Genetic Characteristics and Long-Term Follow-Up of Slovenian Patients with RPGR Retinal Dystrophy
Abstract
1. Introduction
1.1. Molecular Genetics of the RPGR Gene
1.2. Retinal Dystrophies Associated with the RPGR Gene
2. Results
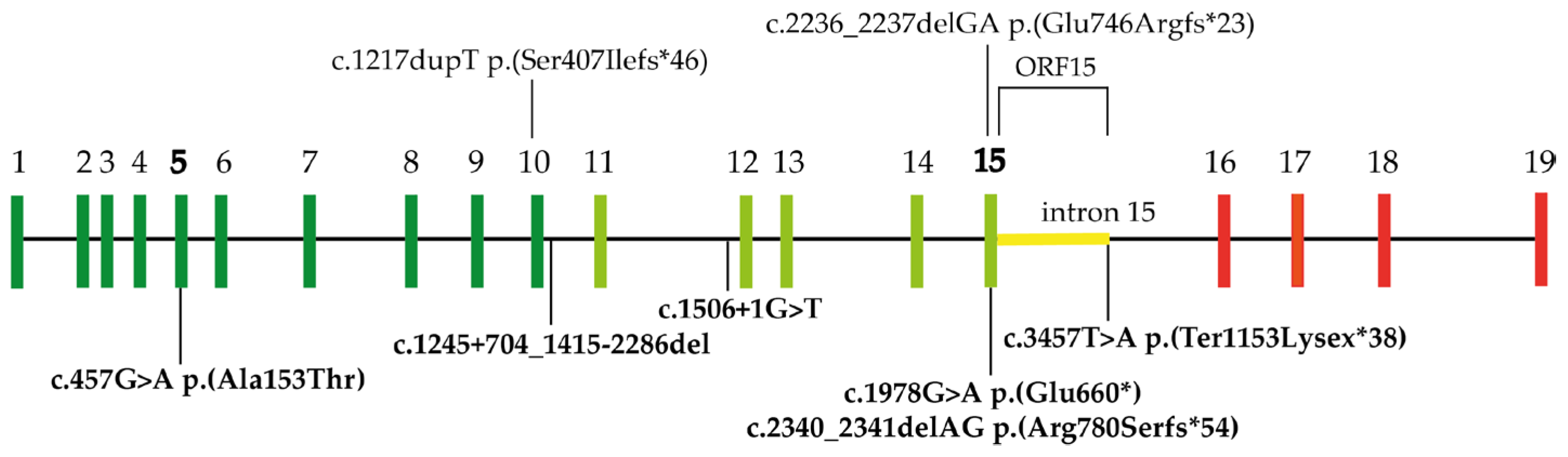
2.1. Genetic Findings
2.2. Clinical Findings
2.2.1. Retinitis Pigmentosa
Male RP Patients
Female RP Patients
2.2.2. Cone Dystrophy
2.2.3. Comparison between Male RP and male COD Patients
3. Discussion
3.1. RPGR Variants Identified in the Slovenian Cohort
3.2. Phenotypes Observed in the Slovenian RPGR Cohort
3.3. Females Harboring RPGR Variants
3.4. Pathogenesis of RPGR-Associated Retinal Dystrophies
3.5. Significance and Novelties of the Slovenian RPGR Study
4. Materials and Methods
4.1. Patients
4.2. Genetic and Bioinformatic Analysis
4.3. Clinical Examination
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RPGR | Retinitis pigmentosa GTPase regulator |
| RP | retinitis pigmentosa |
| COD | Cone dystrophy |
| CORD | Cone-rod dystrophy |
| GEF | Guanine exchange factor |
| GTP | Guanosine triphosphate |
| ORF | Open reading frame |
| TTLL5 | Tubulin tyrosine ligase like 5 |
| XLRP | X-linked retinitis pigmentosa |
| VF | Visual field |
| VA | Visual acuity |
| FAF | Fundus autofluorescence |
| RPE | Retinal pigment epithelium |
| BCVA | Best corrected visual acuity |
| ERG | Electroretinography |
| BE | Both eyes |
| RE | Right eye |
| LE | Left eye |
| F | Female |
| M | Male |
| OCT | Optical coherent tomography |
| N/A | Not available |
| DA | Dark adapted |
| LA | Light adapted |
| PERG | Pattern ERG |
| mfERG | Multifocal ERG |
| ISe | Inner segment ellipsoid |
| ELM | External limiting membrane |
| ONL | Outer nuclear layer |
| BM | Bruch’s membrane |
| HM | Hand movement |
| LP | Light perception |
| LOVD | Leiden Open Variation Database |
| RCC1 | Regulator of chromosome condensation |
| USH2A | Usherin |
| BEST1 | Bestrophin 1 |
| ABCA4 | ATP binding cassette subfamily A member 4 |
| DRAM2 | DNA damage-regulated autophagy modulator protein 2 |
| RHO | Rhodopsin |
| RLD | RCC1-like domain |
| ROS | Reactive oxygen species |
| RNA | Ribonucleic acid |
| RPGRIP1 | RPGR interacting protein 1 |
| CEP290 | Centrosomal protein 290 |
| NPHP5 | Nephrocystin 5 |
| NPHP6 | Nephrocystin 6 |
References
- Meindl, A.; Dry, K.; Herrmann, K.; Manson, F.; Ciccodicola, A.; Edgar, A. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nat. Genet. 1996, 13, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Roepman, R.; van Duijnhoven, G.; Rosenberg, T.; Pinckers, A.J.; Bleeker-Wagemakers, L.M.; Bergen, A.A. Positional cloning of the gene for X-linked retinitis pigmentosa 3: Homology with the guanine-nucleotide-exchange factor RCC1. Hum. Mol. Genet. 1996, 7, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.H.; Pawlyk, B.; Sokolov, M.; Strissel, K.J.; Yang, J.; Tulloch, B.; Wright, A.F.; Arshavsky, V.Y.; Li, T. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Fry, A.M.; Tulloch, B.; Manson, F.D.; Crabb, J.W.; Khanna, H.; Faragher, A.J.; Lennon, A.; He, S.; Trojan, P.; et al. RPGR ORF15 isoform co-localizes with RPGRIP1 at centrioles and basal bodies and interacts with nucleophosmin. Hum. Mol. Genet. 2005, 14, 1183–1197. [Google Scholar] [CrossRef]
- Gakovic, M.; Shu, X.; Kasioulis, I.; Carpanini, S.; Moraga, I.; Wright, A.F. The role of RPGR in cilia formation and actin stability. Hum. Mol. Genet. 2011, 20, 4840–4850. [Google Scholar] [CrossRef]
- Khanna, H. More than Meets the Eye: Current Understanding of RPGR Function. Adv. Exp. Med. Biol. 2018, 1074, 521–538. [Google Scholar] [CrossRef]
- Megaw, R.D.; Soares, D.C.; Wright, A.F. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 2015, 138, 32–41. [Google Scholar] [CrossRef]
- Rao, K.N.; Li, L.; Anand, M.; Khanna, H. Ablation of retinal ciliopathy protein RPGR results in altered photoreceptor ciliary composition. Sci. Rep. 2015, 5, 11137. [Google Scholar] [CrossRef]
- Hong, D.H.; Pawlyk, B.S.; Shang, J.; Sandberg, M.A.; Berson, E.L.; Li, T. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc. Natl. Acad. Sci. USA 2000, 97, 3649–3654. [Google Scholar] [CrossRef]
- Rao, K.N.; Li, L.; Zhang, W.; Brush, R.S.; Rajala, R.V.; Khanna, H. Loss of human disease protein retinitis pigmentosa GTPase regulator (RPGR) differentially affects rod or cone-enriched retina. Hum. Mol. Genet. 2016, 25, 1345–1356. [Google Scholar] [CrossRef]
- Vössing, C.; Atigbire, P.; Eilers, J.; Markus, F.; Stieger, K.; Song, F.; Neidhardt, J. The Major Ciliary Isoforms of RPGR Build Different Interaction Complexes with INPP5E and RPGRIP1L. Int. J. Mol. Sci. 2021, 22, 3583. [Google Scholar] [CrossRef]
- He, S.; Parapuram, S.K.; Hurd, T.W.; Behnam, B.; Margolis, B.; Swaroop, A.; Khanna, H. Retinitis Pigmentosa GTPase Regulator (RPGR) protein isoforms in mammalian retina: Insights into X-linked Retinitis Pigmentosa and associated ciliopathies. Vision Res. 2008, 48, 366–376. [Google Scholar] [CrossRef]
- Hong, D.H.; Li, T. Complex expression pattern of RPGR reveals a role for purine-rich exonic splicing enhancers. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3373–3382. [Google Scholar]
- Hosch, J.; Lorenz, B.; Stieger, K. RPGR: Role in the photoreceptor cilium, human retinal disease, and gene therapy. Ophthalmic. Genet. 2011, 32, 1–11. [Google Scholar] [CrossRef]
- Schmid, F.; Glaus, E.; Cremers, F.P.; Kloeckener-Gruissem, B.; Berger, W.; Neidhardt, J. Mutation- and tissue-specific alterations of RPGR transcripts. Investig. Ophthalmol. Vis. Sci. 2010, 3, 1628–1635. [Google Scholar] [CrossRef]
- Sun, X.; Park, J.H.; Gumerson, J.; Wu, Z.; Swaroop, A.; Qian, H.; Roll-Mecak, A.; Li, T. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc. Natl. Acad. Sci. USA 2016, 113, E2925–E2934. [Google Scholar] [CrossRef]
- Sharon, D.; Sandberg, M.A.; Rabe, V.W.; Stillberger, M.; Dryja, T.P.; Berson, E.L. RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am. J. Hum. Genet. 2003, 73, 1131–1146. [Google Scholar] [CrossRef]
- Talib, M.; van Schooneveld, M.J.; Thiadens, A.A.; Fiocco, M.; Wijnholds, J.; Florijn, R.J.; Schalij-Delfos, N.E.; van Genderen, M.M.; Putter, H.; Cremers, F.P.M.; et al. Clinical and genetic characteristics of male patients with rpgr-associated retinal dystrophies: A Long-Term Follow-up Study. Retina 2019, 39, 1186–1199. [Google Scholar] [CrossRef]
- Bader, I.; Brandau, O.; Achatz, H.; Apfelstedt-Sylla, E.; Hergersberg, M.; Lorenz, B.; Wissinger, B.; Wittwer, B.; Rudolph, G.; Meindl, A.; et al. X-linked retinitis pigmentosa: RPGR mutations in most families with definite X linkage and clustering of mutations in a short sequence stretch of exon ORF15. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1458–1463. [Google Scholar] [CrossRef]
- Tee, J.L.; Smith, A.J.; Hardcastle, A.J.; Michaelides, M. RPGR-associated retinopathy: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2016, 100, 1022–1027. [Google Scholar] [CrossRef]
- De Silva, S.R.; Arno, G.; Robson, A.G.; Fakin, A.; Pontikos, N.; Mohamed, M.D.; Bird, A.C.; Moore, A.T.; Michaelides, M.; Webster, A.R.; et al. The X-linked retinopathies: Physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog. Retin Eye Res. 2021, 82, 100898. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, R.; Lennon, A.; Bird, A.C.; Tulloch, B.; Axton, R.; Miano, M.G.; Meindl, A.; Meitinger, T.; Ciccodicola, A.; Wright, A.F. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat. Genet. 2000, 25, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Fishman, G.A.; Farber, M.D.; Derlacki, D.J. X-linked retinitis pigmentosa. Profile of clinical findings. Arch. Ophthalmol. 1988, 106, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Lehr, A.W.; Roche, K.W. Neuroligins and Neurodevelopmental Disorders: X-Linked Genetics. Front. Synaptic. Neurosci. 2020, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, J.; Heath Jeffery, R.C.; Thompson, J.A.; Charng, J.; Chelva, E.S.; Constable, I.J.; McLaren, T.L.; Lamey, T.M.; De Roach, J.N.; Chen, F.K. Progressive sector retinitis pigmentosa due to c.440G>T mutation in. Ophthalmic. Genet. 2021, 42, 62–70. [Google Scholar] [CrossRef]
- Sandberg, M.A.; Rosner, B.; Weigel-DiFranco, C.; Dryja, T.P.; Berson, E.L. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1298–1304. [Google Scholar] [CrossRef]
- Birtel, J.; Gliem, M.; Mangold, E.; Müller, P.L.; Holz, F.G.; Neuhaus, C.; Lenzner, S.; Zahnleiter, D.; Betz, C.; Eisenberger, T.; et al. Next-generation sequencing identifies unexpected genotype-phenotype correlations in patients with retinitis pigmentosa. PLoS ONE 2018, 13, e0207958. [Google Scholar] [CrossRef]
- Gill, J.S.; Georgiou, M.; Kalitzeos, A.; Moore, A.T.; Michaelides, M. Progressive cone and cone-rod dystrophies: Clinical features, molecular genetics and prospects for therapy. Br. J. Ophthalmol. 2019, 103, 711–720. [Google Scholar] [CrossRef]
- Ebenezer, N.D.; Michaelides, M.; Jenkins, S.A.; Audo, I.; Webster, A.R.; Cheetham, M.E.; Stockman, A.; Maher, E.R.; Ainsworth, J.R.; Yates, J.R.; et al. Identification of novel RPGR ORF15 mutations in X-linked progressive cone-rod dystrophy (XLCORD) families. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1891–1898. [Google Scholar] [CrossRef]
- Thiadens, A.A.; Soerjoesing, G.G.; Florijn, R.J.; Tjiam, A.G.; den Hollander, A.I.; van den Born, L.I.; Riemslag, F.C.; Bergen, A.A.; Klaver, C.C. Clinical course of cone dystrophy caused by mutations in the RPGR gene. Graefes. Arch. Clin. Exp. Ophthalmol. 2011, 249, 1527–1535. [Google Scholar] [CrossRef]
- Nassisi, M.; De Bartolo, G.; Mohand-Said, S.; Condroyer, C.; Antonio, A.; Lancelot, M.E.; Bujakowska, K.; Smirnov, V.; Pugliese, T.; Neidhardt, J.; et al. Retrospective Natural History Study of RPGR-Related Cone- and Cone-Rod Dystrophies While Expanding the Mutation Spectrum of the Disease. Int. J. Mol. Sci. 2022, 23, 7189. [Google Scholar] [CrossRef]
- Georgiou, M.; Awadh Hashem, S.; Daich Varela, M.; Michaelides, M. Gene Therapy in X-linked Retinitis Pigmentosa Due to Defects in RPGR. Int. Ophthalmol. Clin. 2021, 61, 97–108. [Google Scholar] [CrossRef]
- Fahim, A.T.; Daiger, S.P. The Role of X-Chromosome Inactivation in Retinal Development and Disease. Adv. Exp. Med. Biol. 2016, 854, 325–331. [Google Scholar] [CrossRef]
- Nanda, A.; Salvetti, A.P.; Clouston, P.; Downes, S.M.; MacLaren, R.E. Exploring the Variable Phenotypes of RPGR Carrier Females in Assessing their Potential for Retinal Gene Therapy. Genes 2018, 9, 643. [Google Scholar] [CrossRef]
- Comander, J.; Weigel-DiFranco, C.; Sandberg, M.A.; Berson, E.L. Visual Function in Carriers of X-Linked Retinitis Pigmentosa. Ophthalmology 2015, 122, 1899–1906. [Google Scholar] [CrossRef]
- Grover, S.; Fishman, G.A.; Anderson, R.J.; Lindeman, M. A longitudinal study of visual function in carriers of X-linked recessive retinitis pigmentosa. Ophthalmology 2000, 107, 386–396. [Google Scholar] [CrossRef]
- Talib, M.; van Schooneveld, M.J.; Van Cauwenbergh, C.; Wijnholds, J.; Ten Brink, J.B.; Florijn, R.J.; Schalij-Delfos, N.E.; Dagnelie, G.; van Genderen, M.M.; De Baere, E.; et al. The Spectrum of Structural and Functional Abnormalities in Female Carriers of Pathogenic Variants in the RPGR Gene. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4123–4133. [Google Scholar] [CrossRef]
- Hadalin, V.; Šuštar, M.; Volk, M.; Maver, A.; Sajovic, J.; Jarc-Vidmar, M.; Peterlin, B.; Hawlina, M.; Fakin, A. Cone Dystrophy Associated with a Novel Variant in the Terminal Codon of the RPGR-ORF15. Genes 2021, 12, 499. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Ouyang, J.; Xiao, X.; Sun, W.; Li, S.; Zhang, Q. Genotype-Phenotype Analysis of. Front. Genet. 2021, 12, 600210. [Google Scholar] [CrossRef]
- Tuupanen, S.; Gall, K.; Sistonen, J.; Saarinen, I.; Kämpjärvi, K.; Wells, K.; Merkkiniemi, K.; von Nandelstadh, P.; Sarantaus, L.; Känsäkoski, J.; et al. Prevalence of RPGR-Mediated Retinal Dystrophy in an Unselected Cohort of over 5000 Patients. Transl. Vis. Sci. Technol. 2022, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Branham, K.; Othman, M.; Brumm, M.; Karoukis, A.J.; Atmaca-Sonmez, P.; Yashar, B.M.; Schwartz, S.B.; Stover, N.B.; Trzupek, K.; Wheaton, D.; et al. Mutations in RPGR and RP2 account for 15% of males with simplex retinal degenerative disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8232–8237. [Google Scholar] [CrossRef] [PubMed]
- Murga-Zamalloa, C.; Swaroop, A.; Khanna, H. Multiprotein complexes of Retinitis Pigmentosa GTPase regulator (RPGR), a ciliary protein mutated in X-linked Retinitis Pigmentosa (XLRP). Adv. Exp. Med. Biol. 2010, 664, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, V.; Karali, M.; Melillo, P.; Testa, F.; Brunetti-Pierri, R.; Musacchia, F.; Condroyer, C.; Neidhardt, J.; Audo, I.; Zeitz, C.; et al. Spectrum of Disease Severity in Patients with X-Linked Retinitis Pigmentosa Due to RPGR Mutations. Investig. Ophthalmol. Vis. Sci. 2020, 61, 36. [Google Scholar] [CrossRef]
- Zupan, A.; Fakin, A.; Battelino, S.; Jarc-Vidmar, M.; Hawlina, M.; Bonnet, C.; Petit, C.; Glavač, D. Clinical and Haplotypic Variability of Slovenian. Genes 2019, 10, 1015. [Google Scholar] [CrossRef]
- Glavač, D.; Jarc-Vidmar, M.; Vrabec, K.; Ravnik-Glavač, M.; Fakin, A.; Hawlina, M. Clinical and genetic heterogeneity in Slovenian patients with BEST disease. Acta Ophthalmol. 2016, 94, e786–e794. [Google Scholar] [CrossRef]
- Jaakson, K.; Zernant, J.; Külm, M.; Hutchinson, A.; Tonisson, N.; Glavac, D.; Ravnik-Glavac, M.; Hawlina, M.; Meltzer, M.R.; Caruso, R.C.; et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum. Mutat. 2003, 22, 395–403. [Google Scholar] [CrossRef]
- Krašovec, T.; Volk, M.; Šuštar Habjan, M.; Hawlina, M.; Vidović Valentinčič, N.; Fakin, A. The Clinical Spectrum and Disease Course of DRAM2 Retinopathy. Int. J. Mol. Sci. 2022, 23, 7398. [Google Scholar] [CrossRef]
- Demirci, F.Y.; Rigatti, B.W.; Wen, G.; Radak, A.L.; Mah, T.S.; Baic, C.L.; Traboulsi, E.I.; Alitalo, T.; Ramser, J.; Gorin, M.B. X-linked cone-rod dystrophy (locus COD1): Identification of mutations in RPGR exon ORF15. Am. J. Hum. Genet. 2002, 70, 1049–1053. [Google Scholar] [CrossRef]
- Zahid, S.; Khan, N.; Branham, K.; Othman, M.; Karoukis, A.J.; Sharma, N.; Moncrief, A.; Mahmood, M.N.; Sieving, P.A.; Swaroop, A.; et al. Phenotypic conservation in patients with X-linked retinitis pigmentosa caused by RPGR mutations. JAMA Ophthalmol. 2013, 131, 1016–1025. [Google Scholar] [CrossRef]
- Ayyagari, R.; Demirci, F.Y.; Liu, J.; Bingham, E.L.; Stringham, H.; Kakuk, L.E.; Boehnke, M.; Gorin, M.B.; Richards, J.E.; Sieving, P.A. X-linked recessive atrophic macular degeneration from RPGR mutation. Genomics 2002, 80, 166–171. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Tee, J.J.L.; Kalitzeos, A.; Webster, A.R.; Peto, T.; Michaelides, M. Quantitative analysis of hyperautofluorescent rings to characterize the natural history and progression in rpgr-associated retinopathy. Retina 2018, 38, 2401–2414. [Google Scholar] [CrossRef]
- Tee, J.J.L.; Yang, Y.; Kalitzeos, A.; Webster, A.; Bainbridge, J.; Michaelides, M. Natural History Study of Retinal Structure, Progression, and Symmetry Using Ellipzoid Zone Metrics in RPGR-Associated Retinopathy. Am. J. Ophthalmol. 2019, 198, 111–123. [Google Scholar] [CrossRef]
- Andréasson, S.; Breuer, D.K.; Eksandh, L.; Ponjavic, V.; Frennesson, C.; Hiriyanna, S.; Filippova, E.; Yashar, B.M.; Swaroop, A. Clinical studies of X-linked retinitis pigmentosa in three Swedish families with newly identified mutations in the RP2 and RPGR-ORF15 genes. Ophthalmic. Genet. 2003, 24, 215–223. [Google Scholar] [CrossRef]
- Robson, A.G.; Saihan, Z.; Jenkins, S.A.; Fitzke, F.W.; Bird, A.C.; Webster, A.R.; Holder, G.E. Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br. J. Ophthalmol. 2006, 90, 472–479. [Google Scholar] [CrossRef]
- Robson, A.G.; Michaelides, M.; Luong, V.A.; Holder, G.E.; Bird, A.C.; Webster, A.R.; Moore, A.T.; Fitzke, F.W. Functional correlates of fundus autofluorescence abnormalities in patients with RPGR or RIMS1 mutations causing cone or cone rod dystrophy. Br. J. Ophthalmol. 2008, 92, 95–102. [Google Scholar] [CrossRef]
- Aizawa, S.; Mitamura, Y.; Baba, T.; Hagiwara, A.; Ogata, K.; Yamamoto, S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye (Lond) 2009, 23, 304–308. [Google Scholar] [CrossRef]
- Fakin, A.; Jarc-Vidmar, M.; Glavač, D.; Bonnet, C.; Petit, C.; Hawlina, M. Fundus autofluorescence and optical coherence tomography in relation to visual function in Usher syndrome type 1 and 2. Vision Res. 2012, 75, 60–70. [Google Scholar] [CrossRef]
- Lima, L.H.; Zett, C.; Kniggendorf, V.; Marianelli, B.; de Carvalho, R.A.P.; Farah, M.E.; Sallum, J.M.F. Progressive expansion of the hyperautofluorescent ring in cone-rod dystrophy patients. Ophthalmic. Genet. 2018, 39, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.G.; Michaelides, M.; Saihan, Z.; Bird, A.C.; Webster, A.R.; Moore, A.T.; Fitzke, F.W.; Holder, G.E. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc. Ophthalmol. 2008, 116, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Skosyrski, S.; Kirschner-Schwabe, R.; Knobeloch, K.P.; Neidhardt, J.; Feil, S.; Glaus, E.; Luhmann, U.F.; Rüther, K.; Berger, W. Cone versus rod disease in a mutant Rpgr mouse caused by different genetic backgrounds. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Carver, K.A.; Yang, D. N-Acetylcysteine Amide Protects against Oxidative Stress-Induced Microparticle Release from Human Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 360–371. [Google Scholar] [CrossRef]
- Shen, J.; Yang, X.; Dong, A.; Petters, R.M.; Peng, Y.W.; Wong, F.; Campochiaro, P.A. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell Physiol. 2005, 203, 457–464. [Google Scholar] [CrossRef]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of daylight vision in retinal degeneration: Are oxidative stress and metabolic dysregulation to blame? J. Biol. Chem. 2012, 287, 1642–1648. [Google Scholar] [CrossRef]
- Roepman, R.; Wolfrum, U. Protein networks and complexes in photoreceptor cilia. Subcell Biochem. 2007, 43, 209–235. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Epitranscriptome Analysis of Oxidative Stressed Retinal Epithelial Cells Depicted a Possible RNA Editing Landscape of Retinal Degeneration. Antioxidants 2022, 11, 1967. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Meynert, A.M.; Bicknell, L.S.; Hurles, M.E.; Jackson, A.P.; Taylor, M.S. Quantifying single nucleotide variant detection sensitivity in exome sequencing. BMC Bioinform. 2013, 14, 195. [Google Scholar] [CrossRef]
- Hawlina, M.; Konec, B. New noncorneal HK-loop electrode for clinical electroretinography. Doc. Ophthalmol. 1992, 81, 253–259. [Google Scholar] [CrossRef]
- Robson, A.G.; Frishman, L.J.; Grigg, J.; Hamilton, R.; Jeffrey, B.G.; Kondo, M.; Li, S.; McCulloch, D.L. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc. Ophthalmol. 2022, 144, 165–177. [Google Scholar] [CrossRef]
- Robson, A.G.; Nilsson, J.; Li, S.; Jalali, S.; Fulton, A.B.; Tormene, A.P.; Holder, G.E.; Brodie, S.E. ISCEV guide to visual electrodiagnostic procedures. Doc. Ophthalmol. 2018, 136, 1–26. [Google Scholar] [CrossRef]
- Hood, D.C.; Bach, M.; Brigell, M.; Keating, D.; Kondo, M.; Lyons, J.S.; Marmor, M.F.; McCulloch, D.L.; Palmowski-Wolfe, A.M. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc. Ophthalmol. 2012, 124, 1–13. [Google Scholar] [CrossRef]



















| Family and Patient ID (Center ID) | Sex | Phenotype | RPGR Variant | Variant Published (Author) | Age at the First and Last Examination (years) | Age at Onset: Symptoms | Ishihara at First and Last Examination | Refraction (Dioptre) | BCVA at First and Last Examination: Snellen Decimal (logMAR) | Visual Field at First and Last Examination (°)2 | Fundus Features | FAF | OCT | Horizontal Ise Loss per Year (μm)] | Ring Area at First and Last Examination (mm2) | ERG | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BE | RE | LE | RE | LE | BE | RE | LE | RE | LE | |||||||||||
| F1P1 (00713) | M | RP | c.1978G>A p.(Glu660*) | No | 18; 39 | 18 | 1/15 (0/15) | −4.75 (N/A) | −3.75–1.25/180° (N/A) | 0.6 (0.22); 0.16 (0.79) | 0.7 (0.15); 0.2 (0.69) | BE: Constricted central ring; RE: 29918 (20752); LE: 14811 (14218) | BE: Optic pallor, attenuated and sclerotic vessels, bone spicules in the peripheral retina, atrophic central retina | Hyperautofluorescent spots in the center surrounded by hyperautofluorescent ring | BE: Atrophic changes in the central retina | 54 | 48 | N/A | N/A | Was not performed |
| F1P2 (00714) | F | RPGR-RP | c.1978G>A p.(Glu660*) | No | 45; 66 | 45 | RE: 6/15 (1/15); LE 3/15 (0/15) | −1.0 (−1.0–0.75/105°) | 0 (+0.750.50/180°) | 1.0 (0); 0.4 (0.39) | 1.0 (0); 0.6 (0.22) | RE: Scotoma in upper half; LE: scotoma in temporal half; RE: 887528; LE: 251130 | BE: Optic pallor, attenuated and sclerotic vessels, maculas without reflex, atrophic central retina, bone spiclues in the peripheral retina | Focal pattern BE (sector RP) | RE: Remnants of ELM in the foveola; LE: absent RPE, Ise, and ELM in the central macula | 68 | 15 | 0.49 (852); 0.11 (246) | 0.25 (446); 0.18 (361) | BE: Reduced and delayed DA and LA ERG; RE: normal PERG P50 and mfERG; LE: reduced/undetectable PERG P50 and mfERG |
| F1P3 (01072) | F | RPGR-RP | c.1978G>A p.(Glu660*) | No | 10 | Asymptomatic | 15/15 | 0 | 0 | 1.0 (0) | 1.0 (0) | BE: Within normal values; RE: 2932589; LE: 2946630 | BE: Normal | Normal/near-normal pattern BE | BE: Within normal values | N/A | N/A | N/A | N/A | Was not performed |
| F2P4 (00563) | M | RP | c.1245+704_1415-2286del; ex-11del | No | 16; 27 | Childhood: night blindness, loss of peripheral vision | 15/15 (15/15) | −1.0–3.25/10° (−11.0–4.5/15°) | −1.0–3.25/175° (N/A) | 0.5 (0.3); 0.4 (0.39) | 0.5 (0.3); 0.4 (0.39) | BE: Constricted central ring; RE: 67738; LE: 62140 | BE: Optic pallor, attenuated vessels, maculas without reflex, bone spicules in the peripheral retina | Paramacular hyperautofluorescent ring | BE: Remnants of ELM in the foveola | 48 | 23 | 0.48 (901); 0.45 (689) | 0.44 (658); 0.3 (477) | BE: Undetectable DA and LA ERG; undetectable PERG P50 |
| F2P5 (00600) | F | RPGR-RP | c.1245+704_1415-2286del; ex-11del | No | 51; 55 | Childhood: refraction error (myopia) | RE: 15/15 (N/A); LE: 1/15 (N/A) | −9.0–4.0/17° (N/A) | N/A (N/A) | 1.0 (0); 0.7 (0.15) | 0.4 (0.4); 0.2 (0.69) | RE: Temporal scotoma; LE: constricted central ring; RE: 1102152 (176308); LE: 607884 (105834) | BE: Optic pallor, attenuated vessels, maculas without reflex, bone spicules in the peripheral retina | Focal pattern RE, male pattern LE | N/A | 38 | 39 | 0.41 (744); 0.32 (591) | 0.23 (609); 0.19 (462) | RE: Significantly reduced DA, LA ERG, and PERG; LE: undetectable DA and LA ERG and PERG P50 |
| F2P6 (00374) | M | RP | c.1245+704_1415-2286del; ex-11del | No | 8; 12 | Childhood (6 years): hyperopia | 15/15 (11/15) | +0.25 + 2.0/110° (+1.0+2.25/110°) | +2.25/70° (−2.75/75°) | 0.4 (0.40); 0.6 (0.22) | 0.5 (0.3); 0.7 (0.15) | BE: Constricted central ring | BE: Attenuated vessels, bone spicules in the peripheral retina | Hyperautofluorescent ring surrounding macula encircled by hypoautofluorescent ring | BE: Remnants of ELM in the foveola | N/A | N/A | 0.46 (597) | 0.42 (561) | BE: Undetectable LA, DA, and PERG P50 |
| F2P7 (00888) | F | RPGR-RP | c.1245+704_1415-2286del; ex-11del | No | 35 | Asymptomatic | RE: 13/15; LE: 14/15 | −1.0 | 0 | 1.0 (0) | 1.0 (0) | BE: Some spots of reduced sensitivity | BE: Within normal values | Normal/near-normal pattern BE | BE: Within normal values | N/A | N/A | N/A | N/A | Was not performed |
| F3P8 (00419) | M | RP | c.457G>A p.(Ala153Thr) | No | 35; 47 | 35 | N/A (1/15) | −6.5 + 0.25/5° (N/A) | −6.0 + 1.0/90° (N/A) | 0.7 (0.15); 0.6 (0.22) | 0.6 (0.22); 0.6 (0.22) | BE: Constricted central ring; central scotoma | BE: Optic pallor, paramacular atrophy, bone spicules in the peripheral retina | N/A | RE: Absent ELM, Ise, and RPE; LE: remnants of ELM | 85 | 127 | 0.42 (703); 0.28 (408) | 0.19 (507); 0.18 (360) | Was not performed |
| F4P9 (00595) | F | RPGR-RP | c.1217dupT p.(Ser407Ilefs*46) | No (submitted to ClinVar) | 6; 10 | Childhood (6 years): hyperopia | N/A | +3–4.50/11° (N/A) | +0.25–5.0/3° (N/A) | 0.5 (0.04); 0.9 (0.05) | 0.5 (0.30); 0.5 (0.30) | BE: Constricted central ring (concentric scotoma) | BE: Optic pallor, maculas without reflex | Radial pattern BE | BE: Absent ISe | N/A | N/A | N/A | N/A | BE: Reduced DA ERG and normal LA ERG, mfERG, and PERG P50 |
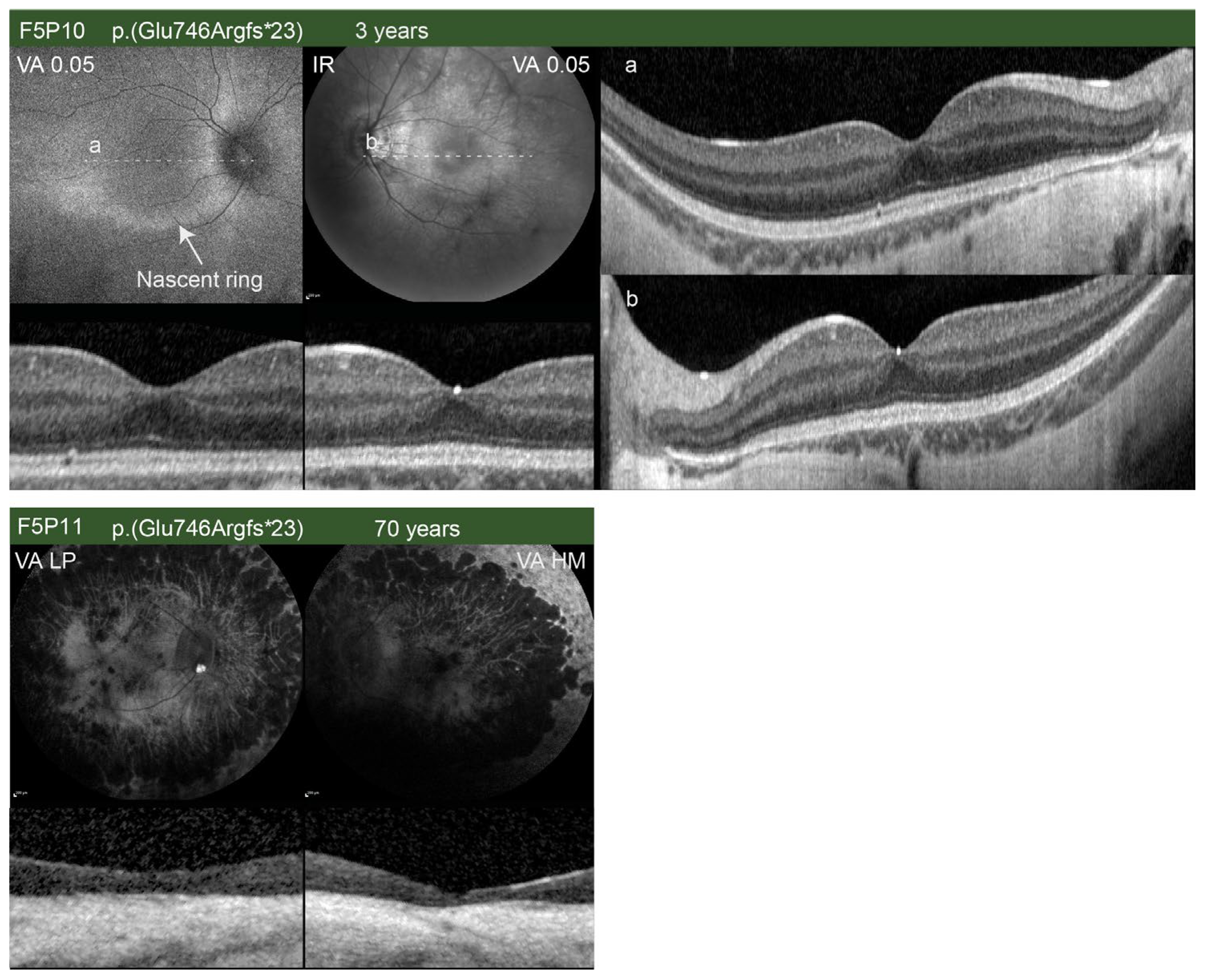
| F5P10 (00380) | M | RP | c.2236_2237delGA p.(Glu746Argfs*23) | Yes [22]) | Less than a year (11 months); 3 | Childhood | N/A | N/A (−9.0) | N/A (−10.0) | 6/130 (0.2); 6/24 (0.60) | 6/130 (0.30); 6/18 (0.47) | N/A | BE: Slight peripapillary atrophy, dystrophic retina | N/A | N/A | N/A | N/A | N/A | N/A | BE: Normal DA ERG; slightly reduced LA ERG |
| F5P11 (01054) | M | RP | c.2236_2237delGA p.(Glu746Argfs*23) | Yes [22] | 61; 70 | N/A | N/A | −11.0–4.50/15° (N/A) | −9.0–4.0/17° (N/A) | 0.01 (2.3); hand movement | 0.005 (2); hand movement | N/A | BE: Optic pallor, attenuated vessels, degenerative changes | N/A | BE: Absent RPE, Ise, and ELM in the central macula | N/A | N/A | N/A | N/A | BE: Undetectable LA and DA ERG |
| F6P12 (00585) | M | RP | c.1506+1G>T | No | 45; 50 | N/A | N/A | −10.0–3.50/140° (−11.0) | −11 (−11.0) | 0.3 (0.52); 0.1 (1) | 0.3 (0.52); 0.1 (1) | N/A | BE: Bone spicules in the peripheral retina | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| F6P13 (01053) | F | RPGR-RP | c.1506+1G>T | No | 66 | N/A | 12/15 | N/A | N/A | 0.6 (0.22) | 0.5 (0.3) | N/A | BE: Macula without reflex, attenuated vessels, bone spicules in the peripheral retina | Focal pattern BE | BE: Spared ISe, RPE, and ELM in the central macula; CME in the LE | N/A | N/A | N/A | N/A | N/A |
| F7P14 (00720) | M | RP | c.2340_2341delAG p.(Arg780Serfs*54) | No | 63 | Childhood | N/A | 0 | 0 | 0.005 (2.30) | 0.005 (2.30) | Could not be performed | BE: Optic pallor, attenuated vessels, chorioretinal atrophy, bone spicules | BE: retinal atrophy | BE: RPE and photoreceptor atrophy, centrally preserved RPE without photoreceptors | N/A | N/A | N/A | N/A | N/A |
| F8P15 (00552) | M | RP | c.1245+704_1415-2286del; ex-11del | No | 32; 39 | Childhood: night blindness, loss of peripheral vision | RE:2/15 (1/15); LE: 3/15 (0/15) | −1.0/40° (−0.5–1.50/37°) | −1.0/150° (−0.25–1.50/145°) | 0.7 p (0.5); 0.4 p (0.39) | 0.7 p (0.3); 0.15 p (0.82) | BE: Concentrically constricted; RE: 11767 (5246); LE: 38716 (10528) | BE: Optic pallor, tilted optic disc, maculas without reflex, attenuated vessels, bone spicules peripherally | BE: hyperautofluorescent ring, hypoautofluorescent spots around vessel archs | BE: Spared ISe, RPE, and ELM in the central macula; epiretinal membrane in LE | 15 | 54 | 0.24 (424) | 0.21 (542) | N/A |
| F9P16 (00611) | M | COD | c.3457T>A p.(Ter1153Lysext*38) | Yes [39] | 31; 33 | Childhood: refraction error (myopia); early 30s: loss of central vision, photophobia | 1/15 (N/A) | −17.00–3.00/75° (N/A) | −2.25–0.75/11° (pseudophakic eye after vitrectomy) (N/A) | 0.015 (1.8); 0.0225 (1.64) | 0.03 (1.52); 0.03 (1.52) | Central scotoma; RE: 1023180 (981382); LE: 116468 (80218) | BE: Bull’s-eye appearance of macula, optic pallor, attenuated vessels; LE: bone spicules in the central and peripheral retina (after retinal detachment) | BE: Hyperautoflourescent ring; LE: RPE mottling in the inferior temporal retina (after retinal detachment) | BE: Absent RPE, Ise, and ELM in the central macula | 147 | 7 | 26.1 (6342); 27.0 (6635) | 21.6 (4267); 22.1 (4280) | BE: Normal DA ERG; undetectable LA ERG; undetectable PERG P50; significantly reduced mfERG |
| F10P17 (00078) | M | COD | c.3457T>A p.(Ter1153Lysext*38) | Yes [39] | 35; 42 | Childhood: refraction error (myopia); early 30s: loss of central vision, difficulties in color discrimination, and night blindness | 1/15 (N/A) | −2.25–1.0/34° (N/A) | −2.0–0.5/139° (N/A) | 0.5 (0.3); 0.1 (1) | 0.6 (0.2); 0.16 (1) | Central scotoma | BE: Bull’s-eye appearance of macula, optic pallor, attenuated vessels | BE: Hyperautofluorescent ring | BE: Absent RPE, Ise, and ELM in the central macula; LE: remnants of ELM in the foveola | 100 | 83 | 8.6 (3618); 11.3 (4284) | 7.5 (3462); 8.6 (3623) | BE: Normal DA ERG; significantly reduced and delayed LA ERG; significantly reduced PERG P50; reduced mfERG |
| F10P18 (00073) | M | COD | c.3457T>A p.(Ter1153Lysext*38) | Yes [39] | 38; 49 | Childhood refraction error (myopia); early 30s: photophobia, difficulties in color discrimination | 1/15 (N/A) | −12.0–2.0/80° (−13) | −14.0–4.0/90° (−16) | 0.03 (1.3); 0.05 (1,3) | 0.1 (1); 0.05 (1,3) | Central scotoma; RE: 540510 (85230); LE: 106274 (55782) | BE: Bull’s-eye appearance of macula, optic pallor, attenuated vessels | BE: Hyperautofluorescent ring | BE: Absent RPE, Ise, and ELM in the central macula with remnants of the ELM in the foveola | 65 | 76 | 10 (3917); 11.2 (4100) | 9.0 (3778); 11.3 (4081) | BE: Undetectable PERG P50; normal DA ERG; LA ERG significantly reduced to undetectable |
| Pathogenic Variant (Change in Base) | Pathogenic Variant (Change in Amino Acid) | Family | Phenotype | Variant Type | ACMG/ACGS Classification | Variant Category | Reference | GnomAD Frequency | HGVS | Exon | Involved Isoforms |
|---|---|---|---|---|---|---|---|---|---|---|---|
| c.1978G>A | p.(Glu660*) | 1 | RP | Stop | PVS1, PM2 | Likely pathogenic | Novel | 0 | NM_001034853.2(RPGR): c.1978G>T (p.Glu660*) | ORF15 | RPGRex1–19, RPGRORF15 |
| c.1245+704_1415-2286del | RPGR: ex-11del | 2, 8 | RP | Exonic Deletion (out of frame) | PVS1, PM2 | Likely pathogenic | Novel | 0 | NM_001034853.2(RPGR): c.1245+704_1415-2286del | 10 | RPGRex1–19, RPGRORF15 RPGRskip14/15 |
| c.457G>A | p.(Ala153Thr) | 3 | RP | Missense | PM2, PP3 | Variant of unknown significance | Novel | 0 | NM_001034853.2(RPGR) :c.457G>A (p.Ala153Thr) | 5 | RPGRex1–19, RPGRORF15 RPGRskip14/15 |
| c.1217dupT | p.(Ser407Ilefs*46) | 4 | RP | Frameshift | PVS1, PM2 | Pathogenic | ClinVarID: 975132 | 0 | NM_001034853.2(RPGR): c.1217dupT (p.Ser407fs) | 10 | RPGRex1–19, RPGRORF15 RPGRskip14/15 |
| c.2236_2237delGA | p.(Glu746Argfs*23) | 5 | RP | Frameshift | PVS1, PM2, PS4_STR | Pathogenic | ClinVarID: 438142, PMID: 32679846, 10932196 | 0 | NM_001034853.2(RPGR): c.2236_2237del (p.Glu746ArgfsTer23) | ORF15 | RPGRex1–19, RPGRORF15 |
| c.1506+1G>T | / | 6 | RP | Splicing | PVS1, PM2, PP3 | Pathogenic | Novel | 0 | NM_001034853.2(RPGR): c.1506+1G>T | 11 | RPGRex1–19, RPGRORF15 RPGRskip14/15 |
| c.2340_2341delAG | p.(Arg780Serfs*54) | 7 | RP | Frameshift | PVS1, PM2 | Likely pathogenic | Novel | 0 | NM_001034853.2(RPGR): c.2340_2341del (p.Arg780SerfsTer54) | ORF15 | RPGRex1–19, RPGRORF15 |
| c.3457T>A | p.(Ter1153Lysext*38) | 9, 10 | COD | Extension | PM2, PM4 | Variant of unknown significance | Novel * | 0 | NM_001034853.2(RPGR): c.3457T>A (p.Ter1153Lysext*38) | ORF15 | RPGRex1–19, RPGRORF15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadalin, V.; Buscarino, M.; Sajovic, J.; Meglič, A.; Jarc-Vidmar, M.; Hawlina, M.; Volk, M.; Fakin, A. Genetic Characteristics and Long-Term Follow-Up of Slovenian Patients with RPGR Retinal Dystrophy. Int. J. Mol. Sci. 2023, 24, 3840. https://doi.org/10.3390/ijms24043840
Hadalin V, Buscarino M, Sajovic J, Meglič A, Jarc-Vidmar M, Hawlina M, Volk M, Fakin A. Genetic Characteristics and Long-Term Follow-Up of Slovenian Patients with RPGR Retinal Dystrophy. International Journal of Molecular Sciences. 2023; 24(4):3840. https://doi.org/10.3390/ijms24043840
Chicago/Turabian StyleHadalin, Vlasta, Maša Buscarino, Jana Sajovic, Andrej Meglič, Martina Jarc-Vidmar, Marko Hawlina, Marija Volk, and Ana Fakin. 2023. "Genetic Characteristics and Long-Term Follow-Up of Slovenian Patients with RPGR Retinal Dystrophy" International Journal of Molecular Sciences 24, no. 4: 3840. https://doi.org/10.3390/ijms24043840
APA StyleHadalin, V., Buscarino, M., Sajovic, J., Meglič, A., Jarc-Vidmar, M., Hawlina, M., Volk, M., & Fakin, A. (2023). Genetic Characteristics and Long-Term Follow-Up of Slovenian Patients with RPGR Retinal Dystrophy. International Journal of Molecular Sciences, 24(4), 3840. https://doi.org/10.3390/ijms24043840







