Evaluation of Genistein as a Mitochondrial Modulator and Its Effects on Sperm Quality
Abstract
:1. Introduction
2. Results
2.1. Water and Diet Consumptions
2.2. Relative Mass of the Sexual Organs
2.3. Plasma Testosterone Levels
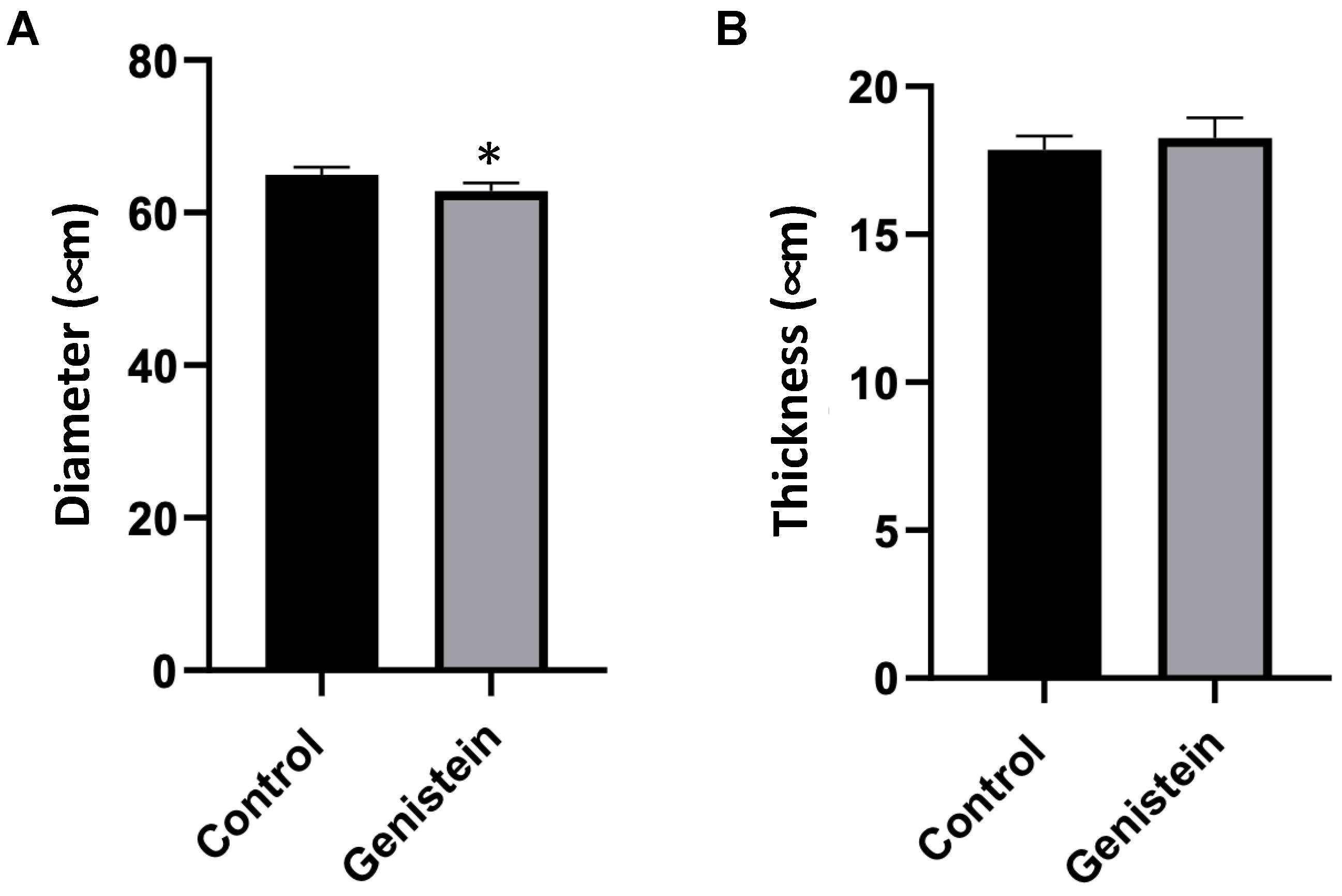
2.4. Evaluation of Seminiferous Tubules
2.5. Morphological Cellular Evaluation
2.6. Mitochondrial Functionality Assays
Epididymal Sperm Mitochondria Respiration Efficiency
3. Discussion
4. Materials and Methods
4.1. Commercial Products
4.2. Preparation of Genistein Solution
4.3. Experimental Model
4.3.1. Treatments
4.3.2. Monitoring of Consumption
4.3.3. Euthanasia of Animals
4.3.4. Relative Weight of Sexual Organs
4.3.5. Histological and Morphological Analysis
4.3.6. Testosterone Quantification
4.3.7. Seminal Collection
4.4. Mitochondrial Assays
4.4.1. Mitochondrial Respiratory Activity
4.4.2. Mitochondrial Respiratory Complexes Activities
4.4.3. ATP and LPO Levels
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.R.; Beyret, E.; Milligan, S.R.; Adeoya-Osiguwa, S.A. Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum. Reprod. 2006, 21, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, H.K.; de Oliveira, J.M.; Langoni de Freitas, G.B. Isoflavones alter male and female fertility in different development windows. Biomed. Pharmacother. 2021, 140, 111448. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Sobue, T.; Sasaki, S.; Kobayashi, M.; Arai, Y.; Uehara, M.; Adlercreutz, H.; Watanabe, S.; Takahashi, T.; Iitoi, Y.; et al. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a japanese population in comparison with dietary records and blood and urine isoflavones. J. Nutr. 2001, 131, 2741–2747. [Google Scholar] [CrossRef]
- FDA Food Labeling. Health Claims; soy protein and coronary heart disease. Fed. Regist. 1999, 64, 57699–57733. [Google Scholar]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Zhou, T.; Meng, C.; He, P. Soy Isoflavones and their Effects on Xenobiotic Metabolism. Curr. Drug Metab. 2019, 20, 46–53. [Google Scholar] [CrossRef]
- Adeoya-Osiguwa, S.A.; Markoulaki, S.; Pocock, V.; Milligan, S.R.; Fraser, L.R. 17beta-Estradiol and environmental estrogens significantly affect mammalian sperm function. Hum. Reprod. 2003, 18, 100–107. [Google Scholar] [CrossRef]
- Ferramosca, A.; Lorenzetti, S.; Di Giacomo, M.; Lunetti, P.; Murrieri, F.; Capobianco, L.; Dolce, V.; Coppola, L.; Zara, V. Modulation of Human Sperm Mitochondrial Respiration Efficiency by Plant Polyphenols. Antioxidants 2021, 10, 217. [Google Scholar] [CrossRef]
- Caceres, S.; Crespo, B.; Alonso-Diez, A.; de Andrés, P.J.; Millan, P.; Silván, G.; Illera, M.J.; Illera, J.C. Long-Term Exposure to Isoflavones Alters the Hormonal Steroid Homeostasis-Impairing Reproductive Function in Adult Male Wistar Rats. Nutrients 2023, 15, 1261. [Google Scholar] [CrossRef] [PubMed]
- Nardi, J.; Moras, P.B.; Koeppe, C.; Dallegrave, E.; Leal, M.B.; Rossato-Grando, L.G. Prepubertal subchronic exposure to soy milk and glyphosate leads to endocrine disruption. Food Chem. Toxicol. 2017, 100, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef]
- Barbagallo, F.; la Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria Quality Control and Male Fertility. Biology 2023, 12, 827. [Google Scholar] [CrossRef]
- Kumar, N. Sperm Mitochondria, the Driving Force behind Human Spermatozoa Activities: Its Functions and Dysfunctions—A Narrative Review. Curr. Mol. Med. 2023, 23, 332–340. [Google Scholar] [CrossRef]
- Hirata, S.; Hoshi, K.; Shoda, T.; Mabuchi, T. Spermatozoon and mitochondrial DNA. Reprod. Med. Biol. 2002, 1, 41–47. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Schulz, M.A.; Sánchez, R.; Villegas, J.V. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia 2009, 41, 51–54. [Google Scholar] [CrossRef]
- Amaral, S.; Tavares, R.S.; Baptista, M.; Sousa, M.I.; Silva, A.; Escada-Rebelo, S.; Paiva, C.P.; Ramalho-Santos, J. Mitochondrial functionality and chemical compound action on sperm function. Curr. Med. Chem. 2016, 23, 3575–3606. [Google Scholar] [CrossRef]
- Ferramosca, A.; Lorenzetti, S.; Di Giacomo, M.; Murrieri, F.; Coppola, L.; Zara, V. Herbicides glyphosate and glufosinate ammonium negatively affect human sperm mitochondria respiration efficiency. Reprod. Toxicol. 2021, 99, 48–55. [Google Scholar] [CrossRef]
- Taniguchi, H.; Matsuo, Y.; Shimoi, K.; Yoshimura, M.; Hirota, K.; Kinoshita, H. Establishment of a novel assessment of the quality of human spermatozoa measuring mitochondrial oxygen metabolism. BMC Res. Notes 2022, 15, 123. [Google Scholar] [CrossRef]
- Escada-Rebelo, S.; Cristo, M.I.; Ramalho-Santos, J.; Amaral, S. Mitochondria-Targeted Compounds to Assess and Improve Human Sperm Function. Antioxid. Redox Signal. 2022, 37, 451–480. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Brunati, A.M.; Clari, G.; Toninello, A. Interaction of genistein with the mitochondrial electron transport chain results in opening of the membrane transition pore. Biochim. Biophys. Acta 2002, 1556, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Sinha-Hikim, A.P.; Ettlin, R.A.; Clegg, E.D. Evaluation of the Testis: Histological and Histopathological, 1st ed.; Cache River Press: Clearwater, FL, USA, 1990; 286p. [Google Scholar]
- Orth, J.M.; Gunsalus, G.L.; Lamperti, A.A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 1988, 122, 787–794. [Google Scholar] [CrossRef]
- O’Hara, L.; Smith, L.B. Androgen receptor roles in spermatogenesis and infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 595–605. [Google Scholar] [CrossRef]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef]
- Sinha Hikim, A.P.; Chakraborty, J.; Jhunjhunwala, J.S. Germ cell quantitation in human testicular biopsy. Urol. Res. 1985, 13, 111–115. [Google Scholar] [CrossRef]
- Misiakiewicz, K.; Kolasa, A.; Kondarewicz, A.; Marchlewicz, M.; Wiszniewska, B. Expression of the c-Kit receptor in germ cells of the seminiferous epithelium in rats with hormonal imbalance. Reprod. Biol. 2013, 13, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Freddo, N.; Nardi, J.; Bertol, C.D.; Dallegrave, E.; Leal, M.B.; Barreto, F.; Frizzo, I.B.; Rossato-Grando, L.G. Isoflavone Quan-titation in Soymilk: Genistein Content and Its Biological Effect. CyTA J. Food 2019, 17, 20–24. [Google Scholar] [CrossRef]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Messina, M.; Mejia, S.B.; Cassidy, A.; Duncan, A.; Kurzer, M.; Nagato, C.; Ronis, M.; Rowland, I.; Sievenpiper, J.; Barnes, S. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: A technical review of the observational and clinical data. Crit. Rev. Food Sci. Nutr. 2022, 62, 5824–5885. [Google Scholar] [CrossRef]
- Campos, A.S.; Diaz, B.L.; Rivera, E.A.B.; Granjeiro, J.M.; Braga, L.M.G.M.B.; Frajblat, M.; Stephano, M.A.; Lima, W.T. Capítulo 1—Introdução Geral. In Guia Brasileiro de Produção, Manutenção ou Utilização de Animais em atividades de Ensino ou Pesquisa Científica/Concea, 1st ed.; Ministério da Ciência, Tecnologia e Inovação: Brasilia, Brazil, 2023; pp. 14–67. [Google Scholar]
- Ferramosca, A.; Conte, A.; Moscatelli, N.; Zara, V. A High-Fat Diet Negatively Affects Rat Sperm Mitochondrial Respiration. Andrology 2016, 4, 520–525. [Google Scholar] [CrossRef]
- Ferramosca, A.; Provenzano, S.P.; Coppola, L.; Zara, V. Mitochondrial Respiratory Efficiency Is Positively Correlated with Human Sperm Motility. Urology 2012, 79, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.M.; Thorburn, D.R.; Turnbull, D.M.; Taylor, R.W. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007, 80, 93–119. [Google Scholar]





| Parameters | Cell Types | Groups | |
|---|---|---|---|
| Control | Genistein | ||
| Viable cells | Elongated spermatid (c/t) | 22.08 ± 8.82 | 9.8 ± 1.88 * |
| Rounded-shaped spermatid (c/t) | 22.48 ± 7.13 | 14.16 ± 2.15 | |
| Primary spermatocytes (c/t) | 37.84 ± 4.00 | 46.24 ± 4.26 * | |
| Spermatogonia (c/t) | 22.76 ± 5.33 | 35.36 ± 3.39 ** | |
| Sertoli Cells (c/t) | 10.08 ± 1.78 | 13.64 ± 2.78 * | |
| Leydig Cells (c/aic) | 35.12 ± 8.80 | 50.88 ± 10.19 * | |
| Degenerated cells | Elongated spermatid (c/t) | 48.72 ± 10.82 | 49.88 ± 4.92 |
| Rounded-shaped spermatid (c/t) | 109.40 ± 7.90 | 119.28 ± 13.50 | |
| Primary spermatocytes (c/t) | 8.85 ± 1.30 | 15.32 ± 3.49 * | |
| Spermatogonia (c/t) | 12.00 ± 2.94 | 18.24 ± 5.42 | |
| Sertoli Cells (c/t) | 4.52 ± 0.63 | 6.96 ± 1.04 ** | |
| Leydig Cells (c/aic) | 17.60 ± 6.71 | 21.26 ± 8.64 | |
| V3 | V4 | RCR | |
|---|---|---|---|
| (nmoles O2/min/mL) | (nmoles O2/min/mL) | ||
| Control | 7.49 ± 0.27 | 4.21 ± 0.39 | 1.79 ± 0.03 |
| Genistein | 7.93 ± 0.35 * | 3.41 ± 0.37 * | 2.35 ± 0.18 * |
| Complex I | Complex II | Complex III | Complex IV | |
|---|---|---|---|---|
| (nmoles of NADH ox/min) | (nmoles of DCPIP red/min) | (nmoles of cyt c red/min) | (nmoles of cyt c ox/min) | |
| Control | 5.3 ± 0.3 | 8.7 ± 0.4 | 11.3 ± 0.7 | 6.3 ± 0.8 |
| Genistein | 5.4 ± 0.3 | 8.8 ± 0.4 | 12.5 ± 0.4 * | 6.4 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferigolo, M.; Nardi, J.; Freddo, N.; Ferramosca, A.; Zara, V.; Dallegrave, E.; Macedo, M.B.; Eller, S.; de Oliveira, A.P.; Biazus, I.C.; et al. Evaluation of Genistein as a Mitochondrial Modulator and Its Effects on Sperm Quality. Int. J. Mol. Sci. 2023, 24, 14260. https://doi.org/10.3390/ijms241814260
Ferigolo M, Nardi J, Freddo N, Ferramosca A, Zara V, Dallegrave E, Macedo MB, Eller S, de Oliveira AP, Biazus IC, et al. Evaluation of Genistein as a Mitochondrial Modulator and Its Effects on Sperm Quality. International Journal of Molecular Sciences. 2023; 24(18):14260. https://doi.org/10.3390/ijms241814260
Chicago/Turabian StyleFerigolo, Marilia, Jessica Nardi, Natália Freddo, Alessandra Ferramosca, Vincenzo Zara, Eliane Dallegrave, Mateus Belmonte Macedo, Sarah Eller, Ana Paula de Oliveira, Inara Carbonera Biazus, and et al. 2023. "Evaluation of Genistein as a Mitochondrial Modulator and Its Effects on Sperm Quality" International Journal of Molecular Sciences 24, no. 18: 14260. https://doi.org/10.3390/ijms241814260







