Phenolic-Based Discrimination between Non-Symptomatic and Symptomatic Leaves of Aesculus hippocastanum Infested by Cameraria ohridella and Erysiphe flexuosa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Visualization of Damage to Horse Chestnut Leaves
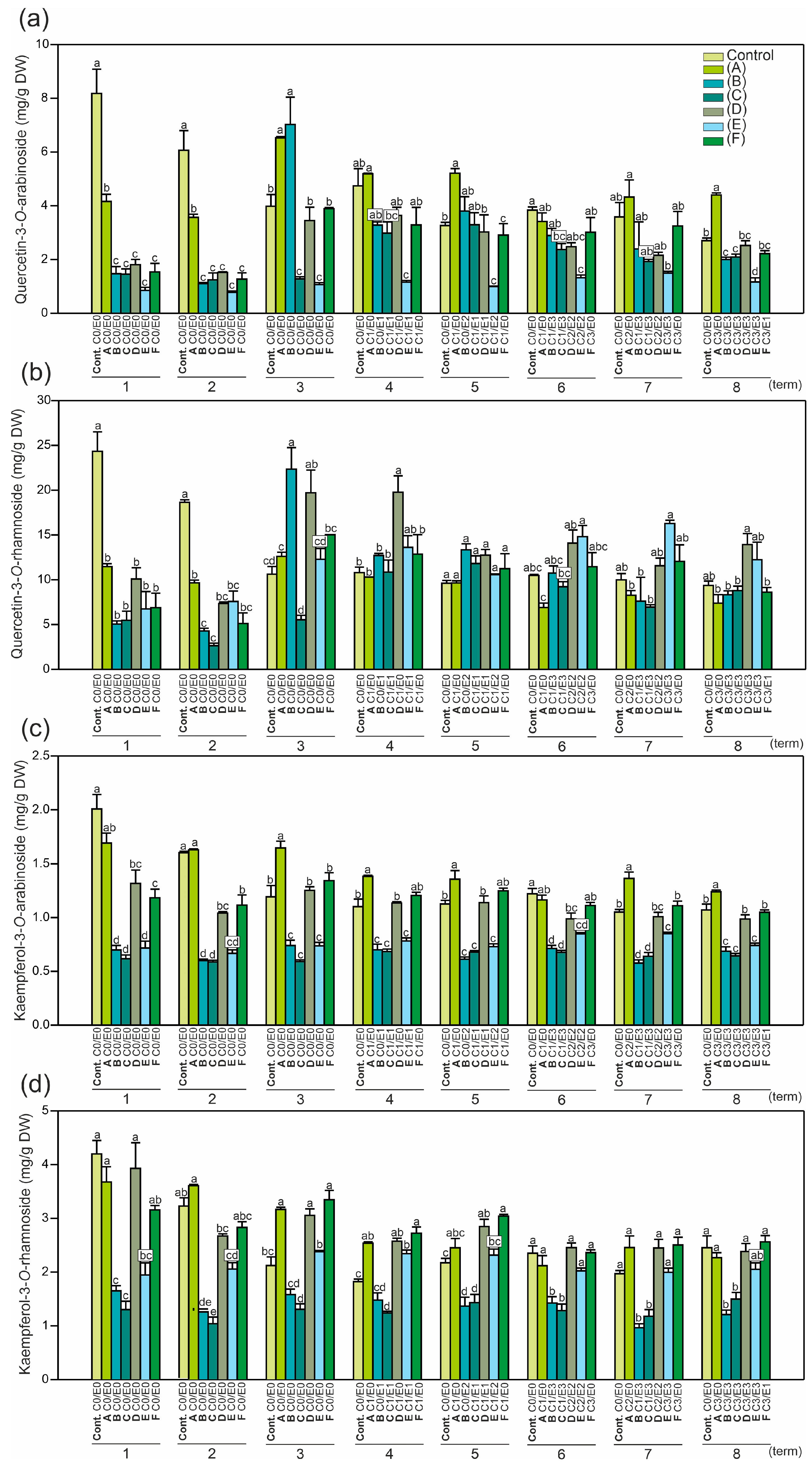
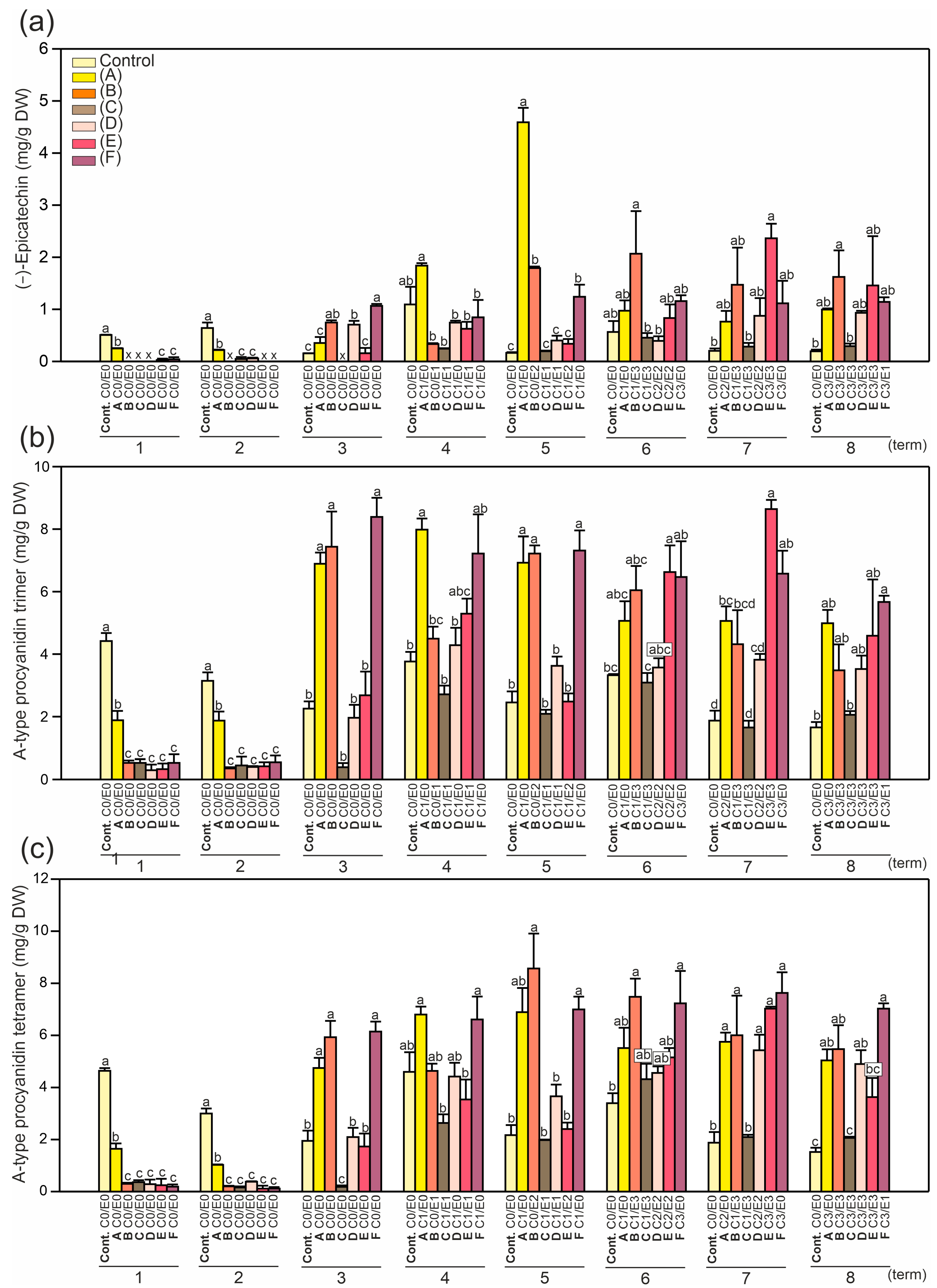
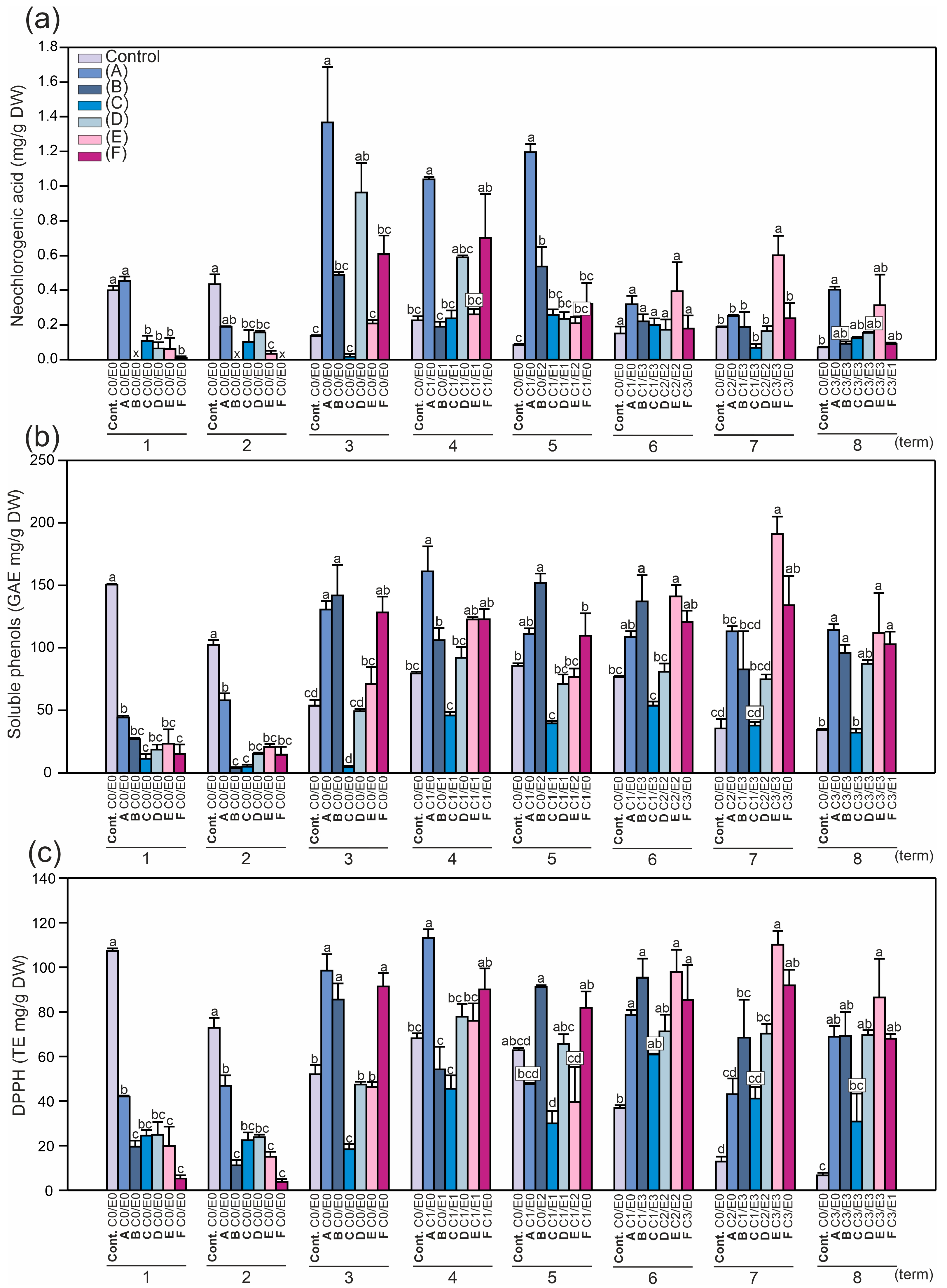
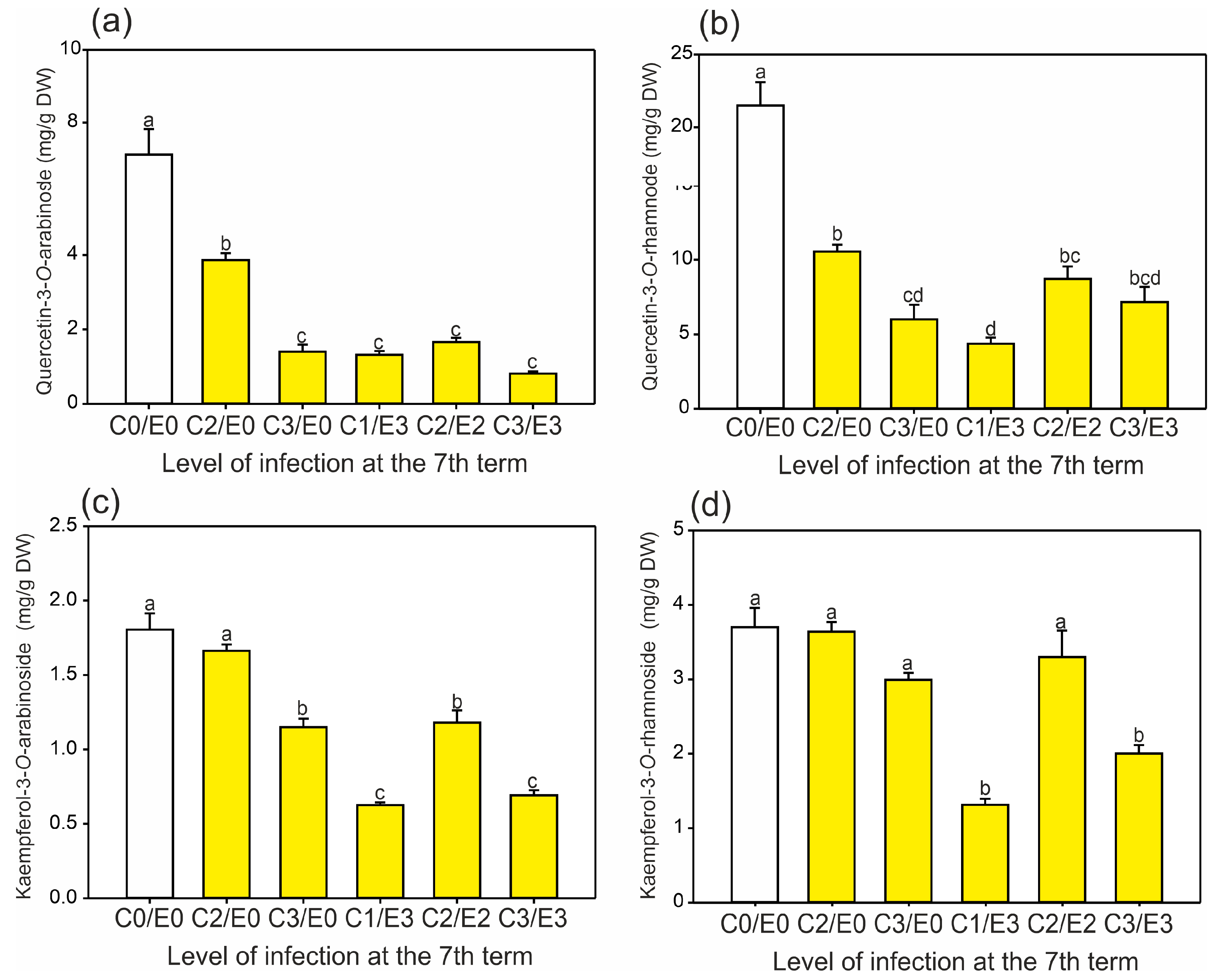
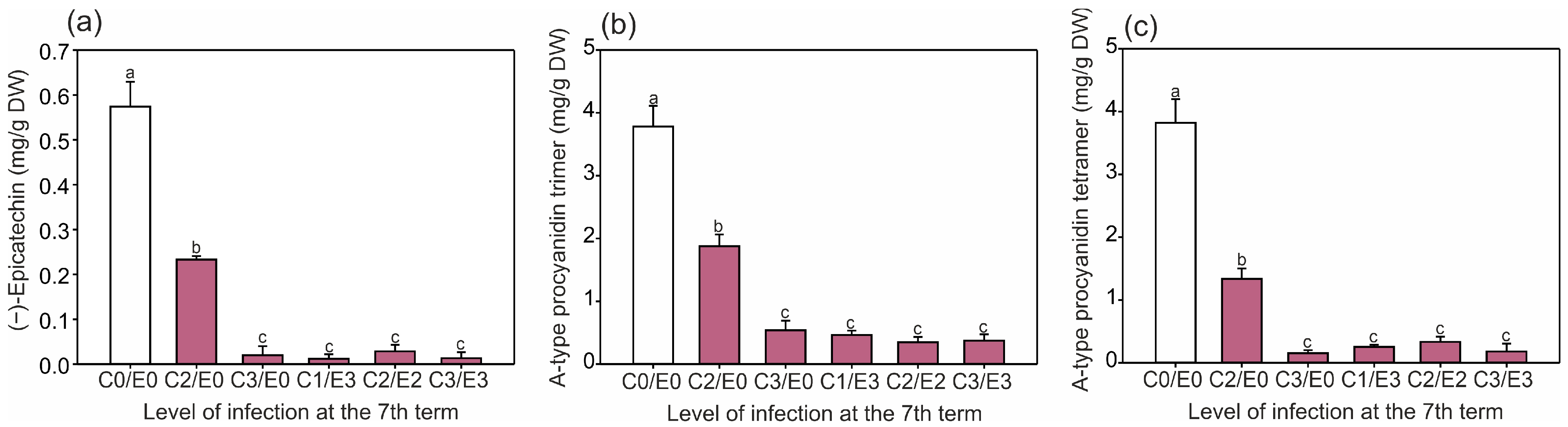
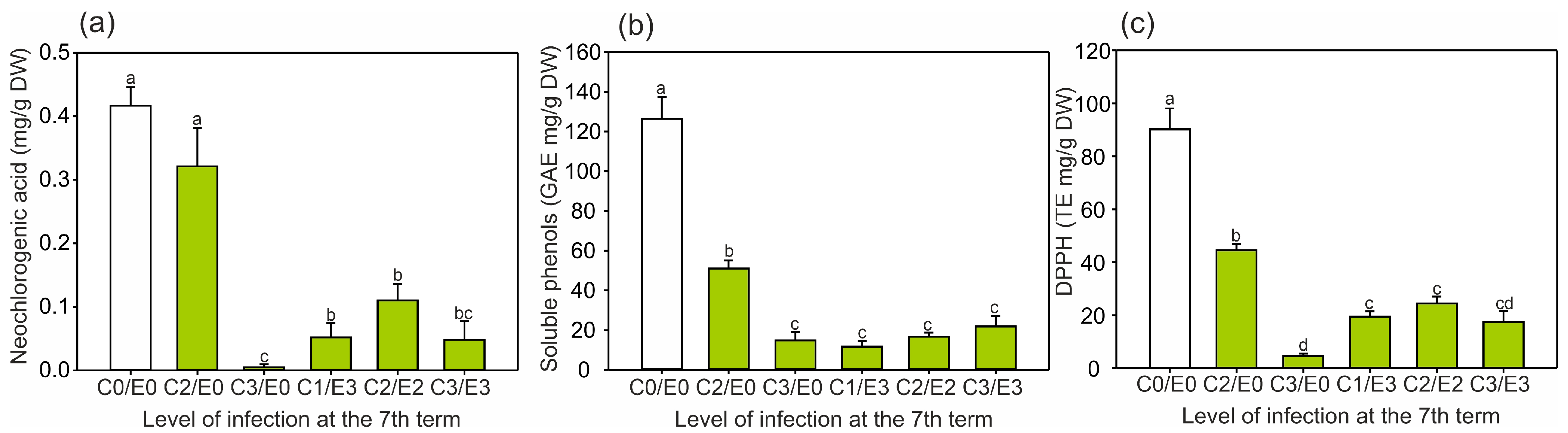
2.2. Identification and Quantification of Phenolics and the Level of Antioxidant Activity in Horse Chestnut Leaves
2.3. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plant Material and Sample Collection
3.2. Scoring Damage to Horse Chestnut Leaves
3.3. Determination of the Specific Polyphenol Content
3.4. Determination of the Total Phenolic Content and Antioxidant Capacity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snieskiene, V.; Stankeviciene, A.; Zeimavicius, K.; Balezentiene, L. Aesculus hippocastanum L. State Changes in Lithuania. Pol. J. Environ. Stud. 2011, 20, 1029–1035. [Google Scholar]
- Oszmiański, J.; Kalisz, S.; Wojtyło, A. The Content of Phenolic Compounds in Leaf Tissues of White (Aesculus hippocastanum L.) and Red Horse Chestnut (Aesculus carea H.) colonized by the Horse Chestnut Leaf Miner (Cameraria ohridella Deschka & Dimić). Molecules 2014, 19, 14625–14636. [Google Scholar] [CrossRef]
- Owczarek-Januszkiewicz, A.; Kicel, A.; Olszewska, M.A. Aesculus hippocastanum in the pharmaceutical industry and beyond—Phytochemistry, bioactivity, present application, and future perspectives. Ind. Crops Prod. 2023, 193, 116187. [Google Scholar] [CrossRef]
- Dridi, A.; Reis, F.S.; Pires, T.C.S.P.; Calhelha, R.C.; Pereira, C.; Zaghdoudi, K.; Ferreira, I.C.F.R.; Barros, L.; Barreira, J.C.M. Aesculus hippocastanum L.: A Simple Ornamental Plant or a Source of Compelling Molecules for Industry? Separations 2023, 10, 160. [Google Scholar] [CrossRef]
- Savarino, P.; Colson, E.; André, J.; Gerbaux, P. Horse Chestnut Saponins–Escins, Isoescins, Transescins, and Desacylescins. Molecules 2023, 28, 2087. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.A.; Alhamd, O.; Iszkuło, G.; Dering, M.; Mukassabi, T.A. Biological Flora of the British Isles: Aesculus hippocastanum. J. Ecol. 2019, 107, 992–1030. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Biernat, A. The Content of Phenolic Compounds in Leaf Tissues of Aesculus glabra and Aesculus parviflora Walt. Molecules 2015, 20, 2176–2189. [Google Scholar] [CrossRef]
- Kücükkurt, I.; Inceb, S.; Keles, H.; Akkold, E.; Avcıa, G.; Yesilada, E.; Bacakf, E. Beneficial effects of Aesculus hippocastanum L. seed extract on the body’s own antioxidant defense system on subacute administration. J. Ethnopharmacol. 2010, 129, 18–22. [Google Scholar] [CrossRef]
- Matysik, G.; Glowniak, K.; Soczewiński, E.; Garbacka, M. Chromatography of Esculin from Stems and Bark of Aesculus hippocastanum L. for Consecutive Vegetative Periods. Chromatographia 1994, 38, 766–770. [Google Scholar] [CrossRef]
- Gülz, P.-G.; Müller, E.; Herrmann, T. Chemical Composition and Surface Structures of Epicuticular Leaf Waxes from Castanea sativa and Aesculus hippocastanum. Z. Naturforsch. 1992, 47c, 661–666. [Google Scholar] [CrossRef]
- Pinon, J.; Frey, P.; Husson, C. Wettability of poplar leaves influences dew formation and infection by Melampsora laricipopulina. Plant Dis. 2006, 90, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Papierowska, E.; Szporak-Wasilewska, S.; Szewińska, J.; Szatyłowicz, J.; Debaene, G.; Utratna, M. Contact angle measurements and water drop behavior on leaf surface for several deciduous shrub and tree species from a temperate zone. Trees 2018, 32, 1253–1266. [Google Scholar] [CrossRef]
- Sucharzewska, E.; Kulesza, K.; Ejdys, E.; Dynowska, M.; Kubiak, D.; Biedunkeiwicz, A. Erysiphe flexuosa (fungi, Erysiphales)—Life strategies and threats to chestnut trees including Cameraria ohridella (lepidoptera, Gracillariidae) pest in the urban environment. Pol. J. Nat. Sci. 2018, 33, 233–246. [Google Scholar]
- Irzykowska, L.; Werner, M.; Bocianowski, J.; Karolewski, Z.; Frużyńska-Jóźwiak, D. Genetic variation of horse chestnut and red horse chestnut and trees susceptibility to Erysiphe flexuosa and Cameraria ohridella. Biologia 2013, 68, 851–860. [Google Scholar] [CrossRef]
- Stankeviciene, A.; Snieskiene, V.; Lugauskas, A. Erysiphe flexuosa—The new pathogen of Aesculus hippocastanum in Lithuania. Phytopathologia 2010, 56, 67–71. [Google Scholar]
- Weryszko-Chmielewska, E.; Haratym, W. Leaf micromorphology of Aesculus hippocastanum L. and damage caused by leaf-mining larvae of Cameraria ohridella Deschka & Dimić. Acta Agrobot. 2012, 65, 25–34. [Google Scholar]
- Weryszko-Chmielewska, E.; Haratym, W. Changes in leaf tissues of common horse chestnut (Aesculus hippocastanum L.) colonised by the horse-chestnut leaf miner (Cameraria ochridella Deschka&Dimić). Acta Agrobot. 2011, 64, 11–22. [Google Scholar]
- Werner, M.; Irzykowska, L.; Karolewski, Z. The occurrence and harmfulness of Erysiphe flexuosa and Cameraria ohridella on aesculus spp.1. Phytopathologia 2012, 65, 5–11. [Google Scholar]
- Mieslerová, B.; Sedlářová, M.; Michutová, M.; Petřeková, V.; Cook, R.; Lebeda, A. Powdery Mildews on Trees and Shrubs in Botanical Gardens, Parks and Urban Green Areas in the Czech Republic. Forests 2020, 11, 967. [Google Scholar] [CrossRef]
- Konarska, A.; Grochowska, M.; Haratym, W.; Tietze, M.; Weryszko-Chmielewska, E.; Lechowski, L. Changes in Aesculus hippocastanum leaves during development of Cameraria ohridella. Urban For. Urban Green. 2020, 56, 126793. [Google Scholar] [CrossRef]
- Żaak, M.; Migula, P.; Stygar, D.; Doleżych, B.; Michalczyk, K. Within and between seasonal changes of detoxifying capabilities of Cameraria ohridella (Lepidoptera: Gracillariidae) larvae. C. R. Biol. 2012, 335, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Pocock, M.J.O.; Evans, D.M. The Success of the Horse-Chestnut Leaf-Miner, Cameraria ohridella, in the UK Revealed with Hypothesis-Led Citizen Science. PLoS ONE 2014, 9, e86226. [Google Scholar] [CrossRef] [PubMed]
- Jansone, D.; Matisons, R.; Jansons, A.; Jaunslaviete, I. Meteorological conditions have a complex effect on the tree-ring width of horse chestnut Aesculus hippocastanum in a forest plantation in Latvia. Dendrochronologia 2023, 77, 126031. [Google Scholar] [CrossRef]
- Dzięgielewska, M.; Adamska, I.; Mikiciuk, M.; Nowak, G.; Ptak, P. Effects of biotic and abiotic factors on the health of horse chestnut trees in an urban area of north-western Poland. Ecol. Quest. 2017, 27, 25–38. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Materska, M.; Pabich, M.; Sachadyn-Król, M.; Konarska, A.; Weryszko-Chmielewska, E.; Chilczuk, B.; Staszowska-Karkut, M.; Jackowska, I.; Dmitruk, M. The Secondary Metabolites Profile in Horse Chestnut Leaves Infested with Horse-Chestnut Leaf Miner. Molecules 2022, 27, 5471. [Google Scholar] [CrossRef]
- Bernards, M.; Båstrup-Spohr, L. Phenylpropanoid metabolism induced by wounding and insect herbivory. In Induced Resistance to Herbivory; Schaller, A., Ed.; Springer: New York, NY, USA, 2008; pp. 189–211. [Google Scholar]
- Paterska, M.; Bandurska, H.; Wysłouch, J.; Molińska-Glura, M.; Moliński, K. Chemical composition of horse-chestnut (Aesculus) leaves and their susceptibility to chestnut leaf miner Cameraria ohridella Deschka and Dimic. Acta Physiol. Plant. 2017, 39, 105. [Google Scholar] [CrossRef]
- Owczarek, A.; Kołodziejczyk-Czepas, J.; Marczuk, P.; Siwek, J.; Wąsowicz, K.; Olszewska, M.A. Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro. Pharmaceuticals 2021, 14, 1301. [Google Scholar] [CrossRef]
- Dias, M.I.; Albiston, C.; Añibarro-Ortega, M.; Ferreira, I.C.F.R.; Pinela, J.; Barros, L. Sonoextraction of phenolic compounds and saponins from Aesculus hippocastanum seed kernels: Modeling and optimization. Ind. Crops Prod. 2022, 185, 115142. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Głowacka, B.; Lipiński, S.; Tarwacki, G. Possibilities of protection of horse chestnut Aesculus hippocastanum L. against horse chestnut leaf-miner Cameraria ohridella Deschka et Dimic. For. Res. Pap. 2009, 70, 317–328. [Google Scholar] [CrossRef]
- Pavan, F.; Barro, P.; Bernardinelli, I.; Gambon, N.; Zandigiacomo, P. Cultural control of Cameraria ohridella on horsechestnut in urban areas by removing fallen leaves in autumn. Arboric. Urban For. 2003, 29, 253–258. [Google Scholar] [CrossRef]
- Menkis, A.; Povilaitienė, A.; Marčiulynas, A.; Lynikienė, J.; Gedminas, A.; Marčiulynienė, D. Occurrence of common phyllosphere fungi of horse-chestnut (Aesculus hippocastanum) is unrelated to degree of damage by leafminer (Cameraria ohridella). Scand. J. For. Res. 2019, 34, 26–32. [Google Scholar] [CrossRef]
- Kimic, K.; Mirzwa-Mróz, E.; Szyndel, M.S. Diagnosis and recommendations for management of trees and shrubs in green squares in Warsaw based on research on fungal diseases. Trees 2023, 37, 161–175. [Google Scholar] [CrossRef]
- Rocznik Meteorologiczny 2019. Instytut Meteorologii i Gospodarki Wodnej, Państwowy Instytut Badawczy. Available online: https://danepubliczne.imgw.pl/data/dane_pomiarowo_obserwacyjne/Roczniki/Rocznik%20meteorologiczny/Rocznik%20Meteorologiczny%202019.pdf (accessed on 15 May 2023).
- Hawrylak-Nowak, B.; Dresler, S.; Stasińska-Jakubas, M.; Wójciak, M.; Sowa, I.; Matraszek-Gawron, R. NaCl-Induced Elicitation Alters Physiology and Increases Accumulation of Phenolic Compounds in Melissa officinalis L. Int. J. Mol. Sci. 2021, 22, 6844. [Google Scholar] [CrossRef]
- Dresler, S.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Bujak, T.; Skic, K.; Feldo, M.; Hanaka, A.; Wójciak, M.; Sowa, I.; et al. A design-of-experiment approach for obtaining Symphytum officinale L. extracts for cosmetic purposes. Ind. Crops Prod. 2023, 199, 116768. [Google Scholar] [CrossRef]
- Hanaka, A.; Nowak, A.; Ozimek, E.; Dresler, S.; Plak, A.; Sujak, A.; Reszczyńska, E.; Strzemski, M. Effect of copper stress on Phaseolus coccineus in the presence of exogenous methyl jasmonate and/or Serratia plymuthica from the Spitsbergen soil. J. Hazard. Mater. 2022, 436, 129232. [Google Scholar] [CrossRef]


| Groups of Metabolites | Examples of Compounds |
|---|---|
| Fatty acids | Lauric, myristic, palmitic, stearic, arachic, and oleic acids |
| Organic acids | Oxalic, quinic, malic, citric, ascorbic, and shikimic acids |
| Carotenoids | Aesculaxanthin, lutein, and citraurin |
| Polyprenols | Undecaprenol, tridecaprenol, dodecaprenol, and castoprenol |
| Triterpene glycosides or saponins | Aescigenin, hippocaesculin and barringtogenol, the mix collectively called aesculin, aescin or escin |
| Coumarin derivatives | Esculetin; coumarin glucosides: esculin and fraxin |
| Flavonoids | Glycosides of quercetin, leucocyanidin, procyanidin, and kaempferol |
| Infection | C. ohridella | E. flexuosa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | 1–3 | 4 | 5 | 6 | 7 | 8 | 1–3 | 4 | 5 | 6 | 7 | 8 | |
| Individuals | |||||||||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| A | 0 | 1 | 1 | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| B | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 1 | 2 | 3 | 3 | 3 | |
| C | 0 | 1 | 1 | 1 | 1 | 3 | 0 | 1 | 1 | 3 | 3 | 3 | |
| D | 0 | 1 | 1 | 2 | 2 | 3 | 0 | 0 | 1 | 2 | 2 | 3 | |
| E | 0 | 1 | 1 | 2 | 3 | 3 | 0 | 1 | 2 | 2 | 3 | 3 | |
| F | 0 | 1 | 1 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Peak No | Rt (min) | Observed Ion Mass [M-H]-/(Fragments) | Δ ppm | Formula | Identified Metabolite |
|---|---|---|---|---|---|
| 1 | 3.01 | 353.08798 (191,179) | 0.49 | C16H18O9 | Neochlorogenic acid * |
| 2 | 9.25 | 289.07189 | 0.44 | C15H14O6 | (−)-Epicatechin * |
| 3 | 14.01 | 863.18319 (289) | 0.35 | C45H36O18 | Procyanidin trimer A-type |
| 4 | 20.71 | 1153.26211 (289) | 0.16 | C60H50O24 | Procyanidin tetramer A-type |
| 5 | 32.84 | 433.07788 (300) | 0.56 | C20H18O11 | Quercetin-3-O-arabinoside |
| 6 | 33.70 | 447.09387 (300) | 1.31 | C21H20O11 | Quercetin-3-O-rhamnoside |
| 7 | 36.77 | 417.08293 (285) | 0.50 | C20H18O10 | Kaempferol-3-O-arabinoside |
| 8 | 37.92 | 431.09871 (285) | 0.79 | C21H20O10 | Kaempferol-3-O-rhamnoside |
| Collection Period | Time Span between Collection Periods [Days] | Date of Collection of Non-Symptomatic Leaves in Mircze | Date of Collection of Symptomatic Leaves in Lublin |
|---|---|---|---|
| 1 | - | 01.05 | 29.04 |
| 2 | 10 | 11.05 | 09.05 |
| 3 | 10 | 21.05 | 19.05 |
| 4 | 10 | 31.05 | 29.05 |
| 5 | 10 | 10.06 | 08.06 |
| 6 | 10 | 20.06 | 18.06 |
| 7 | 25 | 15.07 | 13.07 |
| 8 | 25 | 09.08 | 07.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanaka, A.; Dresler, S.; Mułenko, W.; Wójciak, M.; Sowa, I.; Sawic, M.; Stanisławek, K.; Strzemski, M. Phenolic-Based Discrimination between Non-Symptomatic and Symptomatic Leaves of Aesculus hippocastanum Infested by Cameraria ohridella and Erysiphe flexuosa. Int. J. Mol. Sci. 2023, 24, 14071. https://doi.org/10.3390/ijms241814071
Hanaka A, Dresler S, Mułenko W, Wójciak M, Sowa I, Sawic M, Stanisławek K, Strzemski M. Phenolic-Based Discrimination between Non-Symptomatic and Symptomatic Leaves of Aesculus hippocastanum Infested by Cameraria ohridella and Erysiphe flexuosa. International Journal of Molecular Sciences. 2023; 24(18):14071. https://doi.org/10.3390/ijms241814071
Chicago/Turabian StyleHanaka, Agnieszka, Sławomir Dresler, Wiesław Mułenko, Magdalena Wójciak, Ireneusz Sowa, Magdalena Sawic, Katarzyna Stanisławek, and Maciej Strzemski. 2023. "Phenolic-Based Discrimination between Non-Symptomatic and Symptomatic Leaves of Aesculus hippocastanum Infested by Cameraria ohridella and Erysiphe flexuosa" International Journal of Molecular Sciences 24, no. 18: 14071. https://doi.org/10.3390/ijms241814071
APA StyleHanaka, A., Dresler, S., Mułenko, W., Wójciak, M., Sowa, I., Sawic, M., Stanisławek, K., & Strzemski, M. (2023). Phenolic-Based Discrimination between Non-Symptomatic and Symptomatic Leaves of Aesculus hippocastanum Infested by Cameraria ohridella and Erysiphe flexuosa. International Journal of Molecular Sciences, 24(18), 14071. https://doi.org/10.3390/ijms241814071









