The Porous Structure of Peripheral Nerve Guidance Conduits: Features, Fabrication, and Implications for Peripheral Nerve Regeneration
Abstract
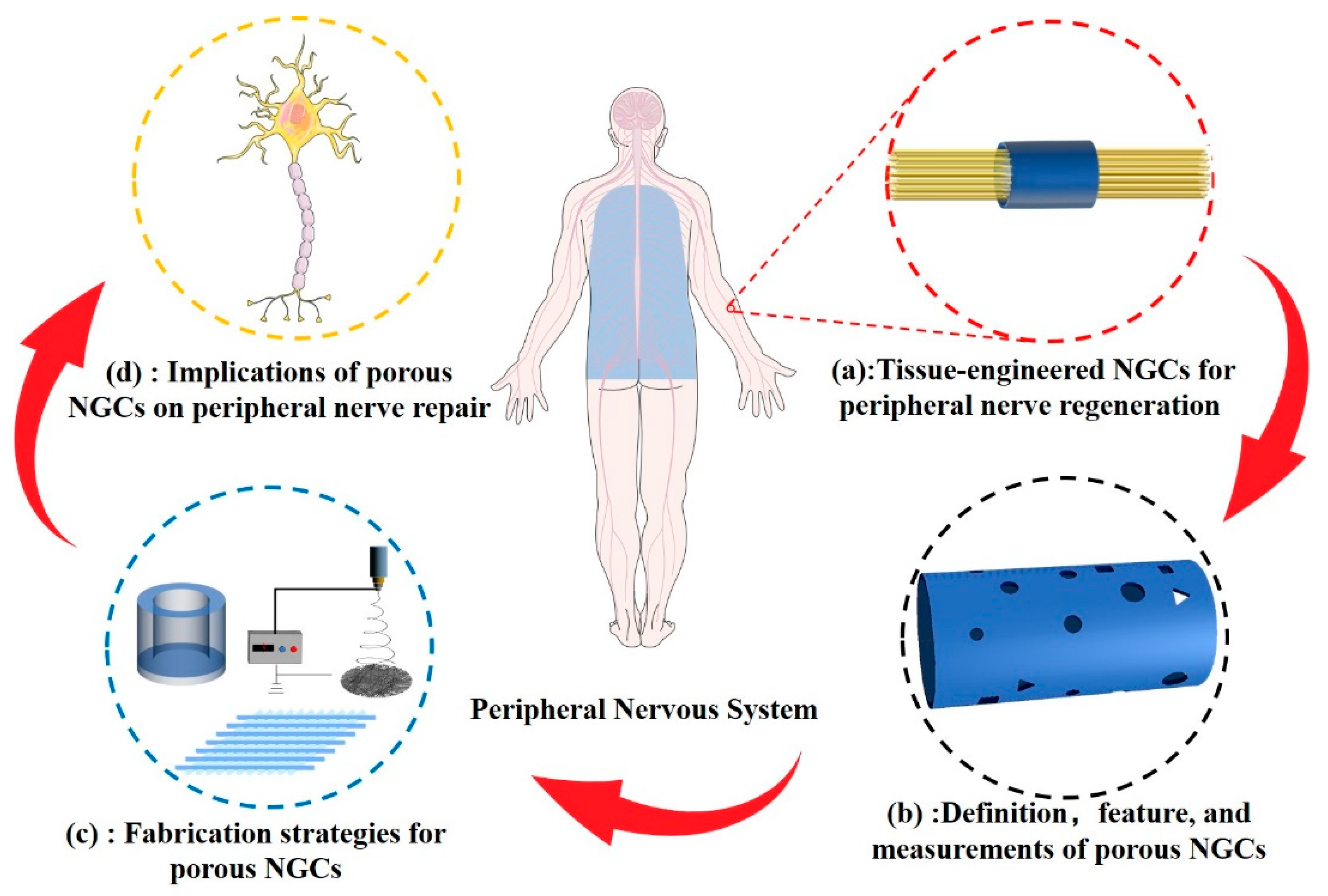
:1. Introduction
2. Definition and Measurement of Pore Structure
3. Fabrication Strategies
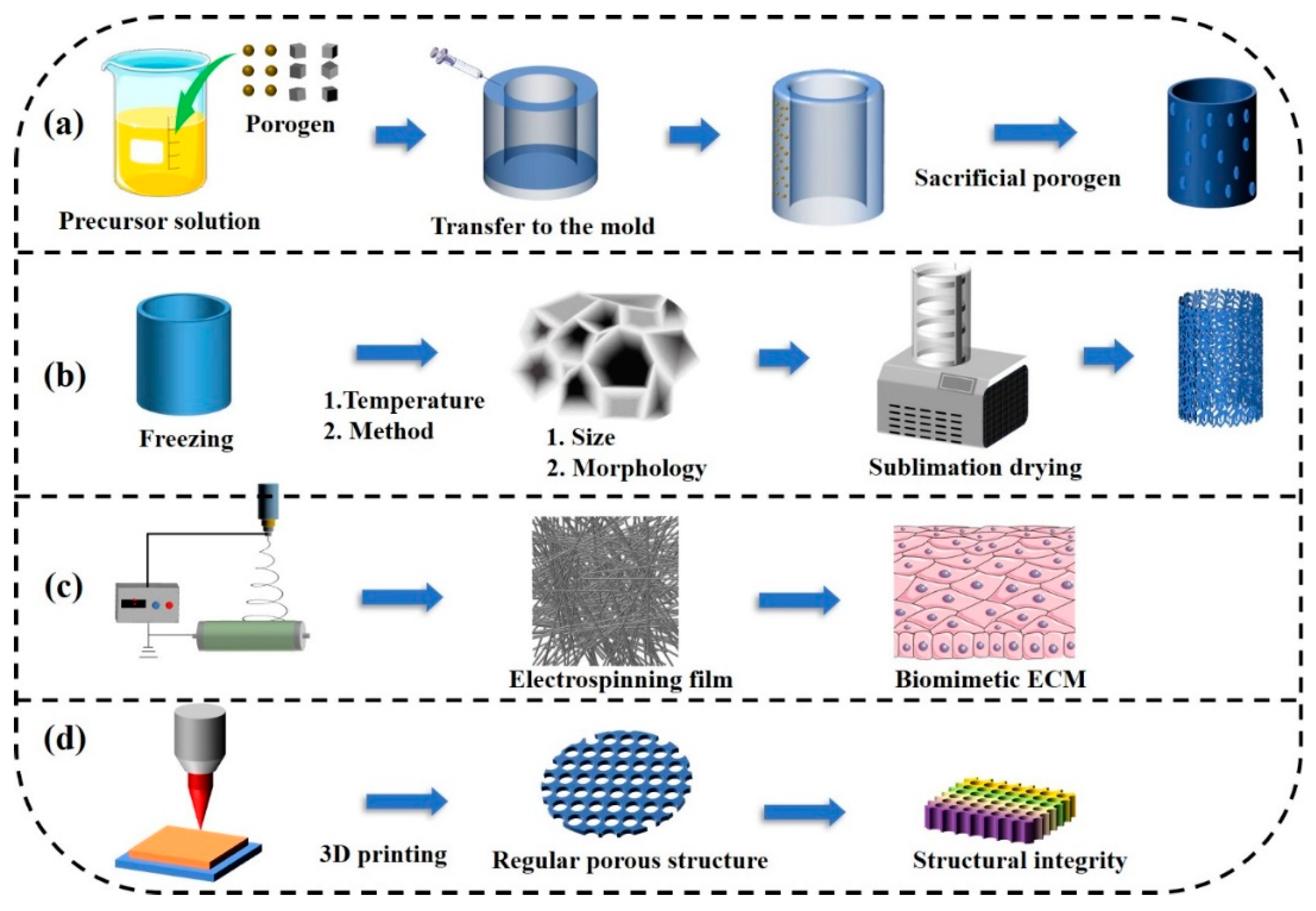
3.1. Templating
3.2. Freeze-Drying
3.3. Electrospinning Fiber Technology
3.4. Three-Dimensional Bioprinting
| Fabrication Strategies | Study | Materials | Pore Characteristics | The Assessment of Nerve Regeneration | Limitation |
|---|---|---|---|---|---|
| Templating | Li et al., 2020 [52] | Gastrodin and polyurethane | Pore size: 10–60 μm. Open and interconnected. | The proliferation and migration of PC12 cells in vitro. | 1. PC12 cells are derived from murine pheochromocytoma and are different from primary neuronal cells; 2. Lacking in vivo animal studies. |
| Jeon et al., 2020 [53] | PLGA | Interior to exterior surfaces interconnected. | 1. The migration and elongation morphology of PC12 cells; 2. A 10 mm sciatic nerve defect. | The assessment is not imprecise because PC12 cells are not fully representative of primary neuronal cells. | |
| Fadia et al., 2020 [54] | PCL | Microsphere leaching forms porous structure. | A 5 mm nerve defect in a rhesus macaque model. | Lacking in vitro cytological studies. | |
| Wang et al., 2015 [55] | PPF-co-PCL | Pore size: 300–400 μm; porosity: 80%. | 1. The spreading of PC12 cells; 2. A 5 mm sciatic nerve defect. | The assessment is not imprecise because PC12 cells are not fully representative of primary neuronal cells. | |
| Cheng et al., 2020 [56] | PVDF/polycaprolactone (PCL) | Pore size: 5.3 μm and 2.7 μm; porosity: 76.2% and 65.5%. | 1. The proliferation and migration of Schwann cells; 2. A 15 mm rat sciatic nerve defect. | Lack of evaluation of neuronal cell regrowth. | |
| Freeze-drying | Li et al., 2018 [22] | Chitosan | Pore size: 20–60 μm and 40–100 μm; porosity: 88.19% and 94.24. | A 10 mm rat sciatic nerve defect. | Lacking in vitro cytological studies. |
| Ma et al., 2022 [57] | Silk fibroin | Interconnected macroporous structure. | 1. Proliferation and differentiation of PC12 cells; 2. A 10 mm rat sciatic nerve defect. | The assessment is not imprecise because PC12 cells are not fully representative of primary neuronal cells. | |
| Choi et al. 2018 [58] | Decellularized matrix | Pore size: 0.5–20 μm. | A 10 cm rat sciatic nerve defect. | Lacking in vitro cytological studies. | |
| Shen et al., 2021 [59] | Silk fibroin and PLGA | Interconnected and open. | 1. Vascularization of human umbilical vein endothelial cells; 2. A 10 mm rat sciatic nerve defect. | Lacking studies on the effects on glial cells and neuronal cells. | |
| Li et al., 2020 [60] | Carbon nanotube (CNT)/sericin | Pore size: 12.84 to 346.46 μm; porosity: 85.80%. | 1. RSC96 cells were applied to evaluate the biocompatibility; 2. A 10 cm rat sciatic nerve defect. | Lacking in vitro cell experiments. | |
| Ye et al., 2020 [61] | Gelatin methacrylate (GelMA) | Pore size: 1151 μm, 1564 μm, and 1915 μm. | Supported the survival, proliferation, and migration of PC12 cells. | Lacking in vivo animal studies. | |
| Electrospinning | Chen et al., 2022 [44] | Methacrylated silk fibroin | Directional pore structure | 1. Adhesion of Schwann cells; 2. axonal regrowth. | Lacking in vivo animal studies. |
| Huang et al., 2017 [62] | PCL | Pore size: 6.5 μm | 1. Schwann cell migration; 2. A 15 mm rat sciatic nerve defect. | Lack of proliferation and extension of Schwann cells. | |
| Zheng et al., 2022 [63] | PCL | Micro–nano morphology | 1. Proliferation of Schwann cells; 2. Extension of dorsal root ganglion cells; 3. A 10 cm rat sciatic nerve defect. | Lack of research on the mechanism of vascularization. | |
| Yoo et al., 2020 [64] | PLCL | Pore size: 2.7 ± 0.6 μm | 1. Spreading morphology of PC12 cells; 2. An 8 mm sciatic nerve defect. | The assessment is not imprecise because of the application of PC12 cells in vitro. | |
| Jaswal et al., 2020 [65] | PCL/gold nanoparticles | The pore size of the spun fiber: 125.85 Å | PC12 cells and Schwann cells were used for biocompatibility and cell morphology studies. | Lacking in vivo animal studies. | |
| 3D printing | Namhongsa et al., 2022 [66] | PLCL and PLGA | Pore size: 165 μm and 215 μm | The proliferation and adhesion of Schwann cells. | 1. Lacking in vivo animal studies; 2. Lacking in vitro neuronal regeneration studies; 3. Lack of migration and morphology of Schwann cells. |
| Qian et al., 2018 [50] | PCL | Pore size: 50 μm | 1. Proliferation and attachment of Schwann cells; 2. A 15 mm rat sciatic nerve defect. | Lacking in vitro neuronal cell regeneration studies. | |
| Vijayavenkataraman et al., 2019 [51] | PCL | Pore size: 125 μm | Proliferation and differentiation of PC12 cells. | 1. The assessment is not imprecise because PC12 cells are not fully representative of primary neuronal cells; 2. Lacking in vivo animal studies. |
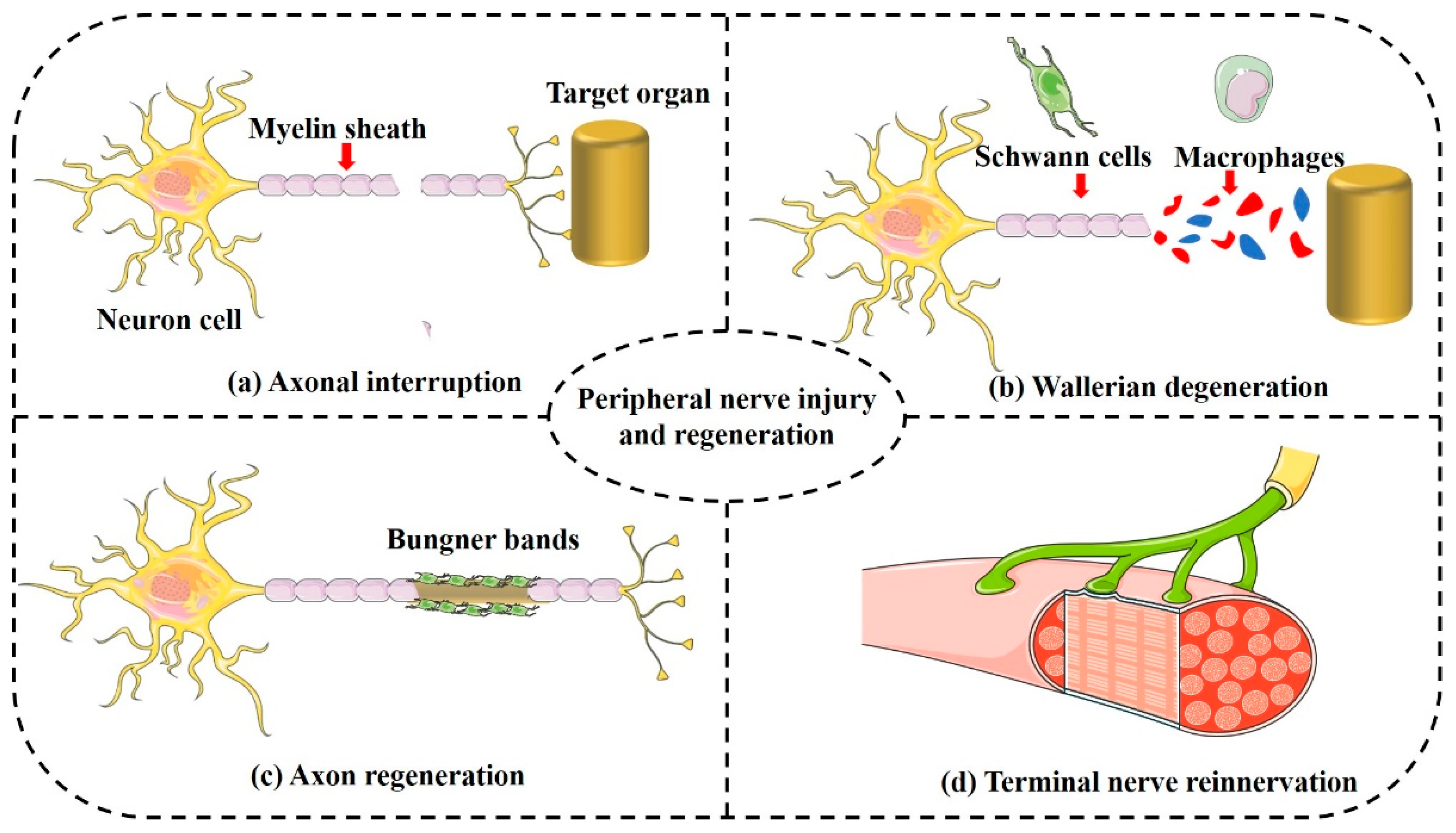
4. Implications of Porous NGCs on Peripheral Nerve Regeneration
4.1. Porous Structure Impact on Physical Properties of NGCs
4.1.1. Degradability
4.1.2. Mechanical Performance
4.1.3. Permeability
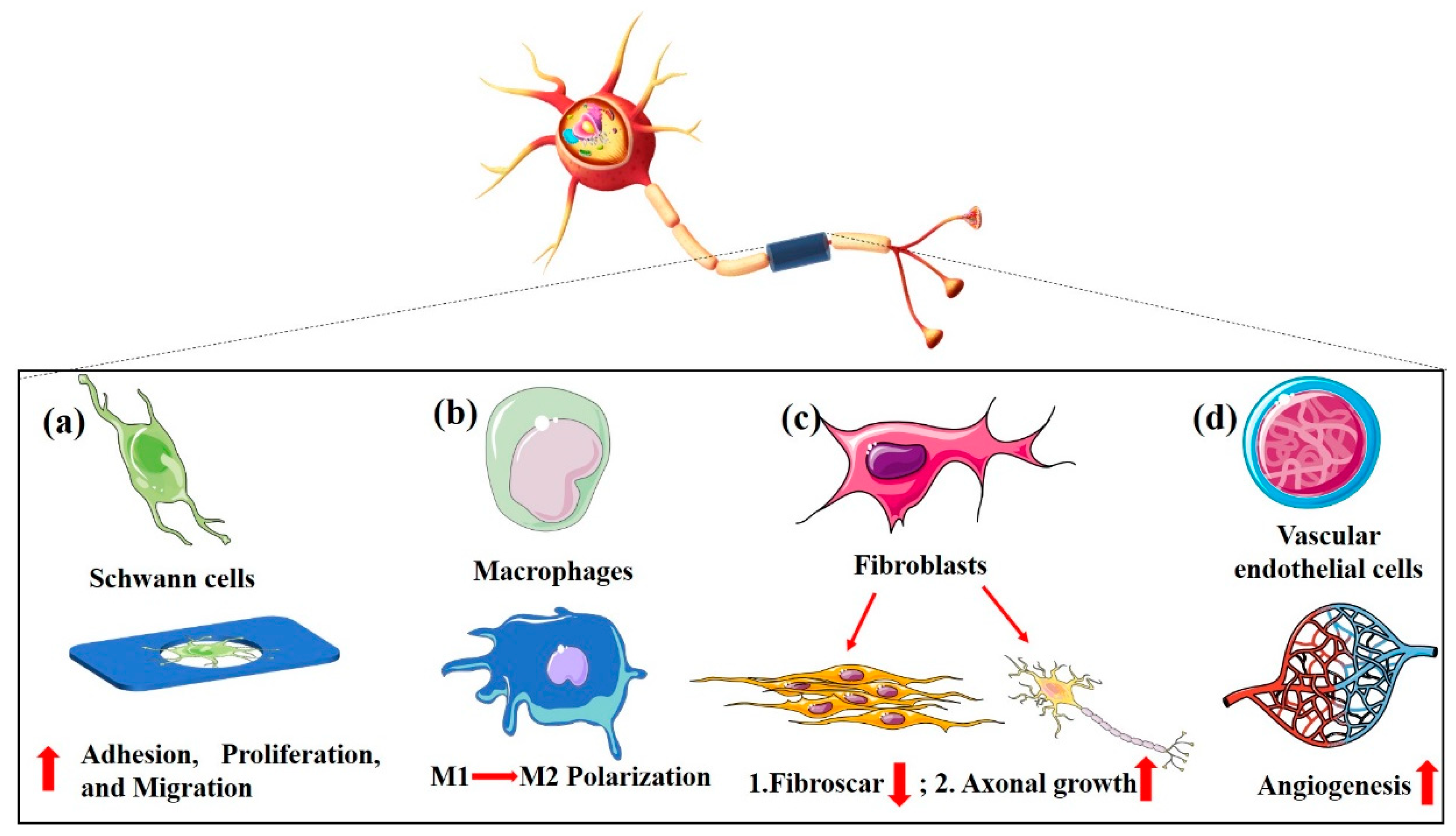
4.2. Porous Structure Determines Bio-Function of NGCs
4.2.1. Schwann Cells
4.2.2. Macrophages
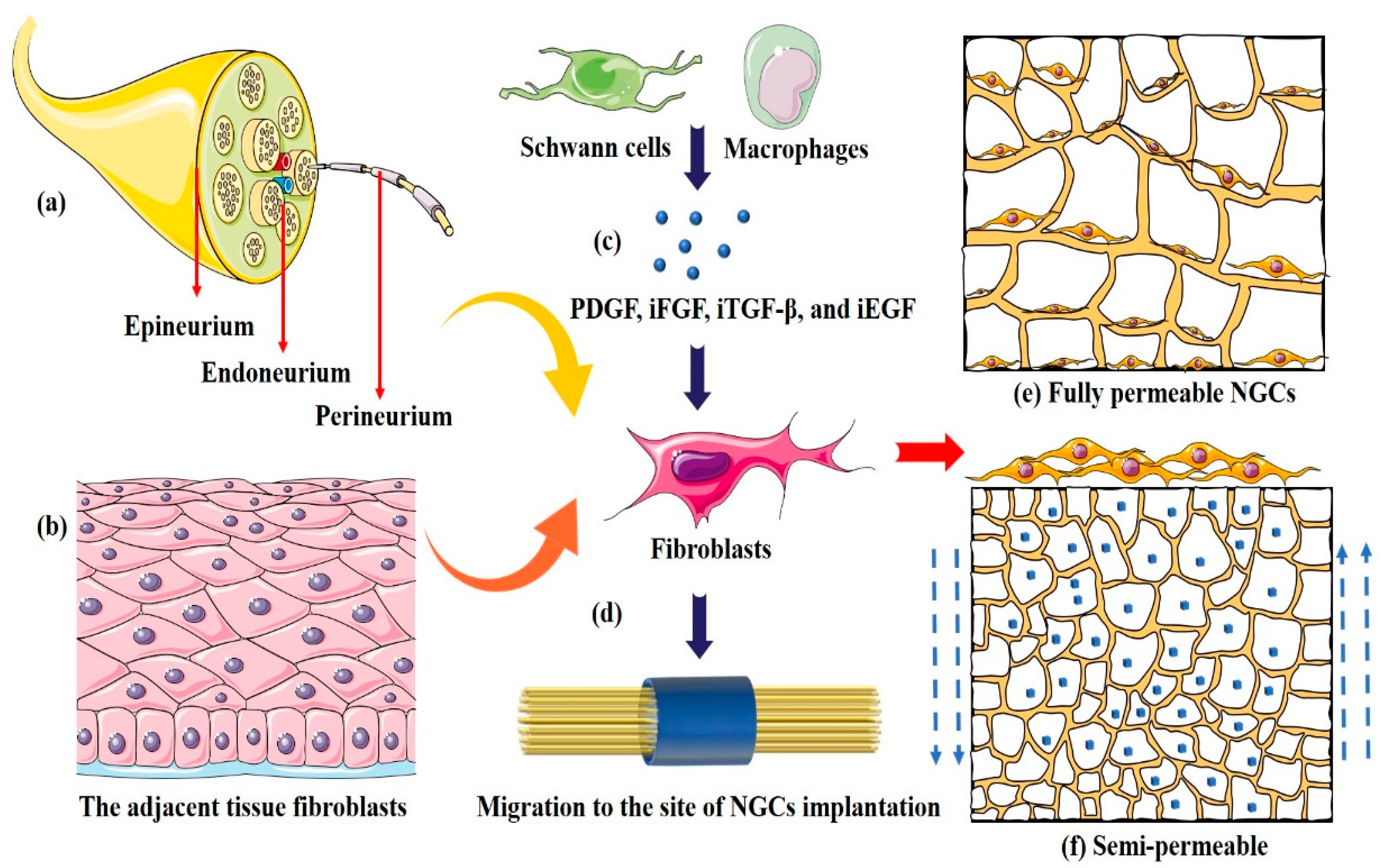
4.2.3. Fibroblasts
4.2.4. Vascular Endothelial Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, J.H.A.; Haycock, J.W. Next Generation Nerve Guides: Materials, Fabrication, Growth Factors, and Cell Delivery. Tissue Eng. Part B Rev. 2012, 18, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.X.; Yao, R.T.; Zhao, J.Y.; Chen, K.L.; Duan, L.R.; Wang, T.; Zhang, S.J.; Guan, J.P.; Zheng, Z.Z.; Wang, X.Q.; et al. Implantable nerve guidance conduits: Material combinations, multi-functional strategies and advanced engineering innovations. Bioact. Mater. 2022, 11, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, P.A.; de Pinho, A.R.G.; Micera, S.; van Wezel, R.J.A.; Moroni, L. Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies. Adv. Healthc. Mater. 2018, 7, 1701164. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, J.H.; Wang, Y.; Shang, L.R.; Chai, R.J.; Zhao, Y.J. Natural Polymer-Derived Bioscaffolds for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2022, 32, 2203829. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.Y.; Young, T.H.; Yang, H.J.; Ji, Y.R. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydr. Polym. 2019, 224, 115112. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, X.D.; Liang, X.; Liu, J.; Wang, B.Y.; Zhao, Y.T.; Huselstein, C.; Feng, X.L.; Tong, Z.; Chen, Y. Composite Hydrogel Conduit Incorporated with Platelet-Rich Plasma Improved the Regenerative Microenvironment for Peripheral Nerve Repair. ACS Appl. Mater. Interfaces 2023, 15, 24120–24133. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zheng, Z.; Yan, J.; Zhang, L.; Li, Y.; Zhang, J.; Li, G.; Wang, X.; Kaplan, D. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair. Acta Biomater. 2021, 134, 116–130. [Google Scholar] [CrossRef]
- Apablaza, J.A.; Lezcano, M.F.; Marquez, A.L.; Sanchez, K.G.; Oporto, G.H.; Dias, F.J. Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration-A Scoping Review. Polymers 2021, 13, 2563. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-Temporal Progress of Peripheral-Nerve Regeneration within a Silicone Chamber—Parameters for a Bioassay. J. Comp. Neurol. 1983, 218, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Uz, M.; Sharma, A.D.; Adhikari, P.; Sakaguchi, D.S.; Mallapragada, S.K. Development of multifunctional films for peripheral nerve regeneration. Acta Biomater. 2017, 56, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Kannan, S.; Cao, T.; Fuh, J.Y.H.; Sriram, G.; Lu, W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019, 7, 266. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, J.H.; Song, K.S.; Jeon, B.H.; Yoon, J.H.; Seo, T.B.; Narngung, U.; Lee, I.W.; Lee, J.H. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 2008, 29, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Chang, C.P.; Lin, S.M. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf. B 2007, 57, 250–255. [Google Scholar] [CrossRef]
- Sayed, E.; Haj-Ahmad, R.; Ruparelia, K.; Arshad, M.S.; Chang, M.W.; Ahmad, Z. Porous Inorganic Drug Delivery Systems-a Review. AAPS PharmSciTech 2017, 18, 1507–1525. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chan, S.H.; Chiang, C.M.; Chen, C.C.C.; Jiang, C.F. Peripheral nerve regeneration using a microporous polylactic acid asymmetric conduit in a rabbit long-gap sciatic nerve transection model. Biomaterials 2011, 32, 3764–3775. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Chakkravarthy, V.; Jose, S.P.; Lakshmanan, M.; Manojkumar, P.; Narayan, R.L.; Kumaran, M. Additive manufacturing of novel Ti-30Nb-2Zr biomimetic scaffolds for successful limb salvage. Mater. Today Proc. 2022, 64, 1711–1716. [Google Scholar] [CrossRef]
- Saltzman, W.M.; Langer, R. Transport Rates of Proteins in Porous Materials with Known Microgeometry. Biophys. J. 1989, 55, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Li, G.C.; Xue, C.B.; Wang, H.K.; Yang, X.M.; Zhao, Y.X.; Zhang, L.Z.; Yang, Y.M. Spatially featured porous chitosan conduits with micropatterned inner wall and seamless sidewall for bridging peripheral nerve regeneration. Carbohydr. Polym. 2018, 194, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Jiao, Z.X.; Guo, M.; Wang, Z.L.; Wan, Y.Z.; Lin, K.L.; Liu, Q.Y.; Zhang, P.B. Gaseous sulfur trioxide induced controllable sulfonation promoting biomineralization and osseointegration of polyetheretherketone implants. Bioact. Mater. 2020, 5, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Menezes, I.R.S.; Sakai, T.; Kaneko, K. Evaluation of graphene oxide nanoporosity by multiprobe gas adsorption analysis. J. Mater. Sci. 2023, 58, 4439–4449. [Google Scholar] [CrossRef]
- Chang, C.J.; Hsu, S.H. The effect of high outflow permeability in asymmetric poly(DL-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. Biomaterials 2006, 27, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.L.; Woodrow, K.A. Medical Applications of Porous Biomaterials: Features of Porosity and Tissue-Specific Implications for Biocompatibility. Adv. Healthc. Mater. 2022, 11, e2102087. [Google Scholar] [CrossRef]
- Cox, S.C.; Jamshidi, P.; Eisenstein, N.M.; Webber, M.A.; Burton, H.; Moakes, R.J.A.; Addison, O.; Attallah, M.; Shepherd, D.E.T.; Grover, L.M. Surface Finish has a Critical Influence on Biofilm Formation and Mammalian Cell Attachment to Additively Manufactured Prosthetics. ACS Biomater. Sci. Eng. 2017, 3, 1616–1626. [Google Scholar] [CrossRef]
- Galperin, A.; Long, T.J.; Garty, S.; Ratner, B.D. Synthesis and fabrication of a degradable poly(N-isopropyl acrylamide) scaffold for tissue engineering applications. J. Biomed. Mater. Res. A 2013, 101, 775–786. [Google Scholar] [CrossRef]
- Huczko, A. Template-based synthesis of nanomaterials. Appl. Phys. A 2000, 70, 365–376. [Google Scholar] [CrossRef]
- Stein, A. Sphere templating methods for periodic porous solids. Microporous Mesoporous Mater. 2001, 44, 227–239. [Google Scholar] [CrossRef]
- Draghi, L.; Resta, S.; Pirozzolo, M.G.; Tanzi, M.C. Microspheres leaching for scaffold porosity control. J. Mater. Sci. Mater. Med. 2005, 16, 1093–1097. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel Freeze-Drying Methods to Produce a Range of Collagen-Glycosaminoglycan Scaffolds with Tailored Mean Pore Sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef]
- Hou, Q.P.; Grijpma, D.W.; Feijen, J. Preparation of interconnected highly porous polymeric structures by a replication and freeze-drying process. J. Biomed. Mater. Res. B 2003, 67, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.P. A Review of the Freeze-Drying Process. Cryobiology 1988, 25, 574. [Google Scholar] [CrossRef]
- De Waard, H.; De Beer, T.; Hinrichs, W.L.J.; Vervaet, C.; Remon, J.P.; Frijlink, H.W. Controlled Crystallization of the Lipophilic Drug Fenofibrate During Freeze-Drying: Elucidation of the Mechanism by In-Line Raman Spectroscopy. AAPS J. 2010, 12, 569–575. [Google Scholar] [CrossRef]
- Jiang, S.M.; Lyu, C.; Zhao, P.; Li, W.J.; Kong, W.Y.; Huang, C.Y.; Genin, G.M.; Du, Y.N. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019, 10, 3491. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, O.S.; Rudraiah, S.; Arul, M.R.; Bartley, J.M.; Baker, J.T.; Yu, X.J.; Kumbar, S.G. Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: In vivo structural and functional assessment. Bioact. Mater. 2021, 6, 2881–2893. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Sun, R.Y.; Wang, B.L.; Zhang, L.F.; Lang, Y.A.; Chang, M.W. Engineering Three-Dimensional Bendable Helix Conduits for Peripheral Nerve Regeneration via Hybrid Electrotechnologies. ACS Mater. Lett. 2022, 4, 2210–2218. [Google Scholar] [CrossRef]
- Zhan, L.; Deng, J.X.; Ke, Q.F.; Li, X.; Ouyang, Y.M.; Huang, C.; Liu, X.Q.; Qian, Y. Grooved Fibers: Preparation Principles Through Electrospinning and Potential Applications. Adv. Fiber Mater. 2022, 4, 203–213. [Google Scholar] [CrossRef]
- Wei, Z.D.; Jin, F.; Li, T.; Qian, L.L.; Zheng, W.Y.; Wang, T.; Feng, Z.Q. Physical Cue-Based Strategies on Peripheral Nerve Regeneration. Adv. Funct. Mater. 2022, 33, 2209658. [Google Scholar] [CrossRef]
- Chen, X.L.; Tang, X.X.; Wang, Y.L.; Gu, X.Y.; Huang, T.T.; Yang, Y.M.; Ling, J. Silk-inspired fiber implant with multi-cues enhanced bionic microenvironment for promoting peripheral nerve repair. Biomater. Adv. 2022, 135, 112674. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.L.; Luo, Z.L.; Rao, Z.L.; Lin, Z.D.; Chen, S.H.; Zhou, J.; Zhu, Q.T.; Liu, X.L.; Bai, Y.; Quan, D.P. Decellularized Extracellular Matrix Containing Electrospun Fibers for Nerve Regeneration: A Comparison Between Core-Shell Structured and Preblended Composites. Adv. Fiber Mater. 2022, 4, 503–519. [Google Scholar] [CrossRef]
- Puhl, D.L.; Funnell, J.L.; Fink, T.D.; Swaminathan, A.; Oudega, M.; Zha, R.H.; Gilbert, R.J. Electrospun fiber-mediated delivery of neurotrophin-3 mRNA for neural tissue engineering applications. Acta Biomater. 2023, 155, 370–385. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D.; Faisal, N.; Sharma, A.; Ansu, A.K.; Goyal, A.; Saxena, K.K.; Prakash, C.; Kumar, D. Application of 3D printing technology for medical implants: A state-of-the-art review. Adv. Mater. Process. Technol. 2023, 1–16. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.J.; Jimenez, A.; Yang, J.Z.; Akpek, A.; Liu, X.; Pi, Q.M.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, e1805510. [Google Scholar] [CrossRef]
- Petcu, E.B.; Midha, R.; McColl, E.; Popa-Wagner, A.; Chirila, T.V.; Dalton, P.D. 3D printing strategies for peripheral nerve regeneration. Biofabrication 2018, 10, 032001. [Google Scholar] [CrossRef]
- Guzzi, E.A.; Tibbitt, M.W. Additive Manufacturing of Precision Biomaterials. Adv. Mater. 2020, 32, 1901994. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.T.; Han, Q.X.; Chen, W.; Li, H.; Yuan, W.E. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. Electrohydrodynamic jet 3D-printed PCL/PAA conductive scaffolds with tunable biodegradability as nerve guide conduits (NGCs) for peripheral nerve injury repair. Mater. Des. 2019, 162, 171–184. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.M.; Yu, M.L.; Zheng, M.; Li, Y.; Yang, J.; Dai, M.; Zhong, L.M.; Sun, L.; Lu, D. Elastomeric polyurethane porous film functionalized with gastrodin for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2020, 108, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lee, M.S.; Lim, J.; Park, S.; Kim, S.M.; Kim, D.I.; Tae, G.; Yang, H.S. Micro-grooved nerve guidance conduits combined with microfiber for rat sciatic nerve regeneration. J. Ind. Eng. Chem. 2020, 90, 214–223. [Google Scholar] [CrossRef]
- Fadia, N.B.; Bliley, J.M.; DiBernardo, G.A.; Crammond, D.J.; Schilling, B.K.; Sivak, W.N.; Spiess, A.M.; Washington, K.M.; Waldner, M.; Liao, H.T.; et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci. Transl. Med. 2020, 12, eaav7753, Erratum in Sci. Transl. Med. 2020, 12, eabc4054. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Kempen, D.H.R.; de Ruiter, G.C.W.; Cai, L.; Spinner, R.J.; Windebank, A.J.; Yaszemski, M.J.; Lu, L.C. Molecularly Engineered Biodegradable Polymer Networks with a Wide Range of Stiffness for Bone and Peripheral Nerve Regeneration. Adv. Funct. Mater. 2015, 25, 2715–2724. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Y.; Qian, Y.; Chen, X.; Ouyang, Y.M.; Yuan, W.E. 3D structured self-powered PVDF/PCL scaffolds for peripheral nerve regeneration. Nano Energy 2020, 69, 104411. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Wang, Q.Q.; Cao, X.D.; Gao, H.C. Piezoelectric conduit combined with multi-channel conductive scaffold for peripheral nerve regeneration. Chem. Eng. J. 2023, 452, 139424. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.H.; Jang, J.W.; Kim, H.J.; Choi, S.N.; Kwon, S.W. Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration. Neural Regen. Res. 2018, 13, 1796–1803. [Google Scholar] [CrossRef]
- Shen, J.J.; Wang, J.Y.; Liu, X.Z.; Sun, Y.; Yin, A.L.; Chai, Y.M.; Zhang, K.H.; Wang, C.Y.; Zheng, X.Y. In Situ Prevascularization Strategy with Three-Dimensional Porous Conduits for Neural Tissue Engineering. ACS Appl. Mater. Inter. 2021, 13, 50785–50801. [Google Scholar] [CrossRef]
- Li, X.L.; Yang, W.; Xie, H.J.; Wang, J.; Zhang, L.; Wang, Z.; Wang, L. CNT/Sericin Conductive Nerve Guidance Conduit Promotes Functional Recovery of Transected Peripheral Nerve Injury in a Rat Model. ACS Appl. Mater. Inter. 2020, 12, 36860–36872. [Google Scholar] [CrossRef]
- Ye, W.S.; Li, H.B.; Yu, K.; Xie, C.Q.; Wang, P.; Zheng, Y.T.; Zhang, P.; Xiu, J.F.; Yang, Y.; Zhang, F.; et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757. [Google Scholar] [CrossRef]
- Huang, L.L.; Zhu, L.; Shi, X.W.; Xia, B.; Liu, Z.Y.; Zhu, S.; Yang, Y.F.; Ma, T.; Cheng, P.Z.; Luo, K.; et al. A compound scaffold with uniform longitudinally oriented guidance cues and a porous sheath promotes peripheral nerve regeneration in vivo. Acta Biomater. 2018, 68, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.T.; Wu, L.L.; Xu, J.W.; Sun, S.L.; Guan, W.C.; Han, Q.; Zhang, L.Z.; Gu, X.S.; Yang, Y.M.; Li, G.C. YR/DFO@DCNT functionalized anisotropic micro/nano composite topography scaffolds for accelerating long-distance peripheral nerve regeneration. Compos. Part B Eng. 2022, 246, 110242. [Google Scholar] [CrossRef]
- Yoo, J.; Park, J.H.; Kwon, Y.W.; Chung, J.J.; Choi, I.C.; Nam, J.J.; Lee, H.S.; Jeon, E.Y.; Lee, K.; Kim, S.H.; et al. Augmented peripheral nerve regeneration through elastic nerve guidance conduits prepared using a porous PLCL membrane with a 3D printed collagen hydrogel. Biomater. Sci. 2020, 8, 6261–6271. [Google Scholar] [CrossRef]
- Jaswal, R.; Shrestha, S.; Shrestha, B.K.; Kumar, D.; Park, C.H.; Kim, C.S. Nanographene enfolded AuNPs sophisticatedly synchronized polycaprolactone based electrospun nanofibre scaffold for peripheral nerve regeneration. Mat. Sci. Eng. C 2020, 116, 111213. [Google Scholar] [CrossRef]
- Namhongsa, M.; Daranarong, D.; Sriyai, M.; Molloy, R.; Ross, S.; Ross, G.M.; Tuantranont, A.; Tocharus, J.; Sivasinprasasn, S.; Topham, P.D.; et al. Surface-Modified Polypyrrole-Coated PLCL and PLGA Nerve Guide Conduits Fabricated by 3D Printing and Electrospinning. Biomacromolecules 2022, 23, 4532–4546. [Google Scholar] [CrossRef]
- Qian, Y.; Lin, H.; Yan, Z.W.; Shi, J.L.; Fan, C.Y. Functional nanomaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater. Today 2021, 51, 165–187. [Google Scholar] [CrossRef]
- Zheng, F.R.; Li, R.; He, Q.D.; Koral, K.; Tao, J.Y.; Fan, L.H.; Xiang, R.Z.; Ma, J.Y.; Wang, N.; Yin, Y.X.; et al. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mat. Sci. Eng. C 2020, 109, 110560. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef]
- Maksoud, F.; De la Paz, M.F.V.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: A review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.Y.; Wu, S.; Fan, Y.B.; Li, X.M. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Fan, C.M.; Chen, Y.W.; Ye, J.C.; Yang, Y.W.; Tang, C.Q.; Zhang, H.; Fei, Y.; An, C.R.; Xie, Y.H.; et al. Single-cell RNA-seq reveals functionally distinct biomaterial degradation-related macrophage populations. Biomaterials 2021, 277, 121116. [Google Scholar] [CrossRef] [PubMed]
- Chakkravarthy, V.; Manojkumar, P.; Lakshmanan, M.; Prasad, K.E.; Dafale, R.; Vadhana, V.C.; Narayan, R.L. Comparing bio-tribocorrosion of selective laser melted Titanium-25% Niobium and conventionally manufactured Ti-6Al-4 V in inflammatory conditions. J. Alloys Compd. 2023, 952, 169852. [Google Scholar] [CrossRef]
- Lu, X.Z.; Lai, C.P.; Chan, L.C. Novel design of a coral-like open-cell porous degradable magnesium implant for orthopaedic application. Mater. Des. 2020, 188, 108474. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, J.; Luo, Y.; Zhou, T.; Wu, H. Preparation and degradation of chitosan-poly(p-dioxanone)/silk fibroin porous conduits. Polym. Degrad. Stab. 2015, 119, 46–55. [Google Scholar] [CrossRef]
- Wu, L.B.; Ding, J.D. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. J. Biomed. Mater. Res. A 2005, 75, 767–777. [Google Scholar] [CrossRef]
- Song, C.B.; Zhang, J.P.; Cen, L.; Xi, Z.H.; Zhao, L.; Yuan, W.K. Modeling Strategies for the Degradation Behavior of Porous Polyester Materials Based on Their Key Structural Features. Ind. Eng. Chem. Res. 2020, 59, 14806–14816. [Google Scholar] [CrossRef]
- Odelius, K.; Hoglund, A.; Kumar, S.; Hakkarainen, M.; Ghosh, A.K.; Bhatnagar, N.; Albertsson, A.C. Porosity and Pore Size Regulate the Degradation Product Profile of Polylactide. Biomacromolecules 2011, 12, 1250–1258. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, B.; Li, L.; Yin, J.; Fu, J.Z. Additive-lathe 3D bioprinting of bilayered nerve conduits incorporated with supportive cells. Bioact. Mater. 2021, 6, 219–229. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Kaplan, D.L.; Kim, H.W.; Kundu, S.C. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. Int. Mater. Rev. 2017, 62, 367–391. [Google Scholar] [CrossRef]
- Yucel, D.; Kose, G.T.; Hasirci, V. Polyester based nerve guidance conduit design. Biomaterials 2010, 31, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Barrera, C.M.; Dauer, E.A.; Gu, W.Y.; Andreopoulos, F.; Huang, C.Y.C. Systematic characterization of porosity and mass transport and mechanical properties of porous polyurethane scaffolds. J. Mech. Behav. Biomed. 2017, 65, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Jiao, C.; Liang, H.X.; Xie, D.Q.; Shen, L.D.; Liu, Z.D. Analysis of Mechanical Properties and Permeability of Trabecular-Like Porous Scaffold by Additive Manufacturing. Front. Bioeng. Biotechnol. 2021, 9, 779854. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jeon, E.Y.; Nam, J.J.; Park, J.H.; Choi, I.C.; Kim, S.H.; Chung, J.J.; Lee, K.; Park, J.W.; Jung, Y. Development of a regenerative porous PLCL nerve guidance conduit with swellable hydrogel-based microgrooved surface pattern via 3D printing. Acta Biomater. 2022, 141, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.Z.; Wang, Y.; Aibaidoula, G.; Chen, G.Q.; Wu, Q. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials 2009, 30, 217–225. [Google Scholar] [CrossRef]
- Vleggeert-Lankamp, C.L.A.M.; de Ruiter, G.C.W.; Wolfs, J.F.C.; Pego, A.P.; van den Berg, R.J.; Feirabend, H.K.P.; Malessy, M.J.A.; Lakke, E.A.J.F. Pores in synthetic nerve conduits are beneficial to regeneration. J. Biomed. Mater. Res. A 2007, 80, 965–982. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liu, J.N.; Liu, S.; Yang, P.P.; Liang, Y.Y.; Ma, J.Y.; Mao, S.S.; Sun, C.; Yang, Y.M. Fibroblast exosomal TFAP2C induced by chitosan oligosaccharides promotes peripheral axon regeneration via the miR-132-5p/CAMKK1 axis. Bioact. Mater. 2023, 26, 249–263. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Wang, H.; Zhao, Y.J.; Chai, R.J. Natural proteins-derived asymmetric porous conduit for peripheral nerve regeneration. Appl. Mater. Today 2022, 27, 101431. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gong, J.X.; Zhang, J.Y.; Zhu, Z.Y.; Qian, Y.; Lu, K.J.; Zhou, S.Y.; Gu, T.Y.; Wang, H.M.; He, Y.; et al. Three Potential Elements of Developing Nerve Guidance Conduit for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2023, 2302251. [Google Scholar] [CrossRef]
- Babu, S.; Krishnan, M.; Panneerselvam, A.; Chinnaiyan, M. A comprehensive review on therapeutic application of mesenchymal stem cells in neuroregeneration. Life Sci. 2023, 327, 121785. [Google Scholar] [CrossRef]
- Liu, J.M.; Li, L.X.; Zou, Y.; Fu, L.Y.; Ma, X.R.; Zhang, H.W.; Xu, Y.Z.; Xu, J.W.; Zhang, J.Q.; Li, M.; et al. Role of microtubule dynamics in Wallerian degeneration and nerve regeneration after peripheral nerve injury. Neural Regen. Res. 2022, 17, 673. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Powell, R.; Phillips, J.B.; Haastert-Talini, K. Perspective on Schwann Cells Derived from Induced Pluripotent Stem Cells in Peripheral Nerve Tissue Engineering. Cells 2020, 9, 2497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.N.; Xiao, C.S.; Liu, B. Engineered hydrogels for peripheral nerve repair. Mater. Today Bio 2023, 20, 100668. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, Y.; Liu, Z.; He, D.; Xu, W.; Li, S.; Zhang, C.; Zhang, Z. Li-Mg-Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioact. Mater. 2023, 28, 227–242. [Google Scholar] [CrossRef]
- Ma, T.; Hao, Y.M.; Li, S.Y.; Xia, B.; Gao, X.; Zheng, Y.; Mei, L.W.; Wei, Y.T.; Yang, C.B.; Lu, L.; et al. Sequential oxygen supply system promotes peripheral nerve regeneration by enhancing Schwann cells survival and angiogenesis. Biomaterials 2022, 289, 121755. [Google Scholar] [CrossRef]
- Borger, A.; Stadlmayr, S.; Haertinger, M.; Semmler, L.; Supper, P.; Millesi, F.; Radtke, C. How miRNAs Regulate Schwann Cells during Peripheral Nerve Regeneration-A Systemic Review. Int. J. Mol. Sci. 2022, 23, 3440. [Google Scholar] [CrossRef]
- Jones, S.; Eisenberg, H.M.; Jia, X.F. Advances and Future Applications of Augmented Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2016, 17, 1494. [Google Scholar] [CrossRef]
- Hercher, D.; Nguyen, M.Q.; Dworak, H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp. Neurol. 2022, 350, 113968. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zheng, T.T.; Wu, L.L.; Han, Q.; Chen, S.Y.; Kong, Y.; Li, G.C.; Ma, L.; Wu, H.; Zhao, Y.H.; et al. Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth. Nanotechnol. Rev. 2021, 10, 50–61. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, L.T.; Su, W.T. Three dimensional chitosan scaffolds influence the extra cellular matrix expression in Schwann cells. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 42, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.Z.; Wang, X.R.; Fan, S.N.; Yao, X.; Zhang, Y.P.; Shao, H.L. Electrospun regenerated Antheraea pernyi silk fibroin scaffolds with improved pore size, mechanical properties and cytocompatibility using mesh collectors. J. Mater. Chem. B 2021, 9, 5514–5527. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Yoon, C.; Giger, R.J.; Sakamoto, J. Engineering a platform for nerve regeneration with direct application to nerve repair technology. Biomaterials 2019, 216, 119263. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Xu, Y.Z.; Wang, X.H.; Liu, J.M.; Hu, X.F.; Tan, D.D.; Li, Z.L.; Guo, J.S. Ascorbic acid accelerates Wallerian degeneration after peripheral nerve injury. Neural Regen. Res. 2021, 16, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Wofford, K.L.; Shultz, R.B.; Burrell, J.C.; Cullen, D.K. Neuroimmune interactions and immunoengineering strategies in peripheral nerve repair. Prog. Neurobiol. 2022, 208, 102172. [Google Scholar] [CrossRef]
- Stratton, J.A.; Shah, P.T. Macrophage polarization in nerve injury: Do Schwann cells play a role? Neural Regen. Res. 2016, 11, 53–57. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Zygelyte, E.; Grenier, J.K.; Edwards, M.G.; Cheetham, J. Temporal changes in macrophage phenotype after peripheral nerve injury. J. Neuroinflamm. 2018, 15, 185. [Google Scholar] [CrossRef]
- Cerri, F.; Salvatore, L.; Memon, D.; Boneschi, F.M.; Madaghiele, M.; Brambilla, P.; Del Carro, U.; Taveggia, C.; Riva, N.; Trimarco, A.; et al. Peripheral nerve morphogenesis induced by scaffold micropatterning. Biomaterials 2014, 35, 4035–4045. [Google Scholar] [CrossRef]
- Yu, J.W.; Lin, Y.F.; Wang, G.W.; Song, J.L.; Hayat, U.; Liu, C.; Raza, A.L.; Huang, X.Y.; Lin, H.D.; Wang, J.Y. Zein-induced immune response and modulation by size, pore structure and drug-loading: Application for sciatic nerve regeneration. Acta Biomater. 2022, 140, 289–301. [Google Scholar] [CrossRef]
- Jia, Y.C.; Yang, W.C.; Zhang, K.H.; Qiu, S.; Xu, J.; Wang, C.Y.; Chai, Y.M. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 2019, 83, 291–301. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Gu, X.K.; Zhang, R.R.; Qian, T.M.; Li, S.Y.; Yi, S. Biological characteristics of dynamic expression of nerve regeneration related growth factors in dorsal root ganglia after peripheral nerve injury. Neural Regen. Res. 2020, 15, 1502–1509. [Google Scholar] [CrossRef]
- Yang, H.C.; Li, Q.; Li, L.M.; Chen, S.C.; Zhao, Y.; Hu, Y.R.; Wang, L.; Lan, X.Q.; Zhong, L.M.; Lu, D. Gastrodin modified polyurethane conduit promotes nerve repair via optimizing Schwann cells function. Bioact. Mater. 2022, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- He, Q.R.; Cong, M.; Yu, F.H.; Ji, Y.H.; Yu, S.; Shi, H.Y.; Ding, F. Peripheral nerve fibroblasts secrete neurotrophic factors to promote axon growth of motoneurons. Neural Regen. Res. 2022, 17, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Saio, S.; Konishi, K.; Hohjoh, H.; Tamura, Y.; Masutani, T.; Iddamalgoda, A.; Ichihashi, M.; Hasegawa, H.; Mizutani, K. Extracellular Environment-Controlled Angiogenesis, and Potential Application for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 11169. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nihara, J.; Trakanant, S.; Kudo, T.; Seo, K.; Iida, I.; Izumi, K.; Kurose, M.; Shimomura, Y.; Terunuma, M.; et al. Perivascular Hedgehog responsive cells play a critical role in peripheral nerve regeneration via controlling angiogenesis. Neurosci. Res. 2021, 173, 62–70. [Google Scholar] [CrossRef]
- Yadav, A.; Ramasamy, T.S.; Lin, S.C.; Chen, S.H.; Lu, J.; Liu, Y.H.; Lu, F.I.; Hsueh, Y.Y.; Lin, S.P.; Wu, C.C. Autologous Platelet-Rich Growth Factor Reduces M1 Macrophages and Modulates Inflammatory Microenvironments to Promote Sciatic Nerve Regeneration. Biomedicines 2022, 10, 1991. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in Craniofacial Bone Tissue Engineering. J. Dent. Res. 2018, 97, 969–976. [Google Scholar] [CrossRef]
- Yin, Y.; He, X.T.; Wang, J.; Wu, R.X.; Xu, X.Y.; Hong, Y.L.; Tian, B.M.; Chen, F.M. S Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today 2020, 18, 100466. [Google Scholar] [CrossRef]
- Wang, W.L.Y.; Kent, R.N.; Huang, S.I.A.; Jarman, E.H.; Shikanov, E.H.; Davidson, C.D.; Hiraki, H.L.; Lin, D.E.; Wall, M.A.; Matera, D.L.; et al. Direct comparison of angiogenesis in natural and synthetic biomaterials reveals that matrix porosity regulates endothelial cell invasion speed and sprout diameter. Acta Biomater. 2021, 135, 260–273. [Google Scholar] [CrossRef]
| Classification | Pore Diameter | Permeability | Advantages in Peripheral Nerve Regeneration | Disadvantageous |
|---|---|---|---|---|
| Semi-permeable [85] | <10 μm | Nutrients, metabolic waste, and molecular signals. | Avoiding fibrous scars. | Cannot achieve direct signal communication between cells. |
| Fully permeable [86] | >50 μm | Cells, nutrients, metabolic waste, and molecular signals. | Promote direct signal communication between cells. | Risk of fibrous scar invasion. |
| Asymmetric structures [88] | External surface pore size > lumen surface pore size. | High outflow. | High effluent efficiency of metabolic waste. | Cannot achieve direct signal communication between cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, T.; Wang, Y.-L.; Zhang, F.-S.; Zhang, X.-M.; Zhang, Y.-C.; Jiang, H.-R.; Zhang, M.; Zhang, P.-X. The Porous Structure of Peripheral Nerve Guidance Conduits: Features, Fabrication, and Implications for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 14132. https://doi.org/10.3390/ijms241814132
Wan T, Wang Y-L, Zhang F-S, Zhang X-M, Zhang Y-C, Jiang H-R, Zhang M, Zhang P-X. The Porous Structure of Peripheral Nerve Guidance Conduits: Features, Fabrication, and Implications for Peripheral Nerve Regeneration. International Journal of Molecular Sciences. 2023; 24(18):14132. https://doi.org/10.3390/ijms241814132
Chicago/Turabian StyleWan, Teng, Yi-Lin Wang, Feng-Shi Zhang, Xiao-Meng Zhang, Yi-Chong Zhang, Hao-Ran Jiang, Meng Zhang, and Pei-Xun Zhang. 2023. "The Porous Structure of Peripheral Nerve Guidance Conduits: Features, Fabrication, and Implications for Peripheral Nerve Regeneration" International Journal of Molecular Sciences 24, no. 18: 14132. https://doi.org/10.3390/ijms241814132





