Selective Block of Upregulated Kv1.3 Potassium Channels in ON-Bipolar Cells of the Blind Retina Enhances Optogenetically Restored Signaling
Abstract
1. Introduction
2. Results
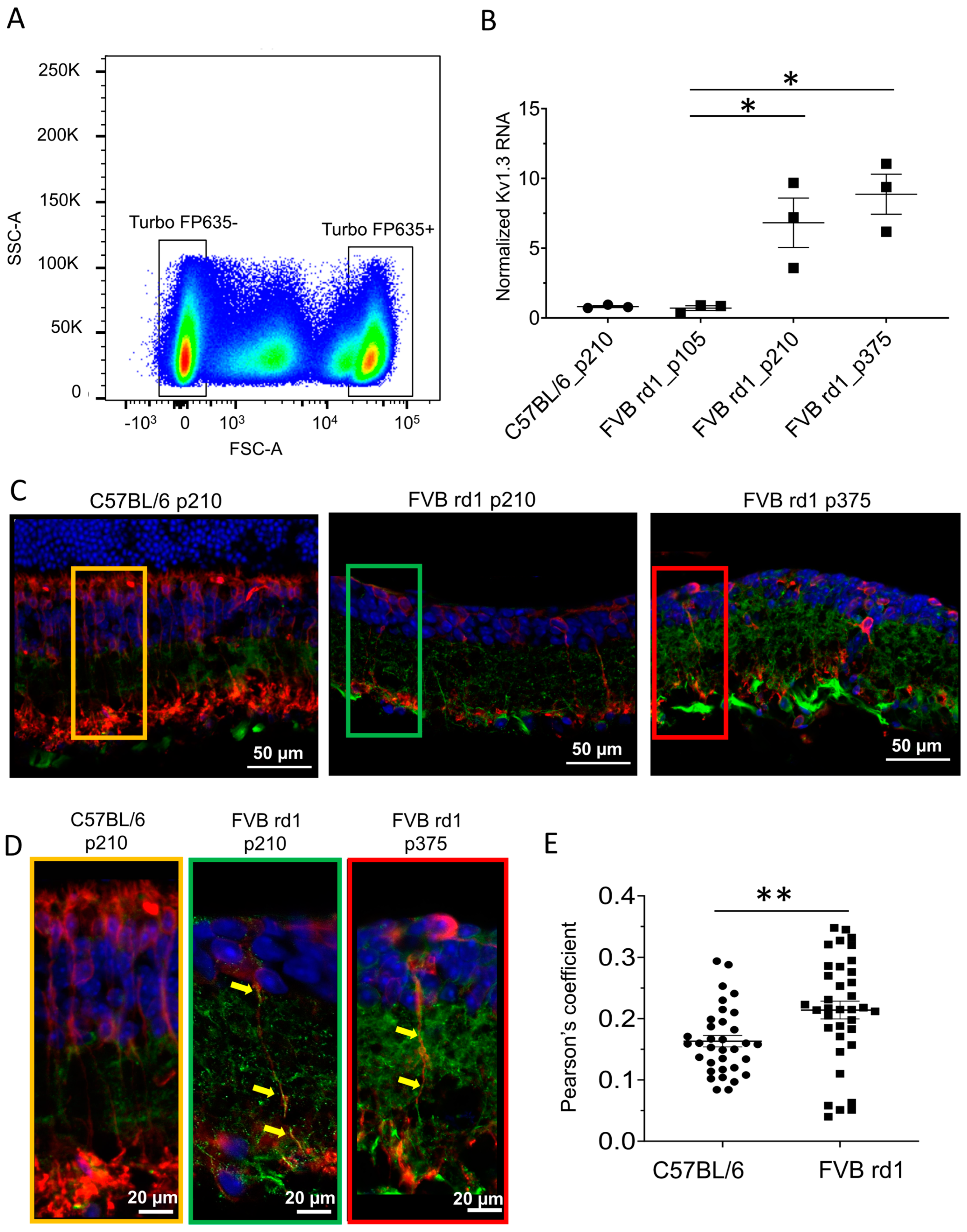
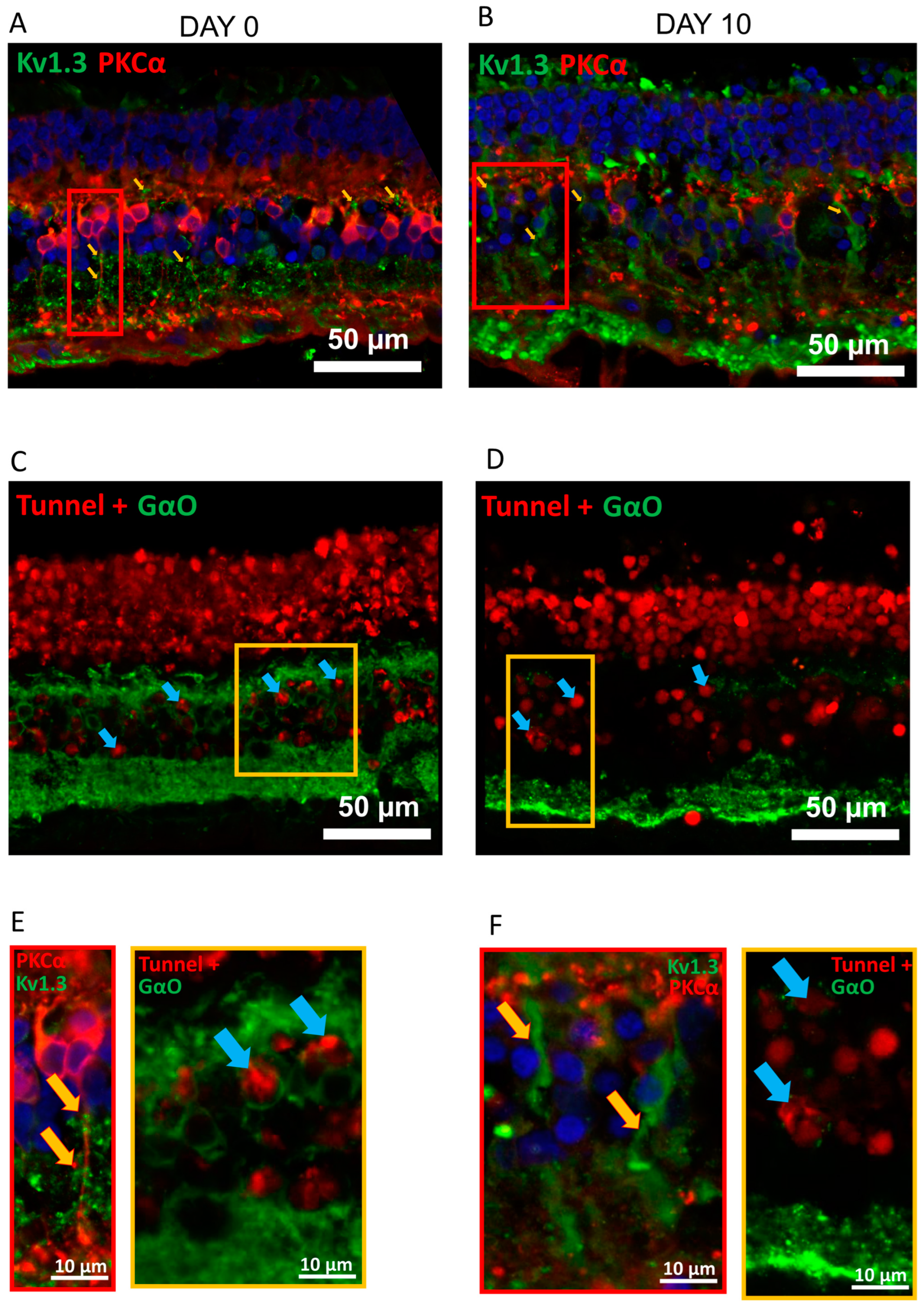
2.1. Kv1.3 Is Expressed in OBC Axons of rd1 (FVB/NCrl_Opto-mGluR6) Mouse Retina
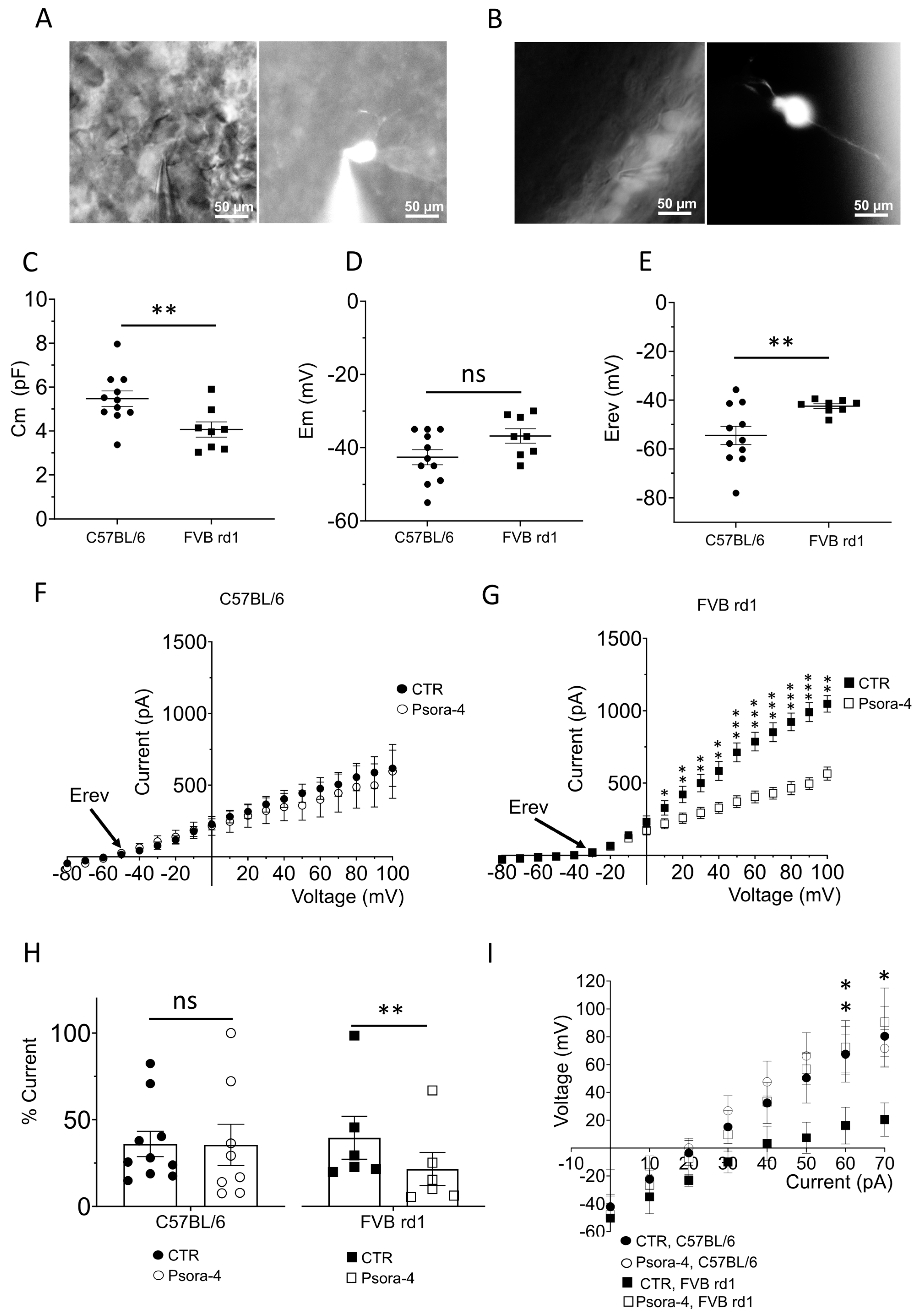
2.2. The Delayed Rectifier Potassium Current Is Driven by Kv1.3 in OBCs of the Degenerated FVB rd1 Retina
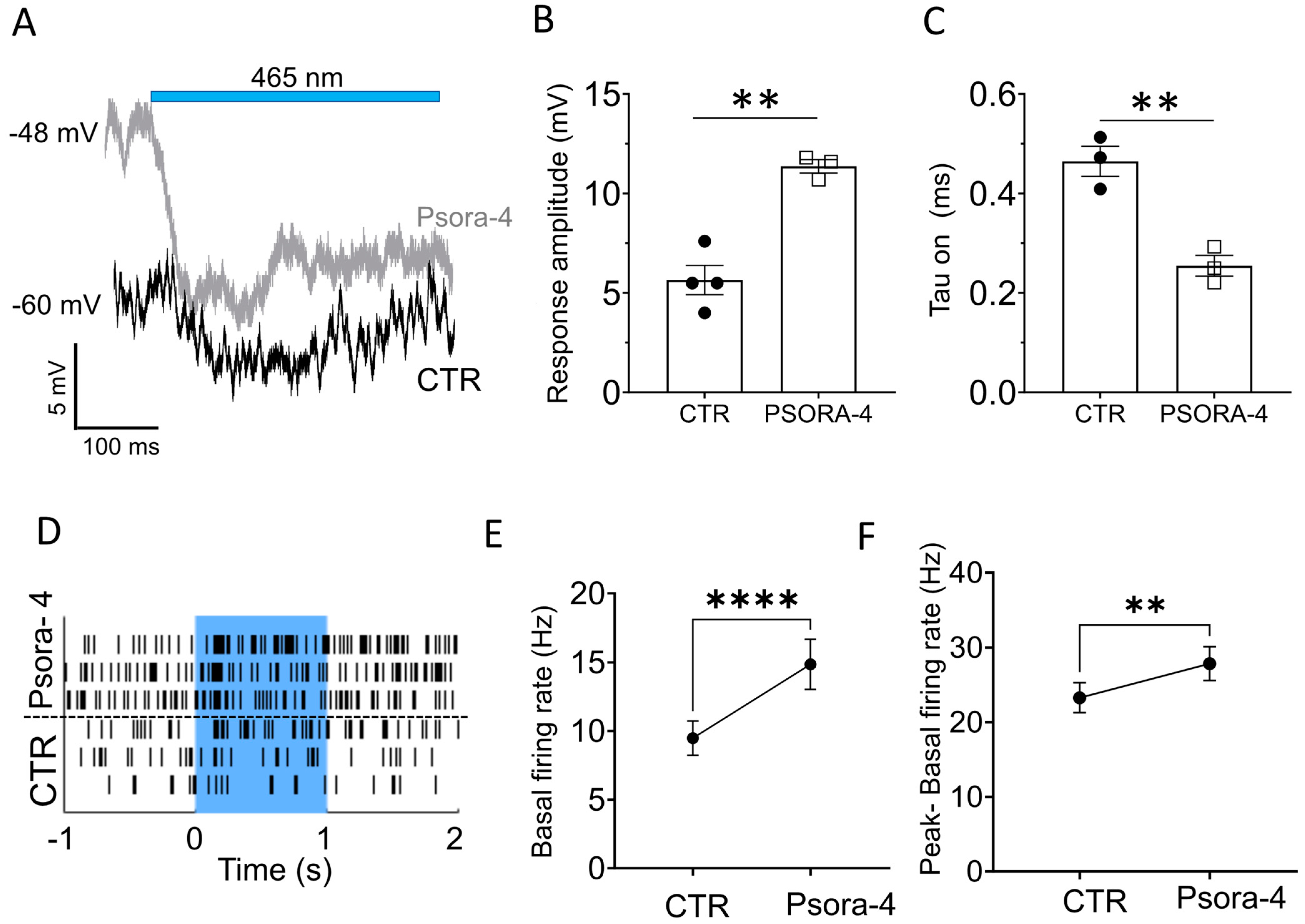
2.3. Kv1.3 Inhibition Accelerates and Enhances the Light-Induced, Opto-mGluR6-Mediated Conductance in OBCs of the Degenerated Retina
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Fluorescence-Activated Cell Sorting (FACS) and qPCR
4.3. Human Retinal Tissue and Culturing
4.4. Immunohistochemistry
4.5. Patch-Clamp Recordings
4.5.1. Solutions and Drugs
4.5.2. OBC Identification
4.5.3. Voltage and Current Clamp Recordings
4.5.4. Light Responses from OBCs in the FVB rd1 Retina
4.6. Multi Electrode Array Recordings
4.7. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, C.-J.; Strettoi, E.; Masland, R.H. The Major Cell Populations of the Mouse Retina. J. Neurosci. 1998, 18, 8936–8946. [Google Scholar] [CrossRef]
- Behrens, C.; Schubert, T.; Haverkamp, S.; Euler, T.; Berens, P. Connectivity map of bipolar cells and photoreceptors in the mouse retina. eLife 2016, 5, e20041. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Fyk-Kolodziej, B.; Cohn, J. Roles of ON Cone Bipolar Cell Subtypes in Temporal Coding in the Mouse Retina. J. Neurosci. 2014, 34, 8761–8771. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, K.; Lapan, S.W.; Whitney, I.E.; Tran, N.M.; Macosko, E.Z.; Kowalczyk, M.; Adiconis, X.; Levin, J.Z.; Nemesh, J.; Goldman, M.; et al. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell 2016, 166, 1308–1323.e30. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Ferrari, S.; Iorio, E.D.; Barbaro, V.; Ponzin, D.; Sorrentino, F.S.; Parmeggiani, F. Retinitis Pigmentosa: Genes and Disease Mechanisms. Curr. Genom. 2011, 12, 238–249. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of Photoreceptor Death in Retinitis Pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef]
- Leroy, B.P.; Fischer, M.D.; Flannery, J.G.; MacLaren, R.E.; Dalkara, D.; Scholl, H.P.N.; Chung, D.C.; Spera, C.; Viriato, D.; Banhazi, J. Gene therapy for inherited retinal disease: Long-term durability of effect. Ophthalmic Res. 2022, 66, 179–196. [Google Scholar] [CrossRef]
- Jang, J.; Kim, H.; Song, Y.M.; Park, J. Implantation of electronic visual prosthesis for blindness restoration. Opt. Mater. Express 2019, 9, 3878–3894. [Google Scholar] [CrossRef]
- Bloch, E.; Luo, Y.; da Cruz, L. Advances in retinal prosthesis systems. Ther. Adv. Ophthalmol. 2019, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Qiu, S.; Wang, Y.V.; Park, S.J.H.; Mohns, E.J.; Mehta, B.; Liu, X.; Chang, B.; Zenisek, D.; Crair, M.C.; et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 2018, 560, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Tochitsky, I.; Kienzler, M.A.; Isacoff, E.; Kramer, R.H. Restoring Vision to the Blind with Chemical Photoswitches. Chem. Rev. 2018, 118, 10748–10773. [Google Scholar] [CrossRef]
- Hüll, K.; Benster, T.; Manookin, M.B.; Trauner, D.; Gelder, R.N.V.; Laprell, L. Photopharmacologic Vision Restoration Reduces Pathological Rhythmic Field Potentials in Blind Mouse Retina. Sci. Rep. 2019, 9, 13561. [Google Scholar] [CrossRef]
- Kralik, J.; Kleinlogel, S. Functional availability of on-bipolar cells in the degenerated retina: Timing and longevity of an optogenetic gene therapy. Int. J. Mol. Sci. 2021, 22, 11515. [Google Scholar] [CrossRef]
- Baker, C.K.; Flannery, J.G. Innovative Optogenetic Strategies for Vision Restoration. Front. Cell. Neurosci. 2018, 12, 316. [Google Scholar] [CrossRef]
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Degli Esposti, S.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Isacoff, E.Y.; Flannery, J.G. Optogenetic Vision Restoration Using Rhodopsin for Enhanced Sensitivity. Mol. Ther. 2015, 23, 1562–1571. [Google Scholar] [CrossRef]
- van Wyk, M.; Pielecka-Fortuna, J.; Löwel, S.; Kleinlogel, S. Restoring the ON Switch in Blind Retinas: Opto-mGluR6, a Next-Generation, Cell-Tailored Optogenetic Tool. PLoS Biol. 2015, 13, e1002143. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Eleftheriou, C.; Allen, A.E.; Milosavljevic, N.; Pienaar, A.; Bedford, R.; Davis, K.E.; Bishop, P.N.; Lucas, R.J. Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr. Biol. 2015, 25, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Kralik, J.; van Wyk, M.; Stocker, N.; Kleinlogel, S. Bipolar cell targeted optogenetic gene therapy restores parallel retinal signaling and high-level vision in the degenerated retina. Commun. Biol. 2022, 5, 1116. [Google Scholar] [CrossRef] [PubMed]
- Strettoi, E.; Pignatelli, V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2000, 97, 11020–11025. [Google Scholar] [CrossRef] [PubMed]
- Strettoi, E.; Porciatti, V.; Falsini, B.; Pignatelli, V.; Rossi, C. Morphological and Functional Abnormalities in the Inner Retina of the rd/rd Mouse. J. Neurosci. 2002, 22, 5492–5504. [Google Scholar] [CrossRef]
- Menzler, J.; Zeck, G. Neurobiology of Disease Network Oscillations in Rod-Degenerated Mouse Retinas. J. Neurosci. 2011, 31, 2280–2291. [Google Scholar] [CrossRef]
- Dagar, S.; Nagar, S.; Goel, M.; Cherukuri, P.; Dhingra, N.K. Loss of photoreceptors results in upregulation of synaptic proteins in bipolar cells and amacrine cells. PLoS ONE 2014, 9, e90250. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B.W.; Anderson, J.R.; Kinard, K.; Marshak, D.W.; Wilson, J.H.; Wensel, T.; Lucas, R.J. Neural reprogramming in retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3364–3371. [Google Scholar] [CrossRef]
- Soto, F.; Kerschensteiner, D. Synaptic remodeling of neuronal circuits in early retinal degeneration. Front. Cell. Neurosci. 2015, 9, 395. [Google Scholar] [CrossRef]
- Pfeiffer, R.L.; Marc, R.E.; Jones, B.W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog. Retin. Eye Res. 2020, 74, 100771. [Google Scholar] [CrossRef]
- Chua, J.; Fletcher, E.L.; Kalloniatis, M. Functional remodeling of glutamate receptors by inner retinal neurons occurs from an early stage of retinal degeneration. J. Comp. Neurol. 2009, 514, 473–491. [Google Scholar] [CrossRef]
- Schilardi, G.; Kleinlogel, S. Two Functional Classes of Rod Bipolar Cells in the Healthy and Degenerated Optogenetically Treated Murine Retina. Front. Cell. Neurosci. 2022, 15, 562. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Müller, F. Retinal bipolar cell types differ in their inventory of ion channels. Vis. Neurosci. 2006, 23, 143–154. [Google Scholar] [CrossRef]
- Hook, M.J.V.; Nawy, S.; Thoreson, W.B. Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog. Retin. Eye Res. 2019, 72, 100760. [Google Scholar] [CrossRef] [PubMed]
- Wässle, H.; Yamashita, M.; Greferath, U.; Grünert, U.; Müller, F. The rod bipolar cell of the mammalian retina. Vis. Neurosci. 1991, 7, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, D.J.; Song, E.J.; Ito, S.; Sheng, M.H.; Jan, L.Y.; Pinto, L.H. The Shaker-Like Potassium Channels of the Mouse Rod Bipolar Cell and Their Contributions to the Membrane Current. J. Neurosci. 1995, 15, 5004–5013. [Google Scholar] [CrossRef]
- van Wyk, M.; Schneider, S.; Kleinlogel, S. Variable phenotypic expressivity in inbred retinal degeneration mouse lines: A comparative study of C3H/HeOu and FVB/N rd1 mice. Mol. Vis. 2015, 21, 811–827. [Google Scholar]
- Chang, B. Mouse models for studies of retinal degeneration and diseases. In Retinal Degeneration; Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 2013; Volume 935, pp. 27–39. [Google Scholar] [CrossRef]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Hulliger, E.C.; Hostettler, S.M.; Kleinlogel, S. Empowering Retinal Gene Therapy with a Specific Promoter for Human Rod and Cone ON-Bipolar Cells. Mol. Ther. Methods Clin. Dev. 2020, 17, 505–519. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Wang, Y.; Schlichter, L.C. Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ. 2010, 17, 134–144. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Schlichter, L.C. Targeting KV channels rescues retinal ganglion cells in vivo directly and by reducing inflammation. Channels 2010, 4, 337–346. [Google Scholar] [CrossRef][Green Version]
- Walston, S.T.; Chow, R.H.; Weiland, J.D. Direct Measurement of Bipolar Cell Responses to Electrical Stimulation in Wholemount Mouse Retina. J. Neural Eng. 2018, 15, 046003. [Google Scholar] [CrossRef] [PubMed]
- Euler, T.; Wässle, H. Immunocytochemical identification of cone bipolar cells in the rat retina. J. Comp. Neurol. 1995, 361, 461–478. [Google Scholar] [CrossRef]
- Euler, T.; Masland, R.H. Light-Evoked Responses of Bipolar Cells in a Mammalian Retina. J. Neurophysiol. 2000, 83, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.X.; Nicol, G.D. Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons. J. Neurophysiol. 2007, 98, 2683–2692. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhang, R.; Liu, Y.; Feng, J.; Wang, W.; Wu, Y.; Ding, J. Physiological role of Kv1.3 channel in T lymphocyte cell investigated quantitatively by kinetic modeling. PLoS ONE 2014, 9, e89975. [Google Scholar] [CrossRef]
- Gilhooley, M.J.; Hickey, D.G.; Lindner, M.; Palumaa, T.; Hughes, S.; Peirson, S.N.; MacLaren, R.E.; Hankins, M.W. ON-bipolar cell gene expression during retinal degeneration: Implications for optogenetic visual restoration. Exp. Eye Res. 2021, 207, 108553. [Google Scholar] [CrossRef]
- Hargrove-Grimes, P.; Mondal, A.K.; Gumerson, J.; Nellissery, J.; Aponte, A.M.; Gieser, L.; Qian, H.; Fariss, R.N.; Bonifacino, J.S.; Li, T.; et al. Loss of endocytosis-associated RabGEF1 causes aberrant morphogenesis and altered autophagy in photoreceptors leading to retinal degeneration. PLoS Genet. 2020, 16, e1009259. [Google Scholar] [CrossRef]
- Bales, K.L.; Gross, A.K. Aberrant protein trafficking in retinal degeneration: The initial phase of retinal remodelling. Exp. Eye Res. 2016, 150, 71–80. [Google Scholar] [CrossRef]
- McLaughlin, T.; Medina, A.; Perkins, J.; Yera, M.; Wang, J.J.; Zhang, S.X. Cellular stress signaling and the unfolded protein response in retinal degeneration: Mechanisms and therapeutic implications. Mol. Neurodegener. 2022, 17, 25. [Google Scholar] [CrossRef]
- Gayet-Primo, J.; Puthussery, T. Alterations in kainate receptor and TRPM1 localization in bipolar cells after retinal photoreceptor degeneration. Front. Cell. Neurosci. 2015, 9, 486. [Google Scholar] [CrossRef]
- Xu, Y.; Dhingra, A.; Fina, M.E.; Koike, C.; Furukawa, T.; Vardi, N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J. Neurophysiol. 2012, 107, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.J.; Cotter, T.G. Oxidative stress induces caspase-independent retinal apoptosis in vitro. Cell Death Differ. 2000, 7, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Pliego, J.A.F.; Pedroarena, C.M. Kv1 potassium channels control action potential firing of putative GABAergic deep cerebellar nuclear neurons. Sci. Rep. 2020, 10, 6954. [Google Scholar] [CrossRef] [PubMed]
- Gazula, V.R.; Strumbos, J.G.; Mei, X.; Chen, H.; Rahner, C.; Kaczmarek, L.K. Localization of Kv1.3 channels in presynaptic terminals of brainstem auditory neurons. J. Comp. Neurol. 2010, 518, 3205–3220. [Google Scholar] [CrossRef]
- Shen, Y.; Rampino, M.A.F.; Carroll, R.C.; Nawy, S. G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gβγ dimer. Proc. Natl. Acad. Sci. USA 2012, 109, 8752–8757. [Google Scholar] [CrossRef]
- Oancea, E.; Vriens, J.; Brauchi, S.; Jun, J.; Splawski, I.; Clapham, D.E. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci. Signal. 2009, 2, ra21. [Google Scholar] [CrossRef]
- Ramesha, S.; Rayaprolu, S.; Bowen, C.A.; Giver, C.R.; Bitarafan, S.; Nguyen, H.M.; Gao, T.; Chen, M.J.; Nwabueze, N.; Dammer, E.B.; et al. Unique Molecular Characteristics and Microglial Origin of Kv1.3 Channel–Positive Brain Myeloid Cells in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2013545118. Available online: https://www.pnas.org/doi/full/10.1073/pnas.2013545118 (accessed on 10 May 2023). [CrossRef]
- Pérez-García, M.T.; Cidad, P.; López-López, J.R. The secret life of ion channels: Kv1.3 potassium channels and proliferation. Am. J. Physiol. Cell Physiol. 2018, 314, 27–42. [Google Scholar] [CrossRef]
- Han, J.; Dinculescu, A.; Dai, X.; Du, W.; Smith, W.C.; Pang, J. Review: The history and role of naturally occurring mouse models with Pde6b mutations. Mol. Vis. 2013, 19, 2579–2589. [Google Scholar]
- Arman, A.C.; Sampath, A.P. Patch Clamp Recordings from Mouse Retinal Neurons in a Dark-adapted Slice Preparation. J. Vis. Exp. JoVE 2010, 43, e2107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilardi, G.; Kralik, J.; Kleinlogel, S. Selective Block of Upregulated Kv1.3 Potassium Channels in ON-Bipolar Cells of the Blind Retina Enhances Optogenetically Restored Signaling. Int. J. Mol. Sci. 2023, 24, 14207. https://doi.org/10.3390/ijms241814207
Schilardi G, Kralik J, Kleinlogel S. Selective Block of Upregulated Kv1.3 Potassium Channels in ON-Bipolar Cells of the Blind Retina Enhances Optogenetically Restored Signaling. International Journal of Molecular Sciences. 2023; 24(18):14207. https://doi.org/10.3390/ijms241814207
Chicago/Turabian StyleSchilardi, Giulia, Jakub Kralik, and Sonja Kleinlogel. 2023. "Selective Block of Upregulated Kv1.3 Potassium Channels in ON-Bipolar Cells of the Blind Retina Enhances Optogenetically Restored Signaling" International Journal of Molecular Sciences 24, no. 18: 14207. https://doi.org/10.3390/ijms241814207
APA StyleSchilardi, G., Kralik, J., & Kleinlogel, S. (2023). Selective Block of Upregulated Kv1.3 Potassium Channels in ON-Bipolar Cells of the Blind Retina Enhances Optogenetically Restored Signaling. International Journal of Molecular Sciences, 24(18), 14207. https://doi.org/10.3390/ijms241814207





