A.A. Qualitative and quantitative morphology of renal nerves in C57BL/6J mice. Anat. Rec. 2002, 268, 399–404. [Google Scholar] [CrossRef]
- Sato, K.L.; do Carmo, J.M.; Fazan, V.P. Ultrastructural anatomy of the renal nerves in rats. Brain Res. 2006, 1119, 94–100. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Milligan, E.D.; Mehmert, K.K.; Hinde, J.L.; Harvey, L.O.; Martin, D.; Tracey, K.J.; Maier, S.F.; Watkins, L.R. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000, 861, 105–116. [Google Scholar] [CrossRef]
- Chacur, M.; Milligan, E.D.; Gazda, L.S.; Armstrong, C.; Wang, H.; Tracey, K.J.; Maier, S.F.; Watkins, L.R. A new model of sciatic inflammatory neuritis (SIN): Induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain 2001, 94, 231–244. [Google Scholar] [CrossRef] [PubMed]
- de Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Yu, Y.C.; Koo, S.T.; Kim, C.H.; Lyu, Y.; Grady, J.J.; Chung, J.M. Two variables that can be used as pain indices in experimental animal models of arthritis. J. Neurosci. Methods 2002, 115, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Skyba, D.A.; Radhakrishnan, R.; Sluka, K.A. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J. Pain 2005, 6, 41–47. [Google Scholar] [CrossRef]
- Anderson, K.D.; Abdul, M.; Steward, O. Quantitative assessment of deficits and recovery of forelimb motor function after cervical spinal cord injury in mice. Exp. Neurol. 2004, 190, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, A.L.P.; Barbieri, C.H.; Mazzer, N. Correlation between different methods of gait functional evaluation in rats with ischiatic nerve crushing injuries. Acta Ortop. Bras. 2007, 15, 285–289. [Google Scholar] [CrossRef]









| Parameter | Value |
|---|---|
| Output power | 30 mW |
| Power density | 0.75 W/cm2 |
| Energy density | 15 J/cm2 |
| Wavelength | 780 nm |
| Application time (per point) | 20 s |
| Number of application points | 3 |
| Wave type | Continuous wave |
| Beam direction | Perpendicular to tissue |
| Dose per treatment spot (per point) | 0.6 J |
| Spot area of application | 0.04 cm2 |
| Number of irradiation sessions | 6 (alternate days) |
| Group | Experimental Protocol Applied |
|---|---|
| Control | Anesthetized animals were subjected to trichotomy in the left hind paw and kept in lateral decubitus for 10 min (n = 18). |
| Exposed | The uninjured exposed sciatic nerve was placed on the lesion support for 10 min (n = 18). |
| Injury | The sciatic nerve was injured via crushing (150 N/15 kgf, 10 min) and repositioned without treatment (n = 18). |
| LLLT | The injured sciatic nerve was repositioned and, after two days, irradiated with LLLT (15 J/cm2, 780 nm) (n = 18). |
| F1 | The injured sciatic nerve was repositioned, and we subsequently applied hyaluronic acid with purified natural latex protein (0.1% concentration) around the nerve at the injury site (n = 18). |
| LLLT + F1 | The injured sciatic nerve was repositioned, and we subsequently applied F1 (0.1%). After 2 days, the animals were irradiated with LLLT (15 J/cm2, 780 nm) (n = 18). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, F.J.; Cury, D.P.; Dias, P.E.; Borie, E.; Alarcón-Apablaza, J.; Lezcano, M.F.; Martínez-Rodríguez, P.; Vargas, D.; Gutiérrez, B.; Fazan, V.P.S. Effects of Low-Level Laser Therapy and Purified Natural Latex (Hevea brasiliensis) Protein on Injured Sciatic Nerve in Rodents: Morpho-Functional Analysis. Int. J. Mol. Sci. 2023, 24, 14031. https://doi.org/10.3390/ijms241814031
Dias FJ, Cury DP, Dias PE, Borie E, Alarcón-Apablaza J, Lezcano MF, Martínez-Rodríguez P, Vargas D, Gutiérrez B, Fazan VPS. Effects of Low-Level Laser Therapy and Purified Natural Latex (Hevea brasiliensis) Protein on Injured Sciatic Nerve in Rodents: Morpho-Functional Analysis. International Journal of Molecular Sciences. 2023; 24(18):14031. https://doi.org/10.3390/ijms241814031
Chicago/Turabian StyleDias, Fernando José, Diego Pulzatto Cury, Paula Elisa Dias, Eduardo Borie, Josefa Alarcón-Apablaza, María Florencia Lezcano, Paulina Martínez-Rodríguez, Daniel Vargas, Brandon Gutiérrez, and Valéria Paula Sassoli Fazan. 2023. "Effects of Low-Level Laser Therapy and Purified Natural Latex (Hevea brasiliensis) Protein on Injured Sciatic Nerve in Rodents: Morpho-Functional Analysis" International Journal of Molecular Sciences 24, no. 18: 14031. https://doi.org/10.3390/ijms241814031
APA StyleDias, F. J., Cury, D. P., Dias, P. E., Borie, E., Alarcón-Apablaza, J., Lezcano, M. F., Martínez-Rodríguez, P., Vargas, D., Gutiérrez, B., & Fazan, V. P. S. (2023). Effects of Low-Level Laser Therapy and Purified Natural Latex (Hevea brasiliensis) Protein on Injured Sciatic Nerve in Rodents: Morpho-Functional Analysis. International Journal of Molecular Sciences, 24(18), 14031. https://doi.org/10.3390/ijms241814031










