Chitosan Particles Complexed with CA5-HIF-1α Plasmids Increase Angiogenesis and Improve Wound Healing
Abstract
:1. Introduction
2. Results
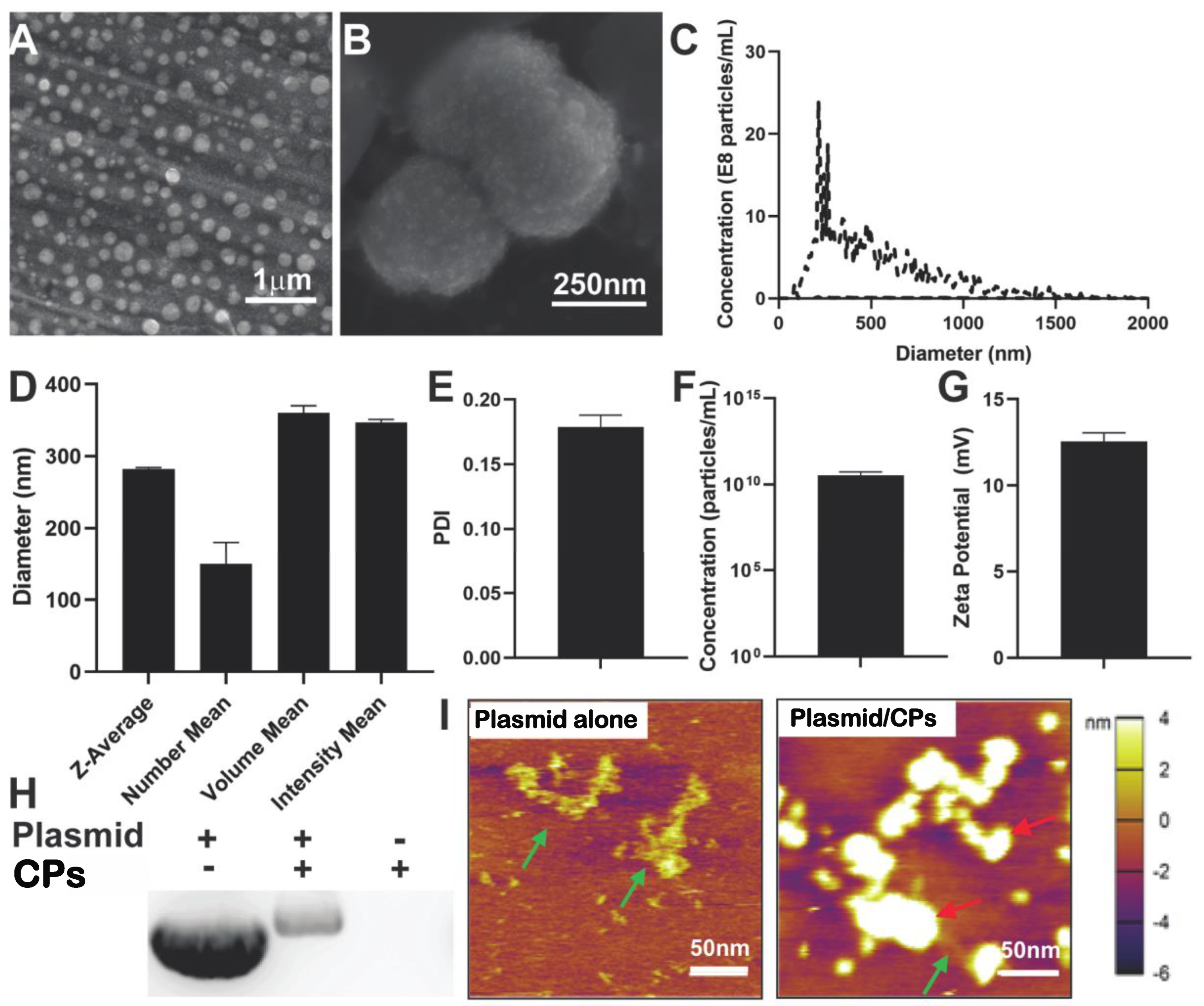
2.1. Characterization of Chitosan Particle/HIF-1α-CA5 Plasmid Complexes
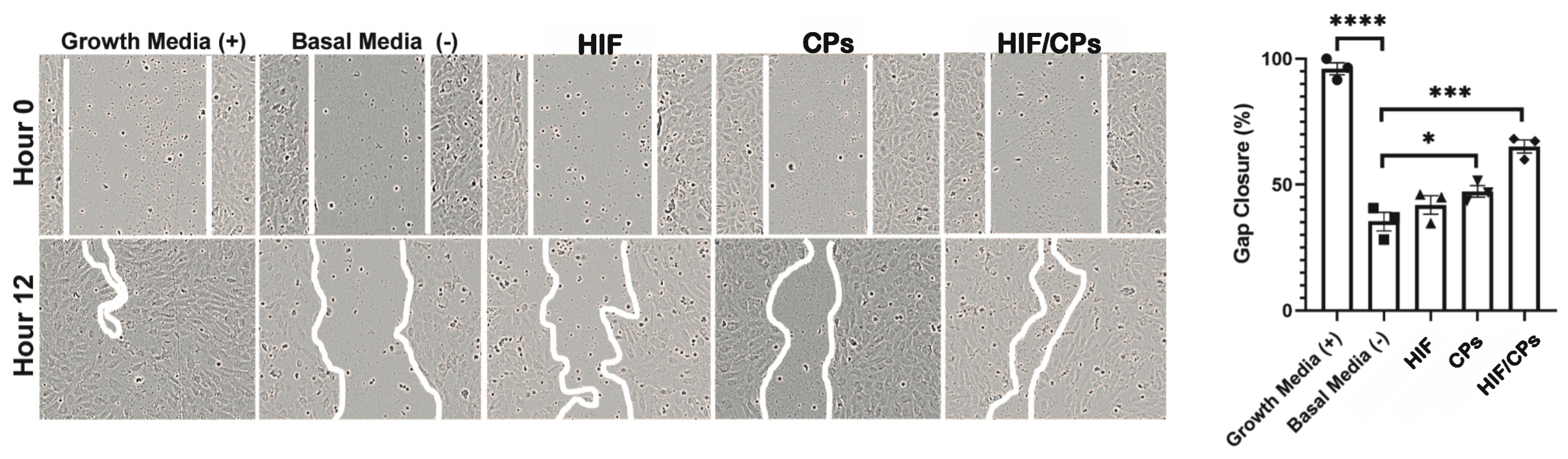
2.2. In Vitro Assessment of HIF/CPs Angiogenic Activity
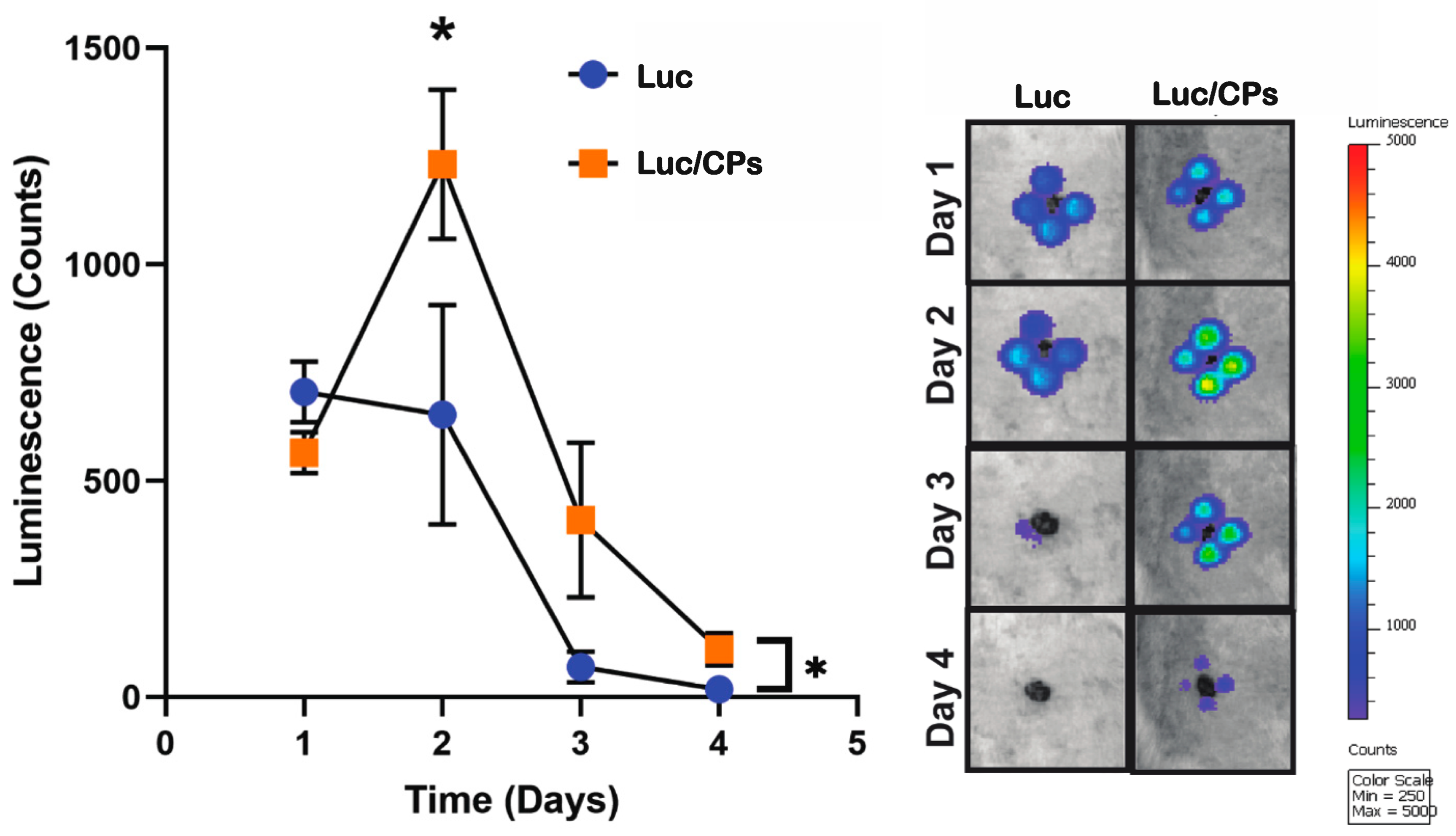
2.3. Verification of In Vivo Transfection Using Luciferase Plasmid/NP Complexes
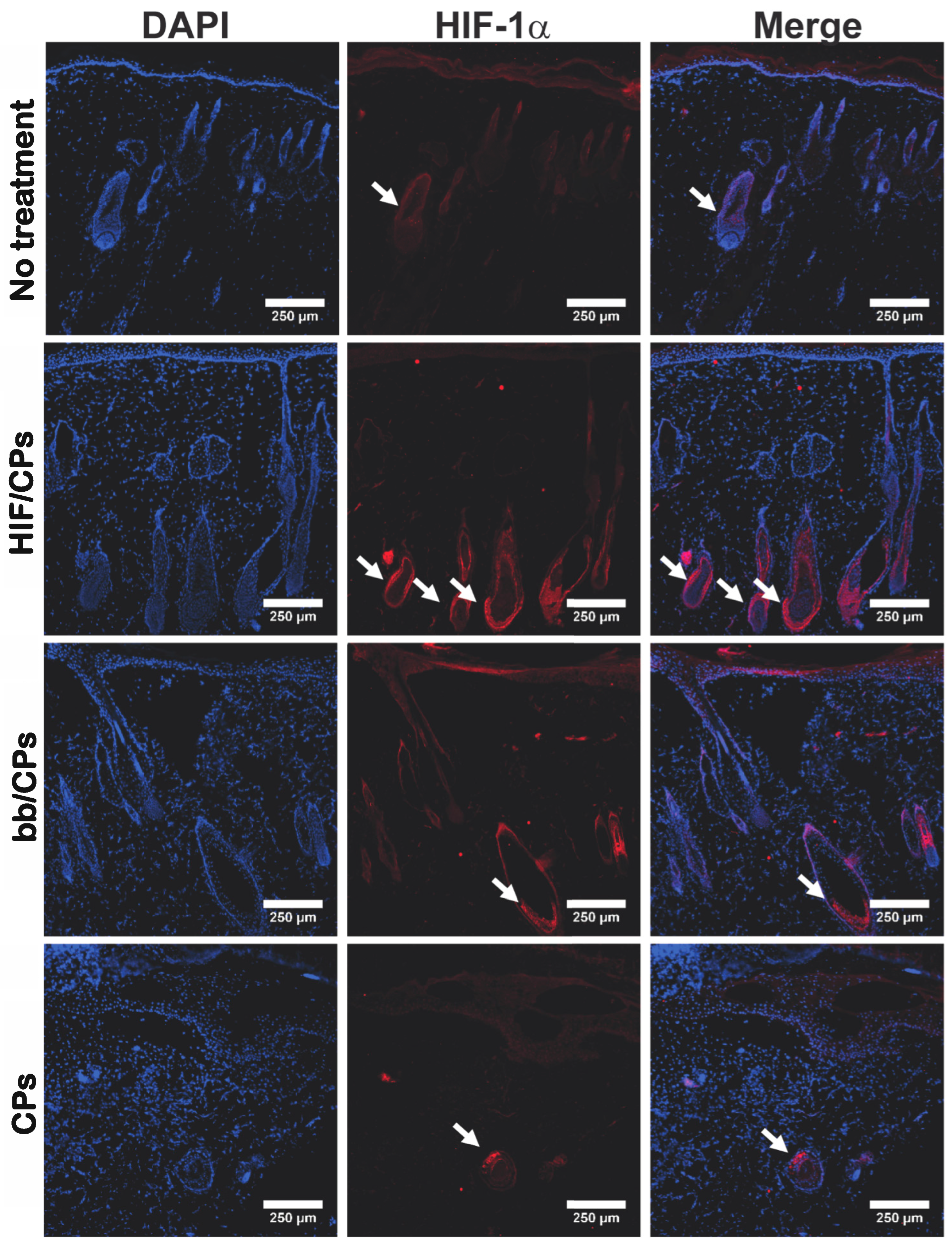
2.4. In Vivo Tissue and Molecular Analysis of HIF/CPs
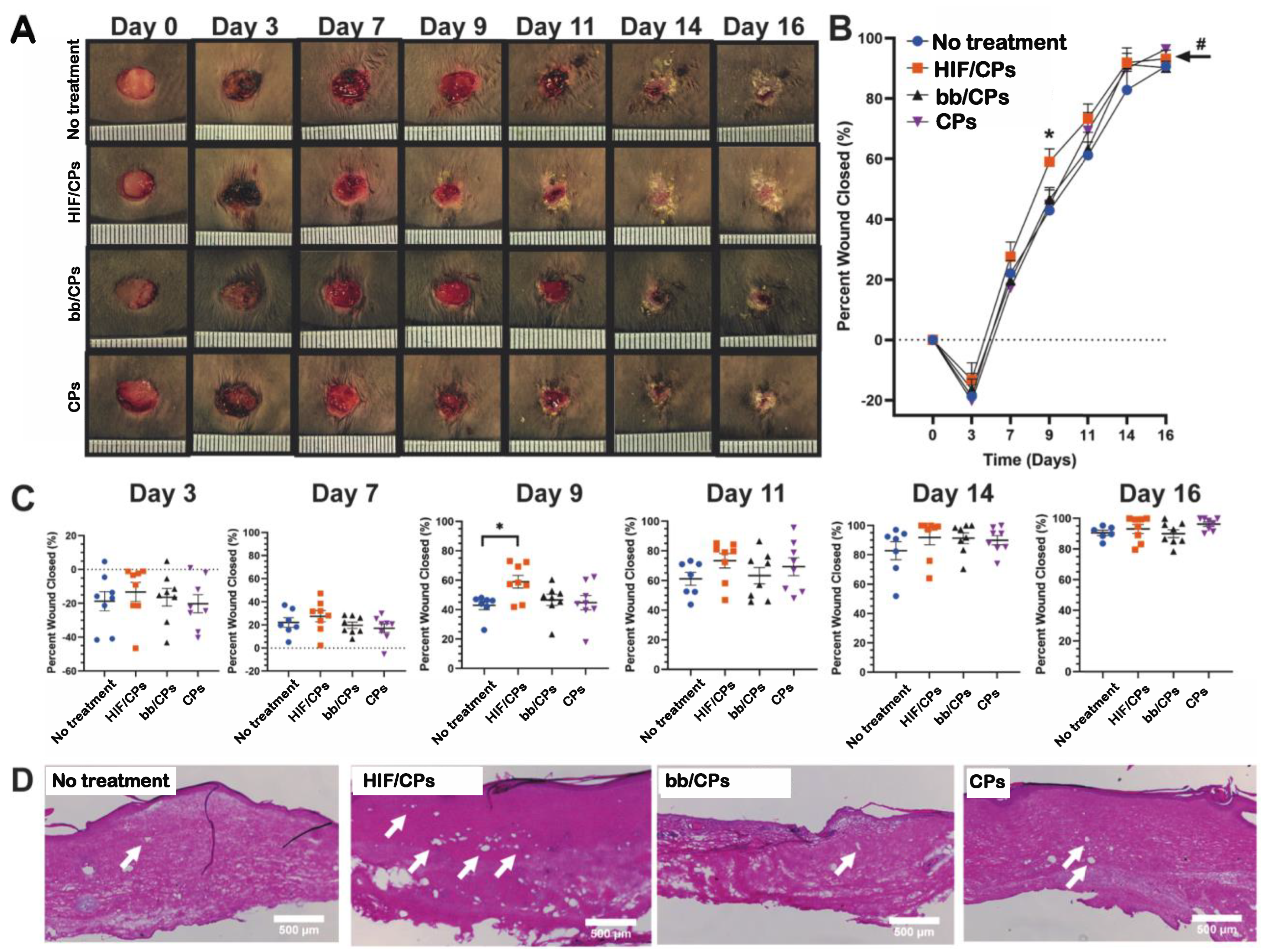
2.5. In Vivo Excisional db/db Wound-Healing Model and Histological Analysis for Angiogenesis
3. Discussion
4. Materials and Methods
4.1. Plasmid Preparation
4.2. Preparation and Characterization of Chitosan Particle/CA5-HIF-1α Plasmid Complexes
4.3. In Vitro Assessment of Angiogenic Activity
4.4. In Vivo Luciferase Reporter Assay Using Chitosan Particles in Sprague Dawley Rats
4.5. In Vivo Molecular Study of HIF/CPs in Sprague Dawley Rats
4.6. In Vivo Functional Study of HIF/CPs in db/db Mice
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.A.; Falanga, V.; Fife, C.E.; Gardner, S.E.; et al. Chronic Wound Repair and Healing in Older Adults: Current Status and Future Research. J. Am. Geriatr. Soc. 2015, 63, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- ADA. Statistics About Diabetes. Available online: https://www.diabetes.org/resources/statistics/statistics-about-diabetes (accessed on 24 November 2020).
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.L.; Borman, H.; Bahar, T.; Ertaş, N.M.; Haberal, M. An In Vivo Comparison of Commonly Used Topical Antimicrobials on Skin Graft Healing After Full-Thickness Burn Injury. J. Burn. Care Res. 2015, 36, e47–e54. [Google Scholar] [CrossRef] [PubMed]
- Senet, P. Bécaplermine gel (Regranex® gel). Ann. Dermatol. Vénéréol. 2004, 131, 351–358. [Google Scholar] [CrossRef]
- Cabral, J.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Extracellular vesicles as modulators of wound healing. Adv. Drug Deliv. Rev. 2018, 129, 394–406. [Google Scholar] [CrossRef]
- Gibbons, G.W.; Karon, M.; Hesp, Z.C.; Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Uveges, T.E.; Jacobstein, D.A.; Danilkovitch, A.; Lei, J.; et al. Grafix®, a Cryopreserved Placental Membrane, for the Treatment of Chronic/Stalled Wounds. Adv. Wound Care 2015, 4, 534–544. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Fahs, F.; Bi, X.-L.; Yu, F.-S.; Zhou, L.; Mi, Q.-S. Small RNAs Play Big Roles: MicroRNAs in Diabetic Wound Healing. Curr. Mol. Med. 2016, 16, 545–552. [Google Scholar] [CrossRef]
- Davis, F.M.; Schaller, M.A.; Dendekker, A.; Joshi, A.D.; Kimball, A.S.; Evanoff, H.; Wilke, C.; Obi, A.T.; Melvin, W.J.; Cavassani, K.; et al. Sepsis Induces Prolonged Epigenetic Modifications in Bone Marrow and Peripheral Macrophages Impairing Inflammation and Wound Healing. Arter. Thromb. Vasc. Biol. 2019, 39, 2353–2366. [Google Scholar] [CrossRef]
- Theocharidis, G.; Baltzis, D.; Roustit, M.; Tellechea, A.; Dangwal, S.; Khetani, R.S.; Shu, B.; Zhao, W.; Fu, J.; Bhasin, S.; et al. Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes 2020, 69, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, A.P.; Stone, R.C.; Brooks, S.R.; Pastar, I.; Jozic, I.; Hasneen, K.; O’Neill, K.; Mehdizadeh, S.; Head, C.R.; Strbo, N.; et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat. Commun. 2020, 11, 4678. [Google Scholar] [CrossRef] [PubMed]
- Gene Expression Profiles in Healing and Non-Healing Wounds. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01101854?term=gene+expression+profiles+in+healing+and+non+healing+wounds (accessed on 24 November 2020).
- Shi, Q.; Luo, X.; Huang, Z.; Midgley, A.C.; Wang, B.; Liu, R.; Zhi, D.; Wei, T.; Zhou, X.; Qiao, M.; et al. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater. 2019, 86, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Bonham, C.A.; Rodrigues, M.; Galvez, M.; Trotsyuk, A.; Stern-Buchbinder, Z.; Inayathullah, M.; Rajadas, J.; Gurtner, G.C. Deferoxamine can prevent pressure ulcers and accelerate healing in aged mice. Wound Repair Regen. 2018, 26, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lv, R.; Yang, X.; Cheng, S.; Xu, J.; Ma, T. Hypoxia enhances the protective effects of placenta-derived mesenchymal stem cells against scar formation through hypoxia-inducible factor-1α. Biotechnol. Lett. 2016, 38, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Sunkari, V.G.; Lind, F.; Botusan, I.R.; Kashif, A.; Liu, Z.-J.; Ylä-Herttuala, S.; Brismar, K.; Velazquez, O.; Catrina, S.-B. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015, 23, 98–103. [Google Scholar] [CrossRef]
- Watanabe, Y.; Murdoch, C.E.; Sano, S.; Ido, Y.; Bachschmid, M.M.; Cohen, R.A.; Matsui, R. Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1α and improve limb revascularization. Proc. Natl. Acad. Sci. USA 2016, 113, 6011–6016. [Google Scholar] [CrossRef]
- Costa, M.; Cerqueira, M.T.; Santos, T.C.; Sampaio-Marques, B.; Ludovico, P.; Marques, A.P.; Pirraco, R.P.; Reis, R.L. Cell sheet engineering using the stromal vascular fraction of adipose tissue as a vascularization strategy. Acta Biomater. 2017, 55, 131–143. [Google Scholar] [CrossRef]
- Zhang, X.; Sarkar, K.; Rey, S.; Sebastian, R.; Andrikopoulou, E.; Marti, G.P.; Fox-Talbot, K.; Semenza, G.L.; Harmon, J.W. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. J. Mol. Med. 2011, 89, 985–995. [Google Scholar] [CrossRef]
- Liu, L.; Marti, G.P.; Wei, X.; Zhang, X.; Zhang, H.; Liu, Y.V.; Nastai, M.; Semenza, G.L.; Harmon, J.W. Age-dependent impairment of HIF-1α expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J. Cell. Physiol. 2008, 217, 319–327. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Wei, X.; Tan, Y.S.; Tong, L.; Chang, B.R.; Ghanamah, M.S.; Reinblatt, M.; Marti, G.P.; Harmon, J.W.; et al. Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1α heterozygous-null mice after burn wounding. Wound Repair Regen. 2010, 18, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Semenza, G.L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 2010, 86, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Shoureshi, P.; Lay, F.; Sebastian, R.; Habibabady, Z.A.; Born, L.J.; Marti, G.P.; Meltzer, S.J.; Abraham, J.M.; Harmon, J.W. Preconditioning of surgical pedicle flaps with DNA plasmid expressing hypoxia-inducible factor-1α (HIF-1α) promotes tissue viability. Gene Ther. 2020, 28, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Synthesis of intracellular reduction-sensitive amphiphilic polyethyleneimine and poly(ε-caprolactone) graft copolymer for on-demand release of doxorubicin and p53 plasmid DNA. Acta Biomater. 2016, 39, 79–93. [Google Scholar] [CrossRef]
- Lackington, W.A.; Raftery, R.M.; O’Brien, F.J. In vitro efficacy of a gene-activated nerve guidance conduit incorporating non-viral PEI-pDNA nanoparticles carrying genes encoding for NGF, GDNF and c-Jun. Acta Biomater. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, Y.; Hu, Q.; Guo, Z.; Liu, T.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. Injectable supramolecular hydrogel formed from α-cyclodextrin and PEGylated arginine-functionalized poly(l-lysine) dendron for sustained MMP-9 shRNA plasmid delivery. Acta Biomater. 2017, 49, 456–471. [Google Scholar] [CrossRef]
- Lin, W.; Hanson, S.; Han, W.; Zhang, X.; Yao, N.; Li, H.; Zhang, L.; Wang, C. Well-defined star polymers for co-delivery of plasmid DNA and imiquimod to dendritic cells. Acta Biomater. 2017, 48, 378–389. [Google Scholar] [CrossRef]
- Cutlar, L.; Zhou, D.; Gao, Y.; Zhao, T.; Greiser, U.; Wang, W.; Wang, W. Highly Branched Poly(β-Amino Esters): Synthesis and Application in Gene Delivery. Biomacromolecules 2015, 16, 2609–2617. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, D.; Yang, J.; Zhou, H.; Chen, J.; Guo, T. Bioreducible Zinc(II)-Coordinative Polyethylenimine with Low Molecular Weight for Robust Gene Delivery of Primary and Stem Cells. J. Am. Chem. Soc. 2017, 139, 5102–5109. [Google Scholar] [CrossRef]
- Goldshtein, M.; Shamir, S.; Vinogradov, E.; Monsonego, A.; Cohen, S. Co-assembled Ca2+ Alginate-Sulfate Nanoparticles for Intracellular Plasmid DNA Delivery. Mol. Ther.-Nucleic Acids 2019, 16, 378–390. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Dhaliwal, H.K.; Aljaeid, B.M.; Alhakamy, N.A.; Banjar, Z.M.; Amiji, M.M. Optimization of the Conditions for Plasmid DNA Delivery and Transfection with Self-Assembled Hyaluronic Acid-Based Nanoparticles. Mol. Pharm. 2018, 16, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Gwak, S.-J.; Koo, H.; Yun, Y.; Yhee, J.Y.; Lee, H.Y.; Yoon, D.H.; Kim, K.; Ha, Y. Multifunctional nanoparticles for gene delivery and spinal cord injury. J. Biomed. Mater. Res. Part A 2015, 103, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Q.; Wu, W.; Song, H.-Q.; Ren, Y.; Yang, M.; Li, J.; Xu, F.-J. Well-defined reducible cationic nanogels based on functionalized low-molecular-weight PGMA for effective pDNA and siRNA delivery. Acta Biomater. 2016, 41, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.; Varela-Moreira, A.; Leiro, V.; Lopes, C.D.; Moreno, P.M.; Gomez-Lazaro, M.; Pêgo, A.P. A high-throughput bioimaging study to assess the impact of chitosan-based nanoparticle degradation on DNA delivery performance. Acta Biomater. 2016, 46, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Alsarra, I.A. Chitosan topical gel formulation in the management of burn wounds. Int. J. Biol. Macromol. 2009, 45, 16–21. [Google Scholar] [CrossRef]
- Lord, M.S.; Ellis, A.L.; Farrugia, B.L.; Whitelock, J.M.; Grenett, H.; Li, C.; O’Grady, R.L.; DeCarlo, A.A. Perlecan and vascular endothelial growth factor-encoding DNA-loaded chitosan scaffolds promote angiogenesis and wound healing. J. Control. Release 2017, 250, 48–61. [Google Scholar] [CrossRef]
- Katas, H.; Wen, C.Y.; Siddique, M.I.; Hussain, Z.; Fadhil, F.H.M.; E Pullan, J.; Pullan, A.T.; Taylor, V.B.; Brooks, B.D.; Ewert, D.; et al. Thermoresponsive curcumin/DsiRNA nanoparticle gels for the treatment of diabetic wounds: Synthesis and drug release. Ther. Deliv. 2017, 8, 137–150. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Pestov, A.; Nazirov, A.; Modin, E.; Mironenko, A.; Bratskaya, S. Mechanism of Au(III) reduction by chitosan: Comprehensive study with 13C and 1H NMR analysis of chitosan degradation products. Carbohydr. Polym. 2015, 117, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Synthesis and Evaluation of Boronated Chitosan as a Mucoadhesive Polymer for Intravesical Drug Delivery. J. Pharm. Sci. 2019, 108, 3046–3053. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ge, Y.; Xu, Z.; Aa, N.; Gu, X.; Meng, H.; Lin, Z.; Zhu, D.; Shi, J.; Zhuang, R.; et al. Hypoxia-Inducible Factor 1 alpha (HIF-1α)/Vascular Endothelial Growth Factor (VEGF) Pathway Participates in Angiogenesis of Myocardial Infarction in Muscone-Treated Mice: Preliminary Study. Experiment 2018, 24, 8870–8877. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, J. Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymer and Their Nanomicelles for Nonviral Gene Delivery Applications. Bioconjugate Chem. 2017, 28, 2772–2783. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Correa, V.L.R.; da Silva, F.K.L.; Casas, A.A.; Chagas, A.d.L.d.; de Oliveira, L.P.; Miguel, M.P.; Diniz, D.G.A.; Amaral, A.C.; de Menezes, L.B. Wound healing treatment using insulin within polymeric nanoparticles in the diabetes animal model. Eur. J. Pharm. Sci. 2020, 150, 105330. [Google Scholar] [CrossRef]
- Piran, M.; Vakilian, S.; Piran, M.; Mohammadi-Sangcheshmeh, A.; Hosseinzadeh, S.; Ardeshirylajimi, A. In vitro fibroblast migration by sustained release of PDGF-BB loaded in chitosan nanoparticles incorporated in electrospun nanofibers for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 511–520. [Google Scholar] [CrossRef]
- Correa, V.L.R.; Martins, J.A.; de Souza, T.R.; Rincon, G.d.C.N.; Miguel, M.P.; de Menezes, L.B.; Amaral, A.C. Melatonin loaded lecithin-chitosan nanoparticles improved the wound healing in diabetic rats. Int. J. Biol. Macromol. 2020, 162, 1465–1475. [Google Scholar] [CrossRef]
- Li, F.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-loaded chitosan nanoparticles promote diabetic wound healing via attenuating inflammation in a diabetic rat model. J. Biomater. Appl. 2019, 34, 476–486. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Y.; Yang, G. The Modulation of Chitosan-DNA Interaction by Concentration and pH in Solution. Polymers 2019, 11, 646. [Google Scholar] [CrossRef]
- Kedjarune-Leggat, U.; Supaprutsakul, C.; Chotigeat, W. Ultrasound Treatment Increases Transfection Efficiency of Low Molecular Weight Chitosan in Fibroblasts but Not in KB Cells. PLoS ONE 2014, 9, e92076. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair Follicle Dermal Stem Cells Regenerate the Dermal Sheath, Repopulate the Dermal Papilla, and Modulate Hair Type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Agabalyan, N.A.; Rosin, N.L.; Rahmani, W.; Biernaskie, J. Hair follicle dermal stem cells and skin-derived precursor cells: Exciting tools for endogenous and exogenous therapies. Exp. Dermatol. 2017, 26, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.B.; Luthers, C.R.; Lerman, M.J.; Fisher, J.P.; Jay, S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2018, 95, 236–244. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Born, L.J.; Bengali, S.; Hsu, A.T.W.; Abadchi, S.N.; Chang, K.-H.; Lay, F.; Matsangos, A.; Johnson, C.; Jay, S.M.; Harmon, J.W. Chitosan Particles Complexed with CA5-HIF-1α Plasmids Increase Angiogenesis and Improve Wound Healing. Int. J. Mol. Sci. 2023, 24, 14095. https://doi.org/10.3390/ijms241814095
Born LJ, Bengali S, Hsu ATW, Abadchi SN, Chang K-H, Lay F, Matsangos A, Johnson C, Jay SM, Harmon JW. Chitosan Particles Complexed with CA5-HIF-1α Plasmids Increase Angiogenesis and Improve Wound Healing. International Journal of Molecular Sciences. 2023; 24(18):14095. https://doi.org/10.3390/ijms241814095
Chicago/Turabian StyleBorn, Louis J., Sameer Bengali, Angela Ting Wei Hsu, Sanaz Nourmohammadi Abadchi, Kai-Hua Chang, Frank Lay, Aerielle Matsangos, Christopher Johnson, Steven M. Jay, and John W. Harmon. 2023. "Chitosan Particles Complexed with CA5-HIF-1α Plasmids Increase Angiogenesis and Improve Wound Healing" International Journal of Molecular Sciences 24, no. 18: 14095. https://doi.org/10.3390/ijms241814095
APA StyleBorn, L. J., Bengali, S., Hsu, A. T. W., Abadchi, S. N., Chang, K.-H., Lay, F., Matsangos, A., Johnson, C., Jay, S. M., & Harmon, J. W. (2023). Chitosan Particles Complexed with CA5-HIF-1α Plasmids Increase Angiogenesis and Improve Wound Healing. International Journal of Molecular Sciences, 24(18), 14095. https://doi.org/10.3390/ijms241814095




