Abstract
Rheumatoid arthritis (RA) and osteoarthritis (OA) have a significant impact on the quality of life of patients around the world, causing significant pain and disability. Furthermore, the drugs used to treat these conditions frequently have side effects that add to the patient’s burden. Photobiomodulation (PBM) has emerged as a promising treatment approach in recent years. PBM effectively reduces inflammation by utilizing near-infrared light emitted by lasers or LEDs. In contrast to photothermal effects, PBM causes a photobiological response in cells, which regulates their functional response to light and reduces inflammation. PBM’s anti-inflammatory properties and beneficial effects in arthritis treatment have been reported in numerous studies, including animal experiments and clinical trials. PBM’s effectiveness in arthritis treatment has been extensively researched in arthritis-specific cells. Despite the positive results of PBM treatment, questions about specific parameters such as wavelength, dose, power density, irradiation time, and treatment site remain. The goal of this comprehensive review is to systematically summarize the mechanisms of PBM in arthritis treatment, the development of animal arthritis models, and the anti-inflammatory and joint function recovery effects seen in these models. The review also goes over the evaluation methods used in clinical trials. Overall, this review provides valuable insights for researchers investigating PBM treatment for arthritis, providing important references for parameters, model techniques, and evaluation methods in future studies.
1. Introduction
The two most common types of arthritis are osteoarthritis (OA) and rheumatoid arthritis (RA). OA is a common degenerative joint disease that is characterized by progressive and uneven loss of articular cartilage, bone spurs, and hardening of the underlying bone, as well as a variety of abnormalities in the synovium and around the joint. Aging, obesity, genetics, and previous joint injuries are all risk factors for OA, which primarily affects women [1,2].
RA, on the other hand, is a chronic autoimmune disease that primarily affects the elderly, with women being more affected than men. RA patients’ immune systems attack healthy synovial joints, resulting in continuous polyarticular synovitis, cartilage and bone damage, and joint failure, which causes severe pain, swelling, and fever. As the disease progresses, it can cause disability, reducing the patient’s quality of life and increasing the financial burden [3,4].
Unfortunately, there are currently no effective treatments for RA and OA that are free of side effects. Nonsteroidal anti-inflammatory drugs and steroids (NSAIDs) are commonly used by experts to treat pain and joint stiffness caused by inflammation. Although these medications effectively relieve RA symptoms, they do not provide a long-term cure. The European League Against Rheumatism proposed the use of disease-modifying anti-rheumatic drugs (DMARDs) to treat RA in 2010 [5]. DMARDs take time to work; they can significantly slow down disease progression, prevent the development of RA, and improve joint deformity. However, all current drug treatments for RA and OA have negative side effects, and long-term use of these drugs can put financial strain on patients [6]. As life expectancy rises, so does the number of elderly patients with arthritis, creating an urgent need for effective, side-effect-free treatments for RA and OA.
Photobiomodulation (PBM) has shown promising results in the treatment of neurodegenerative diseases, burns, wounds, and trauma in recent years by exerting anti-inflammatory, antioxidative, and ion channel-regulating effects [7,8,9,10]. PBM uses red or near-infrared light to activate cytochrome C oxidase in mitochondria, resulting in a variety of biological responses. Low-dose laser or LED light has been shown to reduce inflammation, increase ATP production, and regulate enzyme and gene expression in PBM patients [11,12]. The anti-inflammatory properties of PBM provide a theoretical foundation for its potential to alleviate arthritis-associated symptoms [13]. Notably, PBM does not require heat to be effective, and temperature changes during light irradiation are minimal [14,15]. Despite promising results in the treatment of a variety of clinical diseases, including neurodegenerative diseases and alopecia [16,17], the efficacy of PBM remains debatable, and research on PBM parameters is inconsistent. The efficacy of PBM treatments for RA, in particular, is still being debated. While some studies have shown that PBM can reduce inflammation and repair cartilage in arthritis [18,19], others have failed to find significant differences between PBM treatment and placebo groups [20,21,22]. Disparities in PBM efficacy may be explained by differences in PBM parameters such as wavelength, power density, light dose, and treatment duration.
In this review, “Low-level laser therapy”, “rheumatoid arthritis disease”, and “photobiomodulation” keywords were searched in Google Scholar, PubMed, and Medline. And the search for studies on PBM for arthritis ranged from 1987 to 2022.
This review provides an in-depth look at the potential of PBM in arthritis treatment. It covers three levels of investigation: cellular mechanisms, small animal models, and clinical studies. In terms of cellular mechanisms, the review describes how PBM can regulate arthritis-related cells by reducing inflammation, promoting tissue repair, and influencing cellular metabolism and signaling pathways. Moving on to small animal arthritis models, the review emphasizes the importance of quantitative and effective research in understanding the potential benefits of PBM for arthritis treatment. The review then delves into clinical studies of PBM in the treatment of arthritis, providing an accurate summary of the methods used to evaluate arthritis and the outcomes of PBM in clinical settings. Overall, this systematic and informative review is a valuable resource for those interested in learning more about PBM’s applications in arthritis treatment.
2. Arthritis
2.1. Joint
Joints, which are made up of two or more bones, play an important role in facilitating movement within the body. A synovial membrane surrounds movable joints, providing lubrication and nourishment to the joint tissues, including the cartilage. The presence of articular cartilage, a smooth and hard covering on the ends of bones, ensures that joints move smoothly and uninhibitedly as long as the cartilage is free of damage or lesions [23,24]. Joints not only allow for easy and precise movement, but they also support the weight of the body. However, arthritis severely impairs joint function and interferes with daily activities. Pain, swelling, heat, and inflammation are all symptoms of arthritis [25]. RA and OA are the two most common types of arthritis, and they differ in their pathological mechanisms, evaluation, and treatment methods.
2.2. RA
RA is a joint disease that primarily affects the wrists, hands, knees, and ankles. Most of the time, these joints are affected symmetrically [26]. However, the etiology and pathogenesis of RA are complicated, and the precise pathological mechanism is unknown. RA is an autoimmune disease that occurs when the immune system incorrectly attacks joint and organ tissues. Leukocytes and monocytes infiltrate the joint synovium and release cytokines that attack fibroblast-like synoviocytes in RA (FLS). As a result, inflammatory factors are released, causing new blood vessels to form in the synovium. This causes the synovial cavity on both sides to thicken, resulting in the formation of a pannus [27]. The pannus grows and invades the joints over time, causing cartilage and bone destruction [28].
Lymphocytes, white blood cells, monocytes, and macrophages are among the cells that infiltrate the synovial cavity during RA inflammation. These cells release inflammatory mediators that cause joint inflammation, resulting in fluid accumulation and joint swelling [29,30,31]. Furthermore, the swelling puts pressure on the surrounding nerves, causing pain. Inflammatory mediators also play a role in the subsequent stages of the inflammatory response, initiating a cascade of inflammatory effects in different cells that stimulates FLS proliferation, eventually leading to cartilage and bone destruction [28]. As RA progresses, joint inflammation causes a narrowing of the gap between joints, resulting in joint stiffness and reduced mobility, which is also responsible for the RA-specific morning stiffness [32]. The bones between the joints may fuse together in advanced stages of RA, rendering the joints immobile.
RA is a complex disease that is influenced by both genetic and environmental factors. The presence of anti-citrullinated protein antibodies (ACPAs), which are produced by B lymphocytes, divides RA patients into two subtypes. ACPA is a highly specific biomarker used in clinical trials to diagnose and predict the onset of RA. Approximately 67 percent of RA patients test positive for ACPA, and those who are ACPA-positive generally have more severe symptoms than those who are ACPA-negative [32,33,34]. Both genetic and environmental factors influence ACPA production. HLA-DR1 and HLA-DR4 have been identified as the genes most strongly associated with ACPA-positive RA in studies [35]. Furthermore, environmental factors such as smoking and dust exposure can activate immune cells, triggering immune responses that contribute to the development of RA. Furthermore, the gut microbiota has been linked to the pathogenesis of RA via various molecular mechanisms [36,37]. T lymphocytes and B cells become activated in response to genetic and environmental cues, resulting in the production of ACPA and, eventually, the onset of RA. Monocytes can become active and differentiate into macrophages during joint inflammation, with either a pro-inflammatory (M1) or anti-inflammatory (M2) phenotype. According to research, ACPA-negative patients have a higher proportion of the M1/M2 cell phenotype than ACPA-positive patients [38]. This finding suggests that targeting the regulation of macrophage phenotype could be a promising treatment strategy for RA.
External manifestations of synovitis include joint swelling and pain as a result of immune activation. A multitude of immune cells infiltrate the synovial cavity, including B lymphocytes (humoral immunity), T lymphocytes (cell-mediated immunity), monocytes, macrophages, mast cells, dendritic cells, and fibroblast-like synoviocytes (FLS), producing inflammatory factors such as TNF-α and IL-1β [39]. ACPA has been shown to activate NF-kB and increase TNF-α production in macrophages, resulting in a more severe clinical phenotype in ACPA-positive RA patients [40]. These cells require a constant supply of nutrients and oxygen, and the primary pathological process in RA, pannus, provides a supportive environment for synovial lining proliferation and subsequent bone invasion [41,42]. Pannus also causes the formation of new blood vessels, which supply nutrients and oxygen to inflammatory cells, thereby promoting the persistence of RA. VEGF, a potent endothelial cell-specific growth factor, is upregulated by pro-inflammatory cytokines and hypoxia and is synthesized by a variety of cells, including macrophages and FLS. Its concentration is generally high in the serum of RA patients [43]. Inhibiting pannus vascularization is a potential therapeutic strategy for RA blood vessel targeting.
To manage RA, doctors typically use a combination of pharmacological treatments, with the goal of reducing inflammation, relieving pain, and slowing cartilage damage. Nonsteroidal anti-inflammatory drugs (NSAIDs) [44], steroids, and standard disease-modifying antirheumatic drugs (DMARDs) [45] are among the treatment options. When standard DMARDs are ineffective, doctors may prescribe biologic DMARDs [46]. Physical therapy is also frequently recommended as part of the RA treatment regimen to maintain joint flexibility and muscle strength [47]. In severe cases of RA that do not respond to the aforementioned treatments, surgical interventions may be considered. For severely damaged joints, joint replacement surgery, such as total joint replacement, may be performed [48]. When joint replacement is not an option, arthrodesis may be considered, which involves removing the damaged joint and fusing it with pre-grown bone. Another treatment option for severe RA is synovectomy, which involves removing the synovium surrounding the joint and replacing it with artificial joints [49].
2.3. OA
OA is the most common type of arthritis, and its prevalence rises with age, especially in female patients and in weight-bearing joints [50]. The most common type of OA is knee OA. The knee joint is made up of the femur, tibia, and synovial membrane, with articular cartilage covering the end of the femur. The cartilage and bone in a healthy knee joint are smooth and without folds, and the synovial fluid in the synovial cavity acts as a lubricant, allowing painless motion with minimal friction and contact between the upper and lower bones of the knee [51]. OA, on the other hand, affects the cartilage and synovial fluid in the knee joint. Although the joint space appears normal in mild OA, the cartilage matrix has begun to decompose, and dense bone spurs form on the cartilage edge. Moderate exercise and weight loss can help reduce the load on the knee bone and joint, alleviating OA symptoms [52].
Changes in the joints become more noticeable in the middle stage of OA development, and the surface between the bones begins to erode. The cartilage, which is important for lubrication and protection, is also significantly degraded and worn out, resulting in a reduction in joint space. The viscosity and lubricating properties of synovial fluid deteriorate as well. OA typically affects the subchondral bone [53,54]. When the subchondral bone wears flat, oxygen enters the cartilage to try to heal itself. Dendritic cells and lymphocytes release cytokines and nuclear proteins into the synovial fluid, causing an inflammatory environment to form. The size and number of bone spurs may increase at this stage, roughening the bones and causing more severe and persistent joint pain. OA can be treated with pain relievers and steroid medications, as well as moderate exercise and weight loss.
The joint space becomes increasingly narrow in severe OA, resulting in rapid and severe cartilage and bone degradation and wear, a decrease in synovial fluid, inflammation of the knee joint, increased pain, and limited mobility. Inflammatory cells release damaging proteins and cytokines that degrade the cartilage and soft tissue surrounding osteophytes, resulting in an increase in osteophytes and direct contact between the upper and lower bones of the joint. At this point, the joint has lost its ability to move and bear weight, and surgical replacement of a portion or the entire joint is the only viable treatment option [53,55].
3. The Mechanism of PBM on Arthritis Treatment
PBM promotes cell activity and functional normalization by regulating a number of cellular responses, including promoting mitochondrial ATP production, releasing intracellular nitric oxide (NO), and regulating immune cell secretion of inflammatory cytokines such as TNFα, IL-6, and IL-β. Furthermore, the regulation of enzymes and genes is critical. Figure 1 depicts the sequence of events that take place when light is applied.
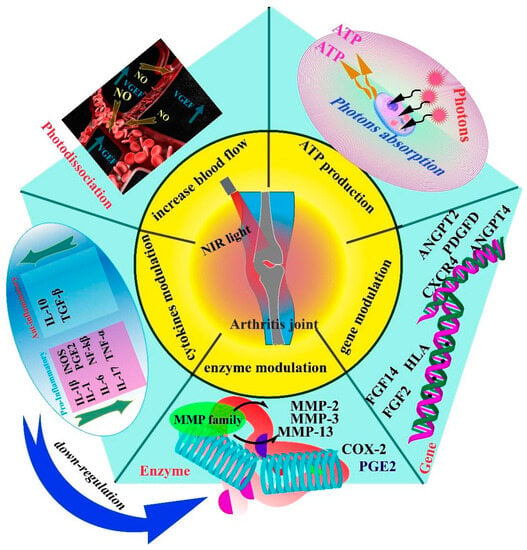
Figure 1.
PBM exerts its therapeutic effects on arthritis through five key mechanisms: regulation of angiogenesis, stimulation of ATP production in cells, modulation of arthritis-related genes, regulation of the secretion of joint-related enzymes, and modulation of the expression of cytokines, including both pro-inflammatory and anti-inflammatory factors. These mechanisms collectively contribute to the efficacy of PBM treatment for arthritis. The regulation of angiogenesis helps reduce the infiltration of inflammatory cells and promotes increased blood flow, aiding in the management of arthritis. Increased ATP production enhances the function and activity of cells involved in arthritis. Modulating gene expression can modify the production of enzymes and inflammatory factors, leading to a reduction in the production of enzymes that damage joint tissues. The overall result is a decrease in inflammation and improvement in arthritis symptoms.
3.1. ATP
According to research, photons emitted by PBM are primarily absorbed by cytochrome C oxidase (CCO) within the fourth chain of the mitochondrial electron transport chain, resulting in a series of complex cellular responses and altered redox states [56]. Two primary redox processes can explain these reactions. First, photoexcitation of specific chromophores within CCO causes changes in their redox properties, which then speeds up electron transfer. Second, upon photo-dissociation, CCO releases NO, increasing oxygen binding and respiration rates [57]. Furthermore, both CCO and NADH flavoproteins function as photoreceptors, causing changes in cytoplasmic proton motility, mitochondrial transmembrane potential, pH, and cellular redox potential by rapidly activating the mitochondrial respiratory chain and NADH oxidation pool [58].
When cells are exposed to red or near-infrared light, CCO absorbs photon energy, causing an electronic transition from a low-energy level to a high-energy level on the chromophore, resulting in the release of an electron that participates in cell respiration and ATP synthesis. As a result, PBM can improve cellular respiration efficiency by promoting cellular metabolism and increasing cell membrane potential [59]. Increased cellular energy levels can contribute to better cellular states, such as cell proliferation and normal functional cell activity.
3.2. Blood Flow
Bone is a highly vascularized tissue, and blood supply is crucial in bone reconstruction [60,61]. It is well known that increased blood flow to bone tissue promotes bone regeneration. PBM has been shown in previous studies to increase blood flow [62]. When photons are absorbed by cells, they directly photo-dissociate NO in the cell’s mitochondria, allowing NO to easily cross the cell membrane and stimulate smooth muscle cells in the inner wall of blood and lymphatic vessels, causing vasodilation and increased blood circulation [63]. Poor blood flow in the affected area of arthritis patients can cause nerve compression and pain. PBM, on the other hand, can regulate normal blood flow, reducing pain and promoting healing. Furthermore, the space previously occupied by NO in cells can be replaced by O2, providing raw materials for cell respiration and facilitating ATP production [64]. Notably, the effect of PBM on NO release regulation is transient, as NO release in CCO stops immediately when the light source is removed [63,65,66]. Additionally, Tim et al. [67] found that PBM could regulate the expression of genes involved in inflammation and angiogenesis. Using real-time PCR to detect inflammation and angiogenesis genes in rats, the results showed that angiogenesis genes were significantly upregulated after 36 and 72 h of PBM treatment, thereby stimulating angiogenesis. Taken together, PBM can regulate blood flow by releasing intracellular NO and modulating the expression of angiogenesis genes.
3.3. Regulation of Cytokines
Various cytokines, including interleukins, activate specific cells in arthritis, regulating or hastening inflammatory processes by activating transcription factors [68]. Cytokines are a class of molecules, mainly produced by T cells, macrophages, and endothelial cells, which mediate the activation of cells during immune or inflammatory reactions. Major regulators of the inflammatory response include TNF-α and interleukins, with changes in IL-6 and IL-1β levels commonly used as indicators to evaluate treatment effects [69,70,71,72]. The release of these cytokines can influence the secretion of other cytokines, creating a cascade effect. For instance, TNF-α not only mediates inflammation, immune processes, and proteolysis but also stimulates the production of IL-6 in the cytokine secretion cascade. Excessive TNF-α secretion in RA’s inflammatory synovium causes abnormal immune responses of T cells [73]. While transient synthesis of IL-6 induces beneficial responses to infections and tissue damage, continuous secretion can lead to autoimmune diseases [74]. Knockdown of the IL-1β gene expression significantly reduces the inflammatory response in some autoimmune diseases like RA [75]. Studies on RA treatment with PBM mainly focus on these cytokines to verify therapeutic effects. Different PBM parameters yield significantly different results. For example, in an OA mouse model, Alves et al. found that using 50 mW/cm2 laser power density (808 nm) was more effective than 100 mW/cm2 in reducing inflammation, with more significant downregulation of IL-1β and IL-6. However, at 100 mW/cm2 power density, the downregulation of TNF-α was more prominent. These findings demonstrate that different PBM parameters have varying anti-inflammatory effects, most directly reflected in the production of inflammatory cytokines [69,70,71,72,76,77,78]. In clinical experiments, PBM inhibits the production of inflammatory factors. For instance, Adly et al. measured the level of IL-6 in patients after laser treatment and found that it was significantly reduced after laser irradiation [79]. Additionally, PBM upregulates anti-inflammatory factors, such as transforming growth factors-β (TGF-β) [80]. TGF-β, produced by synovial fibroblasts, inhibits the production of TNF-α and other inflammatory factors [81]. Bartoli et al. [82] reported increased TGF-β content in a Wistar mouse model of arthritis irradiated with 670 nm lasers. Overall, PBM plays a regulatory role in arthritis by reducing the production of pro-inflammatory cytokines and upregulating anti-inflammatory cytokines [83].
3.4. Enzyme
The primary cause of pain in RA patients is the release of inflammatory substances by cells in response to local inflammation, which stimulates the transduction of cell signals to produce pain-causing substances, such as the relative enzyme. Prostaglandin E2 (PGE2) is a key player in the pain-inducing process and has become the primary representative enzyme of inflammation-related pain [84]. NSAIDs and selective cyclooxygenase 2 (COX-2) inhibitors are commonly used to alleviate RA-related swelling and pain by effectively inhibiting COX-2 activity and reducing PGE2 synthesis [85,86]. PBM also exhibits an anti-inflammatory effect by inhibiting COX-2. Lim et al. reported that after 635 nm LED light irradiation of human gingival fibroblasts, the decrease in reactive oxygen species (ROS) in cells inhibited COX-2 production. Additionally, in animal experiments involving tibial deprivation, COX-2 content tended to be downregulated after 7 days of PBM treatment [67].
In RA patients, IL-1β and TNF-α can stimulate matrix metalloproteinase (MMP-13), which degrades all components of the extracellular matrix, including cartilage in joints. MMPs-1 also plays a crucial role in cartilage degradation in both RA and OA. Moreover, other MMPs, such as MMP-2 and MMP-3, are expressed in arthritic joints, and their content is increased [87]. Therefore, inhibiting MMP production is vital for preventing cartilage damage. A recent study demonstrated that irradiation with an 808 nm laser at 50 J/cm2 can significantly reduce the contents of COX-1 and MMP-13 in arthritic mice [77]. PBM plays a critical role in regulating the content and viability of enzymes associated with arthritis, protecting articular cartilage, and reducing the inflammatory effects of arthritis. In a word, PBM effectively reduces COX-2 content, leading to the inhibition of PGE2 production, relieving pain, and swelling in joints. Additionally, it inhibits metalloprotein enzymes, alleviating the erosion and degradation of joint tissues and components, preserving the normal function of joints.
3.5. Gene
Gene expression regulates many cellular functions, including protein secretion and signaling factor expression. As a result, it is critical to investigate whether PBM can modulate gene expression. In arthritis, inflammation is caused by the infiltration of cells such as single cells, macrophages, lymphocytes, and granulocytes into the synovial cavity, with polymorphonuclear (PMN) leukocytes being key factors that contribute to joint injury [88]. PBM has been shown to increase the expression of both anti-apoptotic (p53 gene) and pro-apoptotic (Bcl2) genes in PMN leukocytes, inducing apoptosis in PMN cells at higher PBM concentrations [89]. Additionally, PBM has shown the ability to regulate the inflammatory process, promoting early granulation tissue deposition and new bone tissue in bone injury areas. It upregulates several proinflammatory and pannus genes such as FGF14, FGF2, ANGPT2, ANGPT4, and PDGFD after 36 and 72 h of PBM. Furthermore, PBM regulates the expression of inflammatory and angiogenic genes and the immune expression of COX-2 and VEGF at the initial stage of bone healing, contributing to new bone development [89]. Inflammation is also caused by the infiltration of inflammatory cells from blood vessels into the synovial cavity. CXCR4, a chemokine receptor involved in various inflammatory diseases mediated by peripheral blood immune cells, mediates the abnormal penetration of immune cells into joint and lung tissues, resulting in lymphocytic infiltration in the synovial cavity of the joints. Moreover, the entry of inflammatory cells from blood vessels into the synovial cavity is also a key step causing inflammation. Therein, the chemokine receptor CXCR4 is involved in various inflammatory diseases mediated by peripheral blood immune cells. Studies have shown that CXCR4 and its ligand CXCL12 mediate the abnormal penetration of immune cells into joint and lung tissues [90]. PBM using 830 nm GaAlAs diode irradiation has been found to downregulate the expression of CXCR4 mRNA, which may be one of the mechanisms of PBM inhibiting RA inflammation [91]. Other studies have also shown that PBM can regulate the expression of CCL2 and type II collagen [72,78]. Investigating the gene regulation of PBM is crucial for understanding its mechanism in treating RA, as it involves changes in protein expression and cellular function, which are critical steps in exploring the mechanism of PBM treatment.
4. PBM Regulates Arthritis-Related Cells
This section examines the regulatory effects of PBM on key cells involved in RA pathogenesis, such as macrophages, PMN cells, T cells, and FLS. We gain valuable insights into the therapeutic potential of PBM for RA treatment by elucidating the molecular-level modulation of these cells by PBM. This knowledge could pave the way for more targeted and effective PBM-based therapies, advancing RA management and improving patient outcomes.
4.1. Polymorphonuclear Cells
A variety of chemokines and cytokines activate PMN cells, causing them to migrate from the blood vessels to the lubricating membrane compartment in the synovial cavity. Once there, they release a variety of pro-inflammatory cytokines, causing inflammatory cell proliferation, pannus formation, increased vascular permeability, and stimulation of synovial stromal cells and cartilage cells to secrete proteolytic enzymes (MMP-2, -9, and -13). These enzymes promote the degradation of type II collagen and alter the glycan composition and water storage capacity of articular cartilage, resulting in physiological changes and joint functional destruction [92]. As a result, inhibiting the proliferation and viability of PMN cells is an effective way to treat RA, and there are related studies on PBM regulating the function of PMN cells. For example, one study found that higher light doses (30 J/cm2) with an 830 nm laser increased pro-apoptotic gene expression, while lower doses (3 J/cm2) decreased it. These findings suggest that the biochemical reactions differ significantly depending on the light dose [89]. Furthermore, other laser wavelengths inhibit PMN proliferation. Carlos et al. used 660 nm lasers at a low light dose (2.5 J/cm2) to treat zymosan-induced arthritis mice, and the results showed that the amount of PMN in the synovium of joints was significantly reduced [93]. The levels of pro-inflammatory cytokines such as IL-1β and IL-6 decreased as well. PBM may have a more specific effect on PMN cells due to the additional mechanism of free radical production by these cells during inflammation and their very short half-life, accelerating cellular functions such as cytokine production, which leads to apoptosis of PMN cells [89,94]. As a result, in the treatment of RA, PBM may reduce joint inflammation by inhibiting PMN viability and proliferation.
4.2. Macrophages
Macrophages play a crucial role in the pathogenesis of RA through antigen presentation, osteoclast generation viability, and secretion of proinflammatory cytokines such as TNF-α, IL-1, and IL-6 [95]. TNF-α promotes the expression of other cytokines, while IL-1β induces the release of adenosine from monocytes, activates PMN and oxidative stress, and increases the release of cytokines and chemokines from synovial fibroblasts. IL-1β and TNF-α can also activate the release and production of MMPs. On the other hand, IL-6 facilitates the proliferation of T and B cells, antibody production, hematopoiesis, and platelet formation [96,97]. It is noteworthy that macrophages have two phenotypes with completely different functions in response to inflammation. For example, M1 macrophages regulate inflammation and cell proliferation during muscle repair, while M2 macrophages guide differentiation and remodeling and promote tissue regeneration. However, 780 nm and 660 nm lasers (1 J/cm2) can effectively reduce the M1 phenotype macrophages while increasing the expression of M2 phenotype and reducing the content of proinflammatory cytokines [98]. Therefore, exploring the regulation of macrophage phenotype by PBM is also a promising approach for the treatment of RA. Different light power densities also have different regulatory effects. Alves et al. irradiated OA mice with 808 nm laser at 50 mW/cm2 and 100 mW/cm2 power density, respectively. The results showed that 50 mW/cm2 laser inhibited the secretion of IL-1β and IL-6 in macrophages more effectively. A power density of 100 mW/cm2 was more effective in reducing TNF-α production, suggesting that PBM can directly regulate inflammatory cytokine secretion of macrophages in addition to regulating macrophage phenotypes [76].
4.3. T (Treg) Cells
As RA is a heterogeneous autoimmune disease, immune lymphocytes also play a significant role in its pathogenesis. The infiltration of T cells into the synovial fluid is an important cause of inflammation, including Th17 cells that secrete proinflammatory factors like IL-17 [99]. The immunosuppressive activity of regulatory T (Treg) cells can modulate joint inflammation [100]. The mechanism of T cell immunosuppression involves the secretion of anti-inflammatory cytokines such as TGF-β and IL-10. Additionally, some inflammatory mediators directly activate the secretion of perforin and activate granzyme A, which are common molecules in CD8+ cytotoxic T cells, leading to the inhibition of molecular effects associated with immunosuppression by effector T cells. Moreover, T cells can inhibit the maturation of DC cells, promote the downregulation of CD80/CD86 expression, and compete with effector CD4+ cells for interaction with antigen-capturing and antigen-presenting cells [101].
T cells are mainly differentiated into two phenotypes, namely CD4+ and CD8+ T lymphocytes. Macrophages and DC cells activate T cells through the presentation of CD80/CD86 and histocompatibility complex (MHC) antigens, leading to differentiation into the aforementioned two phenotypes [102]. An increase in the number of macrophages and DCs results in more antigen presentation in lymph nodes, providing a favorable cytokine environment for the differentiation and proliferation of T lymphocytes. CD4+ T cells contribute to a positive feedback loop in the chronic inflammatory response due to the production of cytokines and chemokines. Furthermore, aggravated inflammation stimulates macrophages and DCs to migrate to the inflammatory site, where they present antigens to stimulate the differentiation of CD4+ lymphocytes, thereby exacerbating chronic inflammation [103].
CD8+ T cells demonstrate two opposite responses under different stimulations in the immune response. On the one hand, they maintain the chronic inflammatory process by secreting high levels of proinflammatory cytokines and lysozymes; on the other hand, they can inhibit inflammatory responses in arthritis by secreting IL-10, an anti-inflammatory cytokine [104]. Therefore, the increase of CD8+ lymphocytes can contribute to inhibiting the occurrence of inflammation. A study has demonstrated that an 830 nm laser at a high dose (30 J/cm2) upregulates the expression of CD25, the receptor for IL-2 on the cell surface, and CD8+ T cells with high CD25 expression facilitate the feedback of negative immune responses in the inflammatory microenvironment, leading to the alleviation of inflammation [89].
4.4. Fibroblast-like Synoviocytes
FLS are a crucial cellular component of joint synovial tissue, and there exists a complex network of FLS–macrophage–lymphocyte interactions in the inflammatory system of RA. Despite the fact that numerous inflammatory cells infiltrate the synovial cavity, the primary cause of synovial hyperplasia is the excessive proliferation and activation of FLS [105]. Additionally, FLS produces a plethora of pro-angiogenic factors, forming new blood vessels to provide nutrients and oxygen for inflammatory cells [42]. Thus, a decrease in FLS directly inhibits the inflammatory responses. Hsieh et al. [106] irradiated arthritic mice with 780 nm GaAlAs lasers at 4.5 J/cm2 to explore the effect of PBM on the activity and number of nuclear FLS cells. The results showed that PBM effectively reduced the number of FLS in the affected site, as well as the levels of inflammatory mediators such as TNF-α and MMP.
PBM, as shown in Figure 2, slows arthritis primarily through the following activities. On the one hand, PBM regulates macrophage phenotypes [107,108], increases the production of cytokines such as IL-10 and TGF-β, promotes tissue repair, and reduces the content of inflammatory factors such as TNF-α, IL-1β, and IL-5. This, in turn, inhibits the differentiation of CD4+ T cells [70] and reduces the content of TNF-α, IL-1β, IL-5, and other substances, forming benign, negative anti-inflammatory feedback. On the other hand, PBM reduces the apoptosis of PMN and FLS cells and reduces the infiltration of these inflammatory cells in arthritis joints [89,109,110]. Furthermore, the effects of PMN-mediated oxidative stress and vascular permeability will be inhibited [111,112,113], and VEGF secretion of FLS will also be reduced, inhibiting the cardiovascular production that provides nutrients for infiltrated inflammatory cells. Therefore, PBM slows down the formation of pannus and reduces the destruction of joint cartilage.
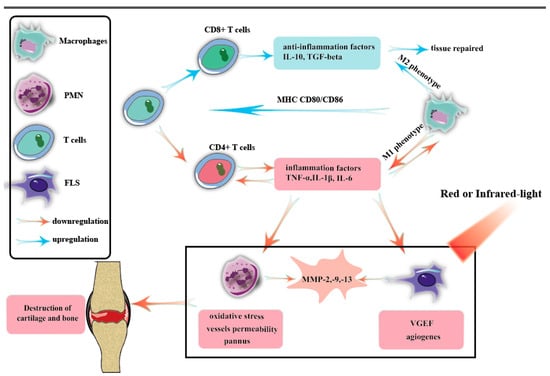
Figure 2.
The activation of innate immunity is the underlying mechanism that triggers RA. Dendritic cells (DCs), macrophages, and activated B and T cells express major histocompatibility complex, CD80/CD86, and other inflammatory stimulatory proteins, which contribute to the differentiation of T cells into T helper 1 (Th1) and Th17 cell phenotypes. Once activated, T and B cells secrete inflammatory cytokines and chemokines, further activating leukocytes, macrophages, fibroblasts, and endothelial cells. This complex network of molecular and cytokine-mediated interactions forms the basis of RA pathogenesis. Each cell involved in the pathogenesis of RA plays a significant role in mediating inflammation and joint destruction. Therefore, inhibiting the function and response of these cells has become a crucial objective in alleviating or potentially curing RA.
5. PBM Regulates Arthritis Animals
5.1. Establish Arthritis Animal Models
The establishment of an effective animal model of arthritis is fundamental for investigating the modulatory effects of PBM on arthritis. Various methods can be used to induce arthritis in mice, such as collagenase-induced arthritis (CIA), Complete Freund’s Adjuvant (CFA), zymosan induction, papain solution-induced arthritis, microcrystalline arthritis (induced by physiological solution, hydroxyapatite, and urate solution), and surgical intervention. These methods induce arthritis through different mechanisms, ultimately resulting in inflammation and structural and functional destruction of arthritic joints. Table 1 presents a summary of various methods utilized for the induction of arthritis, along with their corresponding mechanisms. This table serves as a useful reference for researchers investigating arthritis induction in animal models.

Table 1.
The mechanisms of the methods to arthritis induction.
Among these methods, CIA is a common and effective way to mimic human RA in mouse models. It is widely used to investigate the pathological mechanisms of RA and search for effective treatment approaches [114]. The pathological characteristics of mouse models induced by CIA typically include proliferative synovitis with infiltration of polymorphonuclear and mononuclear cells, pannus formation, cartilage degeneration, and bone erosion [115].
Another common method to establish animal models of arthritis is through the use of CFA solution induction. In 1963, Pearson et al. found that immunizing rats with CFA solution could induce arthritis. In this method, T cells have an immune response to autologous components induced by the adjuvant. However, this animal model is different from real arthritis in terms of reorganization and immunological characteristics. It is typically used to study the mechanism of autoimmune responses induced by external factors [116].
The mechanism by which zymosan solution induces arthritis in animals is by stimulating the secretion of lysosomal enzymes through the activation of macrophages, which induces inflammatory responses in the joints. This is a process mediated by cell surface receptors. During the induction phase of the immune response, phagocytes (monocytes, macrophages, and dendritic cells) bind zymosan with receptors and activate NF-kB channels, leading to the production of inflammatory factors and the expression of co-stimulating molecular CD80. Furthermore, zymosan induces DC maturation and IL-2 production to trigger adaptive immune responses, whereby mature DCs migrate to lymph nodes and induce the activation of T cells, which proliferate via antigen presentation. In the provocation phase, T lymphocytes stimulated by zymosan are recruited and produce a variety of cytokines, leading to the amplification of the inflammatory response into a more intense process [117,118,119]. Zymosan injection also leads to increased vascular permeability and edema, which are important events in maintaining the normal function of inflammatory cells and the source of plasma [120]. Injection of zymosan into mice resulted in inflammatory arthritis with monocyte infiltration, synovial hypertrophy, and pannus formation [119].
Upon injecting papain solution, a common method to establish animal models of osteoarthritis (OA), articular cartilage degeneration is primarily induced [121,122]. The initial symptom is characterized by the elevation and loosening of the cartilage surface, followed by thinning and fibrotic changes. Ultimately, cartilage breakdown and loss near the subchondral bone occur, closely resembling the clinical features of degenerative arthritis [123].
Microcrystal deposition is a common cause of joint diseases that often results in severe pain and inflammation. The main pathogenic agent is sodium urate crystals, responsible for gout, although calcium pyrophosphate can also be deposited in various clinical forms. During the pathogenesis of microcrystal-induced arthritis, a plethora of inflammatory cytokines, such as PGE2 and IL-1, are released [124]. To induce the formation of microcrystalline arthritis, hydroxyapatite and urate are typically injected into animal joints [125].
Transection of joint ligaments is also commonly used to model OA. This procedure stimulates the production of several cytokines, including TGF-β1, IL-1β, MMP-3, and TNF-α, in the subchondral bone [71,126]. Elevated levels of TGF-β1 lead to the formation of osteoid islets in the bone marrow and increased angiogenesis. Similarly, high levels of TGF-β1 have been observed in the subchondral bone of human OA patients [127]. Furthermore, other cytokines contribute to inflammatory responses, exacerbating subarticular osteochondral erosion and bone spur formation.
A recent study proposed an intriguing method to induce arthritis in mice by injecting small amounts of senescent cells into the knee joint [128]. Through the expression of luciferase, researchers could track the activity of senescent cells in the mice, and imaging and histology indicated the development of osteoarthritis. Notably, fibrocytes and mesenchymal stem cells have the potential to differentiate into senescence-related secretory phenotypes, producing inflammatory cytokines, chemokines, and growth factors [129,130]. This unique approach sheds light on the role of senescent cells in arthritis development and adds a novel dimension to arthritis research in animal models.
5.2. Studies on In Vivo Treatment of Arthritis with PBM
The biochemical simplicity of cell environments makes them suitable for investigating the mechanisms of PBM. However, it is crucial to validate whether PBM can effectively regulate arthritis at the in vivo level. Mouse models are widely preferred for arthritis research due to their cost-effectiveness, easy management, and high genetic similarity to humans [131,132], which saves resources while ensuring reliable results. The CD57BL/6 mice strain is particularly common in arthritis research, and most in vivo studies are based on this model. Additionally, rabbits can also serve as research models, given their larger size and distinguishable joint structures [78]. In animal experiments, researchers induce arthritis and administer PBM treatment before euthanizing the animals to collect joint lavage fluid for analysis of cell types, cell numbers, cytokines, and RNA components. Table 2 provides an overview of studies conducted on animal models to investigate the efficacy of PBM in treating arthritis. The table includes information on the type of animal model used, the parameters of PBM treatment, and the study results. By summarizing the available literature on animal studies, this table serves as a valuable resource for researchers interested in exploring the potential of PBM therapy for arthritis treatment.

Table 2.
Study on PBM treatment of animal arthritis models.
Measuring changes in cytokines and inflammatory mediators in mouse joint lavage fluid before and after PBM is the most direct method to evaluate the effect of PBM on arthritis. Commonly measured mediators include MMP, IL cytokines, TNF-α, and VEGF. Several studies have reported significant decreases in the contents of PGE-2, TNF-α, MMP, IL-1, and IL-6 after PBM treatment in mice [67,69,71,72,76,82,110,133,139]. These changes result in the non-activation of response cells involved in arthritis, such as macrophages and lymphocytes that mediate the inflammatory response. This process creates a positive feedback loop for arthritis treatment [143,144]. To understand the reasons behind the decline in these inflammatory mediators, one study found that the number of cells producing these mediators significantly decreased after PBM treatment. Additionally, the number of FLS cells exhibited different responses to higher (72 J/cm2) and lower (4.5 J/cm2) PBM doses, with the latter leading to significantly lower cell numbers and subsequently downregulated TNF-α levels [110]. Another study investigated the effects of different power densities of PBM (50 and 100 mW/cm2 power output) on arthritis treatment in a mouse model. The results showed that the low power density had a more pronounced inhibitory effect on the number of macrophages and neutrophils, as well as the content of IL-6 and IL-1β [76]. However, due to different experimental conditions, such as laser types and equipment, the definition of low power is not clear, and no consensus has been reached.
In animal models, the therapeutic effects of PBM can be evaluated from various perspectives. Assessing angiogenesis, vascular permeability, and articular cartilage protection at the tissue and organ levels is not feasible with cell experiments alone. These arthritis-related symptoms are also targeted for treatment in clinical studies. As a result, some studies have investigated PBM’s therapeutic effects in small animal model species based on the joint’s structural organization and function. For instance, studies using 808 nm and 904 nm lasers on Zymosan- and CFA-induced arthritis mouse models have demonstrated that PBM can reduce angiogenesis, fibrotic tissue production, and protect articular cartilage properties [122,145]. Such evaluations provide valuable insights into the potential therapeutic benefits of PBM for arthritis treatment.
5.3. Effects of PBM Parameters on Animal Models of Arthritis
According to the studies summarized in Table 2, the choice of wavelength and power (dose) in PBM is crucial. Different wavelengths have different modulating mechanisms. For instance, the absorption peak of CCO in mitochondria is located at 600–1000 nm [146], and green light has been found to regulate the selective transmittance of ion channels in cell membranes [147]. However, it appears that the coherence of the PBM light source is not critical. Both LEDs and lasers have been used in studies and have shown certain regulatory effects on arthritis [78,89,134,148,149].
Wavelength is a crucial parameter in PBM, and longer-wavelength infrared light appears to be more effective than red light in terms of anti-inflammatory effects. Morais et al. [134] reported that shorter-wavelength light (628 nm) had no effect on modulating edema and vascular permeability in zymosan-induced arthritis mice, whereas longer wavelengths (685 and 830 nm) demonstrated better performance. In the study by Kuboyama et al. [149], the therapeutic effects of 570 nm LED light and 940 nm light on arthritis in mice were compared, and the results showed that 940 nm light had a better swelling reduction effect and was also more effective in inhibiting proinflammatory factors such as IL-1β, IL-6, and MMP-3. Souza et al. also reported a more effective performance of 780 nm laser in reducing inflammatory cells and promoting muscle fibers than 660 nm laser [98].
Furthermore, researchers have explored the synergistic effects of using two or more wavelengths of light to treat arthritis based on the different modulation effects of each wavelength. For example, Oshima et al. used alternating irradiation of 630 nm (red light) and 870 nm (infrared light) at high frequencies to target specific regulatory sites in arthritis mice. This approach resulted in the inhibition of collagen degradation and a corresponding decrease in inflammation at the joint site [78]. These findings highlight the importance of carefully selecting and matching light wavelengths to achieve the desired therapeutic effect in PBM treatment for arthritis.
On the other hand, it is important to consider that the experimental conditions and specific mouse models used in different studies varied significantly, as shown in Table 2, with a wide range of power densities employed, ranging from single digits to thousands of mW/cm2. This inconsistency in experimental parameters may contribute to the disparate conclusions drawn by various studies. Therefore, the selection of appropriate power density and dose is crucial in PBM research. It is essential to strike a balance between using a high-enough power density and dose to trigger the desired biochemical effects while avoiding tissue thermogenesis and ensuring that the power density and dose are not too low to achieve therapeutic benefits.
Several studies in animal models have indeed compared the effects of PBM at different power densities and doses, consistently indicating that lower power densities tend to be more effective in reducing inflammation and inducing apoptosis in proinflammatory cells [72,76]. However, the definition of “low dose” may vary among studies due to differences in equipment and experimental conditions, leading to potential inconsistency in the interpretation of results. To address this issue, it is recommended for researchers to conduct gradient experiments with varying power densities and doses during their investigations. This approach allows them to systematically evaluate the effects of PBM at different levels and identify the optimal dose based on the specific experimental outcomes. Subsequent experiments can then be conducted with the selected optimal dose to further investigate the effects of PBM in a more controlled manner. Overall, careful consideration and selection of appropriate power densities and doses in PBM experiments are crucial for obtaining meaningful and consistent results.
6. Clinical Studies of PBM in the Treatment of Arthritis
6.1. Clinical Evaluation of Arthritis
In clinical studies, invasive methods for arthritis evaluation should be avoided whenever possible because they are uncomfortable and may not be appropriate for all patients. Instead, a variety of non-invasive assessment methods, including digital approaches such as questionnaires and pain level assessments based on direct patient feedback, are used. Several questionnaires have been developed to assess arthritis symptoms and their impact on the lives of patients. These questionnaires include the visual analogue scale (VAS), the disability of the arm, shoulder, and hand (DASH), the health assessment questionnaire (HAQ), the Osteoarthritis Index (WOMAC) of Western Ontario and McMaster Universities, and the Saudi Knee Function Scale (SKFS) [110,150], rely on the patient’s subjective judgment through questionnaire responses to draw conclusions [22,151]. Each of these questionnaires uses a unique set of questions and conversion methods to assess various aspects of arthritis and its effects on patients. Given the cultural and linguistic differences among patients, questionnaires must be appropriately designed to ensure accurate and meaningful results. For example, the SKFS is tailored to Arabic patients, taking into account their language and comprehension patterns [150].
Among these assessment methods, the VAS is one of the most widely used tools for evaluating the effects of arthritis treatment. It involves a simple vernier ruler approximately 10 cm in length, with 10 scales marked on it, ranging from “0” (no pain) to “10” (most severe and unbearable pain). Patients rate their pain levels on this scale, providing a quantitative measure of pain severity [152]. Due to its simplicity and effectiveness, the VAS is a valuable tool for assessing pain responses in arthritis patients.
Objective evaluation methods are indeed essential in arthritis assessment, and they are often combined with subjective evaluations to provide a comprehensive understanding of the disease and its impact on patients.
One common objective evaluation method is to use a goniometer to measure the range of motion (ROM) of the affected joint, such as the knee joint. This technique evaluates the angle of motion and functional limitations of arthritis patients, providing important information about joint mobility and stiffness [153]. In addition, collecting peripheral blood from arthritis patients is another instructive method for diagnosis and monitoring disease progression. Rheumatoid factors and anti-cyclic citrullinated protein are important indicators for diagnosing RA [154,155]. However, it is essential to consider other clinical and laboratory findings for a confirmed diagnosis of RA, as the presence of these factors alone may not be conclusive. Inflammatory markers in peripheral blood are commonly measured to assess the level of inflammation in arthritis patients. Erythrocyte sedimentation rate (ESR) tests can indicate inflammation if sedimentation is rapid, providing an indication of ongoing inflammatory processes [156]. Additionally, the concentration of inflammatory factors such as C-reactive protein (CRP) tends to increase in response to inflammation events [157]. Using enzyme-linked immunosorbent assays (ELISA) and other methods, researchers and clinicians can measure changes in the levels of inflammatory factors in the peripheral blood serum of patients, including interleukin-6 (IL-6), NF-KB, and CRP [79].
Radiographic analysis, particularly X-ray computed tomography (X-CT), is indeed an essential and effective evaluation method for assessing joint health and structural changes in arthritis patients. X-ray scans can provide a clear picture of the joint space width (JSW) and structural integrity of the affected joints, allowing researchers and clinicians to monitor disease progression and treatment effects. In clinical studies, X-ray scans can be used to select appropriate patients for experimental trials, and researchers often exclude patients with severe genu varus or genu valgus based on X-ray analysis [151]. For example, Gopal et al. [158] conducted X-CT scans on patients with knee OA before and after laser treatment, and the results showed a significant increase in JSW after 8 weeks of laser irradiation compared to patients who did not receive laser treatment (laser: 4.2 ± 0.3, placebo laser: 2.8 ± 0.6). This objective assessment provides valuable quantitative data on the efficacy of PBM in promoting joint health.
Morning stiffness is another useful evaluation indicator in assessing the therapeutic effects of PBM in arthritis patients [19]. It is a common symptom experienced by arthritis patients and can be assessed subjectively to gauge treatment outcomes and improvements in joint function. The assessment methods used in clinical trials investigating the efficacy of PBM in arthritis treatment can be broadly categorized into objective and subjective judgments. Objective evaluations, such as radiographic analysis and laboratory measurements of inflammatory markers, provide scientific and quantitative data, while subjective evaluations, like patient questionnaires and pain level assessments, offer insights into patients’ experiences and perceptions of treatment effects. Table 3 provides a comprehensive overview of the evaluation methods used in clinical trials, outlining the underlying principles of each method. This table serves as a valuable resource for researchers and clinicians interested in utilizing these evaluation methods to obtain scientifically accurate and meaningful results in their own studies.

Table 3.
The arthritis evaluation methods in clinic trials.
By combining both objective and subjective evaluation methods, researchers can gain a comprehensive understanding of the therapeutic effects of PBM in arthritis treatment, enabling them to make informed clinical decisions and develop effective treatment strategies for patients.
6.2. Clinic Trials
Clinical trials examining the therapeutic effects of PBM in the treatment of arthritis have yielded encouraging results, particularly in knee OA and RA. PBM has been shown to reduce pain, swelling, and morning stiffness, indicating its ability to suppress arthritic inflammation [79,151,158,159,160,161,162,163,164,165,166,167,168]. However, it is important to note that some studies have reported no or only partial effects of PBM on arthritis treatment, indicating the need for better-defined treatment parameters and evaluation methods [169,170,171]. For example, Meireles et al. conducted a study with a subset of 82 patients with hand RA who received laser therapy. However, despite using VAS, HAQ, and DASH assessments, they concluded that there was no significant difference between patients who received laser therapy and those who did not [22]. Similarly, other studies on OA have reached similar conclusions to Meireles et al., suggesting that PBM does not provide substantial relief from inflammation or pain [20,21,169,170]. Additionally, specific PBM parameters need to be better defined [172]. The efficacy of PBM remains controversial due to variations in operating methods, treatment parameters (such as wavelength, power density, light source type, and arthritis type), evaluation methods, and irradiation sites in each research project.
Most clinical studies employ wavelengths in the 650–1000 nm range, which corresponds to the “optical window” known to effectively regulate cellular activities [12]. Moreover, the selection of power density is relatively conservative, ranging from 20–3000 mW/cm2, to avoid excessive heat generation that could cause burn damage to the patient’s skin. Studies should consider adhering to the American National Standard Institute (ANSI) standards to select the appropriate optical power density threshold, preventing excessive light-induced heat that may lead to skin or tissue burns. As seen in Table 3, almost all studies use VAS to evaluate the degree of arthritis, as it is a mature and reliable detection method widely used in arthritis treatment evaluation.
By analyzing the data from these studies, researchers can gain a better understanding of the optimal PBM parameters and evaluation methods for arthritis treatment, leading to more standardized and effective clinical approaches. Additionally, further research is needed to address the inconsistencies and explore the potential mechanisms underlying the variable treatment responses, ultimately advancing the field of PBM and its applications in arthritis management.
Table 4 presents a comprehensive summary of studies investigating the efficacy of PBM in OA and RA treatment. It includes detailed information on the PBM parameters and evaluation methods used in each study, along with the final conclusions and effect analysis of each paper. This table serves as a valuable resource for researchers and clinicians interested in utilizing PBM therapy for arthritis treatment, providing a comprehensive overview of the existing literature and guiding future research in this field.

Table 4.
The studies of PBM on the clinic arthritis trials.
7. Conclusions and Prospects
In conclusion, this comprehensive review emphasizes PBM therapy’s potential as an effective and non-invasive arthritis treatment. The detailed overview covers the fundamental mechanisms of PBM treatment, elucidating its cellular function regulation and efficacy in small animal arthritis models. Although there is currently no universally agreed upon optimal PBM treatment parameters, the positive results of clinical trials and extensive research on the underlying mechanisms inspire confidence in the potential of this therapeutic approach. Further research should focus on determining the best parameters for PBM treatment of arthritis. This may involve investigating the effects of different wavelength ranges, dosages, and treatment durations on the treatment’s efficacy. Additionally, future clinical trials should rigorously employ standardized evaluation methods to ensure the safety and efficacy of PBM treatment.
In addition to establishing therapeutic parameters, the exact pathways by which PBM regulates arthritis treatment are not yet fully understood. Future studies could investigate these mechanisms further, particularly in relation to gene regulation and intercellular communication. Investigating these aspects can provide valuable insights that guide the selection of experimental parameters.
Furthermore, accurately assessing treatment efficacy is crucial for future research. Researchers can use multiple assessment methods, combining objective evaluations and subjective questionnaire surveys to enhance the reliability of treatment outcome conclusions, especially in clinical studies.
In summary, PBM therapy shows great promise as an effective and non-invasive treatment for arthritis. This review provides valuable insights and guidance for researchers interested in exploring the use of PBM therapy for arthritis treatment. With continued research and development, PBM therapy has the potential to become a widely adopted and beneficial treatment option for arthritis patients.
Author Contributions
R.Z. researched the literature, wrote the original draft, J.Q. reviewed and supervised the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by the National Natural Science Foundation of China (61835009/62127819); the Shenzhen Key Laboratory of Photonics and Biophotonics (ZDSYS20210623092006020) and the Shenzhen Science and Technology Program (JCYJ20220818100202005).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, Prevalence, Costs, and Impact on Disability of Common Conditions Requiring Rehabilitation in the United States: Stroke, Spinal Cord Injury, Traumatic Brain Injury, Multiple Sclerosis, Osteoarthritis, Rheumatoid Arthritis, Limb Loss, and Back Pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Anzaghe, M.; Schuelke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhou, F.; Chen, W.R. Low-level laser therapy regulates microglial function through Src-mediated signaling pathways: Implications for neurodegenerative diseases. J. Neuroinflamm. 2012, 9, 219. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-Level Laser (Light) Therapy (LLLT) in Skin: Stimulating, Healing, Restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Zhang, R.; Zhou, T.; Liu, L.; Ohulchanskyy, T.Y.; Qu, J. Dose–effect relationships for PBM in the treatment of Alzheimer’s disease. J. Phys. D Appl. Phys. 2021, 54, 353001. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Samanta, S.; Luo, Z.; Li, S.; Xu, H.; Qu, J. Synergistic photobiomodulation with 808-nm and 1064-nm lasers to reduce the β-amyloid neurotoxicity in the in vitro Alzheimer’s disease models. Front. Neuroimaging 2022, 1, 903531. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.H.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Stadler, I.; Lanzafame, R.J.; Oskoui, P.; Zhang, R.Y.; Coleman, J.; Whittaker, M. Alteration of skin temperature during low-level laser irradiation at 830 nm in a mouse model. Photomed. Laser Surg. 2004, 22, 227–231. [Google Scholar] [CrossRef]
- Rochkind, S.; Barrnea, L.; Razon, N.; Bartal, A.; Schwartz, M. Stimulatory effect of he-ne low-dose laser on injured sciatic-nerves of rats. Neurosurgery 1987, 20, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.C.; Gonzalez-Lima, F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem. Pharmacol. 2013, 86, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, G.K.; Clark, J.; Wikonkal, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014, 46, 144–151. [Google Scholar] [CrossRef]
- Hamblin, M.R. Can osteoarthritis be treated with light? Arthritis Res. Ther. 2013, 15, 120. [Google Scholar] [CrossRef]
- Brosseau, L.; Welch, V.; Wells, G.; Tugwell, P.; de Bie, R.; Gam, A.; Harman, K.; Shea, B.; Morin, M. Low level laser therapy for osteoarthritis and rheumatoid arthritis: A metaanalysis. J. Rheumatol. 2000, 27, 1961–1969. [Google Scholar]
- Tascioglu, F.; Armagan, O.; Tabak, Y.; Corapci, I.; Oner, C. Low power laser treatment in patients with knee osteoarthritis. Swiss. Med. Wkly. 2004, 134, 254–258. [Google Scholar] [CrossRef]
- Brosseau, L.; Wells, G.; Marchand, S.; Gaboury, I.; Stokes, B.; Morin, M.; Casimiro, L.; Yonge, K.; Tugwell, P. Randomized controlled trial on low level laser therapy (LLLT) in the treatment of osteoarthritis (OA) of the hand. Lasers Surg. Med. 2005, 36, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.M.; Jones, A.; Jennings, F.; Suda, A.L.; Parizotto, N.A.; Natour, J. Assessment of the effectiveness of low-level laser therapy on the hands of patients with rheumatoid arthritis: A randomized double-blind controlled trial. Clin. Rheumatol. 2010, 29, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Wang, S.C.; Jing, Y.Y.; Su, J.C. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Geenen, R.; Overman, C.L.; Christensen, R.; Asenlof, P.; Capela, S.; Huisinga, K.L.; Husebo, M.E.P.; Koke, A.J.A.; Paskins, Z.; Pitsillidou, I.A.; et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 797–807. [Google Scholar] [CrossRef] [PubMed]
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036. [Google Scholar] [CrossRef]
- Yang, R.; Yao, Y.; Wang, P. Hypoxia-induced the upregulation of stromal cell-derived factor 1 in fibroblast-like synoviocytes contributes to migration of monocytes into synovium tissue in rheumatoid arthritis. Cell Biosci. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Firestein, G.S. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef]
- Mahdi, H.; Fisher, B.A.; Kallberg, H.; Plant, D.; Malmstrom, V.; Ronnelid, J.; Charles, P.; Ding, B.; Alfredsson, L.; Padyukov, L.; et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat. Genet. 2009, 41, 1319–1324. [Google Scholar] [CrossRef]
- Gupta, S.; Kaplan, M.J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 2016, 12, 402–413. [Google Scholar] [CrossRef]
- Navegantes, K.C.; Gomes, R.D.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef]
- Malmstrom, V.; Catrina, A.I.; Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat. Rev. Immunol. 2017, 17, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Kasapçopur, Ö.; Altun, S.; Aslan, M.; Karaarslan, S.; Kamburoglu-Göksel, A.; Sarıbaş, S.; Arısoy, N.; Kocazeybek, B. Diagnostic accuracy of anti-cyclic citrullinated peptide antibodies in juvenile idiopathic arthritis. Ann. Rheum. Dis. 2004, 63, 1687. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Bartoloni, E.; Morozzi, G.; Manganelli, S.; Riccieri, V.; Sabatini, P.; Filippini, M.; Tampoia, M.; Afeltra, A.; Sebastiani, G.; et al. Anti-cyclic citrullinated peptide antibody titer predicts time to rheumatoid arthritis onset in patients with undifferentiated arthritis: Results from a 2-year prospective study. Arthritis Res. Ther. 2013, 15, R16. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Sandor, C.; Stahl, E.A.; Freudenberg, J.; Lee, H.S.; Jia, X.; Alfredsson, L.; Padyukov, L.; Klareskog, L.; Worthington, J.; et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012, 44, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, B.; Liu, J.; Feng, H.; Ma, Y.; Li, D.; Guo, B.; Liang, C.; Dang, L.; Wang, L.; et al. Molecular Insight into Gut Microbiota and Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 431. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front. Immunol. 2017, 8, 1958. [Google Scholar] [CrossRef]
- Burmester, G.R.; Dimitriu-Bona, A.; Waters, S.J.; Winchester, R.J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand. J. Immunol. 1983, 17, 69–82. [Google Scholar] [CrossRef]
- Lu, M.C.; Lai, N.S.; Yu, H.C.; Huang, H.B.; Hsieh, S.C.; Yu, C.L. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010, 62, 1213–1223. [Google Scholar] [CrossRef]
- Paleolog, E.M. Angiogenesis in rheumatoid arthritis. Arthritis Res. Ther. 2002, 4, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct. 2001, 26, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef] [PubMed]
- Hoes, J.N.; Jacobs, J.W.G.; Buttgereit, F.; Bijlsma, J.W.J. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat. Rev. Rheumatol. 2010, 6, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; de Wit, M.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, A.K.R.; Niedermann, K.; Braun, J.; Adams, J.; Brodin, N.; Dagfinrud, H.; Duruoz, T.; Esbensen, B.A.; Gunther, K.P.; Hurkmans, E.; et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 1251–1260. [Google Scholar] [CrossRef]
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef]
- Knupp, M.; Skoog, A.; Tornkvist, H.; Ponzer, S. Triple arthrodesis in rheumatoid arthritis. Foot Ankle Int. 2008, 29, 293–297. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis A Review. JAMA-J. Am. Med. Assoc. 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Getgood, A.; Brown, C.; Lording, T.; Amis, A.; Claes, S.; Geeslin, A.; Musahl, V.; Amis, A.; Brown, C.; Cavaignac, E.; et al. The anterolateral complex of the knee: Results from the International ALC Consensus Group Meeting. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Vicente, E.F. Osteoarthritis: More than Cartilage Degeneration. Clin. Rev. Bone Miner. Metab. 2017, 15, 69–81. [Google Scholar] [CrossRef]
- Intema, F.; Sniekers, Y.H.; Weinans, H.; Vianen, M.E.; Yocum, S.A.; Zuurmond, A.M.; DeGroot, J.; Lafeber, F.P.; Mastbergen, S.C. Similarities and discrepancies in subchondral bone structure in two differently induced canine models of osteoarthritis. J. Bone Min. Res. 2010, 25, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, S.; Fairbank, A. The orthopaedic approach to managing osteoarthritis of the knee. BMJ-Brit. Med. J. 2004, 329, 1220–1224a. [Google Scholar] [CrossRef] [PubMed]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B Biol. 1999, 49, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Nicholls, P.; Wilson, M.T.; Cooper, C.E. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2006, 103, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Hamblin, M.; Demidova, T. Mechanisms of Low Level Light Therapy; SPIE: Bellingham, WA, USA, 2006; Volume 6140. [Google Scholar]
- Tomlinson, R.E.; Silva, M.J. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013, 1, 311–322. [Google Scholar] [CrossRef]
- Compston, J.E. Bone marrow and bone: A functional unit. J. Endocrinol. 2002, 173, 387–394. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kharkwal, G.B.; Sajo, M.; Huang, Y.-Y.; De Taboada, L.; McCarthy, T.; Hamblin, M.R. Dose Response Effects of 810 nm Laser Light on Mouse Primary Cortical Neurons. Lasers Surg. Med. 2011, 43, 851–859. [Google Scholar] [CrossRef]
- Lohr, N.L.; Keszler, A.; Pratt, P.; Bienengraber, M.; Warltier, D.C.; Hogg, N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: Potential role in cardioprotection. J. Mol. Cell. Cardiol. 2009, 47, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.; Pyatibrat, L.; Afanasyeva, N. Cellular Effects of Low Power Laser Therapy Can be Mediated by Nitric Oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.A.; Castello, P.R.; Poyton, R.O. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy. J. Photochem. Photobiol. B Biol. 2011, 102, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. The role of nitric oxide in low level light therapy—Art. no. 684602. In Mechanisms for Low-Light Therapy Iii; Hamblin, M.R., Waynant, R.W., Anders, J., Eds.; SPIE: Bellingham, WA, USA, 2008; Volume 6846, p. 84602. [Google Scholar]
- Tim, C.R.; Bossini, P.S.; Kido, H.W.; Malavazi, I.; von Zeska Kress, M.R.; Carazzolle, M.F.; Parizotto, N.A.; Renno, A.C. Effects of low level laser therapy on inflammatory and angiogenic gene expression during the process of bone healing: A microarray analysis. J. Photochem. Photobiol. B 2016, 154, 8–15. [Google Scholar] [CrossRef]
- Aud, D.; Peng, S.L. Mechanisms of disease: Transcription factors in inflammatory arthritis. Nat. Clin. Pract. Rheum 2006, 2, 434–442. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.A.; Alves, A.C.A.; Leal, E.C.P.; Albertini, R.; Vieira, R.D.; Ligeiro, A.P.; Silva, J.A.; de Carvalho, P.D.C. Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med. Sci. 2014, 29, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, L.M.J.; Salvador, P.A.; de Souza, Á, C.; de Souza da Fonseca, A.; de Paoli, F.; Gameiro, J. Modulation of immune response to induced-arthritis by low-level laser therapy. J. Biophotonics 2019, 12, e201800120. [Google Scholar] [CrossRef]
- Fukuda, T.Y.; Tanji, M.M.; Silva, S.R.; Sato, M.N.; Plapler, H. Infrared low-level diode laser on inflammatory process modulation in mice: Pro- and anti-inflammatory cytokines. Lasers Med. Sci. 2013, 28, 1305–1313. [Google Scholar] [CrossRef]
- Torres-Silva, R.; Lopes-Martins, R.A.B.; Bjordal, J.M.; Frigo, L.; Rahouadj, R.; Arnold, G.; Leal, E.C.P.; Magdalou, J.; Pallotta, R.; Marcos, R.L. The low level laser therapy (LLLT) operating in 660 nm reduce gene expression of inflammatory mediators in the experimental model of collagenase-induced rat tendinitis. Lasers Med. Sci. 2015, 30, 1985–1990. [Google Scholar] [CrossRef]
- Nie, H.; Zheng, Y.; Li, R.; Guo, T.B.; He, D.; Fang, L.; Liu, X.; Xiao, L.; Chen, X.; Wan, B.; et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 2013, 19, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Tanaka, T. Interleukin 6 and rheumatoid arthritis. Biomed. Res. Int. 2014, 2014, 698313. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.A.; Vieira, R.d.P.; Leal-Junior, E.C.P.; dos Santos, S.A.; Ligeiro, A.P.; Albertini, R.; Junior, J.A.S.; de Carvalho, P.d.T.C. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res. Ther. 2013, 15, R116. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Muniz Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Coutts, R.D.; Badlani, N.M.; Healey, R.M.; Kubo, T.; Amiel, D. Effect of light-emitting diode (LED) therapy on the development of osteoarthritis (OA) in a rabbit model. Biomed. Pharmacother. 2011, 65, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.S.; Adly, A.S.; Adly, M.S. Effects of laser acupuncture tele-therapy for rheumatoid arthritis elderly patients. Lasers Med. Sci. 2022, 37, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Travis, M.A.; Sheppard, D. TGF-β activation and function in immunity. Annu Rev Immunol 2014, 32, 51–82. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Zhang, Y.-M.; Zhang, L.-Y.; Zhou, T.; Li, Y.-Y.; Zhou, G.-C.; Miao, Z.-M.; Shang, M.; He, J.-P.; Ding, N.; et al. Duality of Interactions Between TGF-β and TNF-α During Tumor Formation. Front. Immunol. 2022, 12, 810286. [Google Scholar] [CrossRef]
- Bartoli, D.M.F.; Felizatti, A.L.; do Bomfim, F.R.C.; Bovo, J.L.; de Aro, A.A.; do Amaral, M.E.C.; Esquisatto, M.A.M. Laser treatment of synovial inflammatory process in experimentally induced microcrystalline arthritis in Wistar rats. Lasers Med. Sci. 2021, 36, 529–540. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. Aims Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Larkin, S.; Williams, T.J. Cyclooxygenase-2—Regulation and Relevance in Inflammation. Biochem. Pharmacol. 1995, 50, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. COX-2 selective inhibitors in the treatment of arthritis: A rheumatologist perspective. Curr. Top. Med. Chem. 2005, 5, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci-Landmrk 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Pope, R.M. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat. Rev. Immunol. 2002, 2, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, L.M.J.; da Fonseca, A.S.; Gameiro, J.; de Paoli, F. Apoptosis induced by low-level laser in polymorphonuclear cells of acute joint inflammation: Comparative analysis of two energy densities. Lasers Med. Sci. 2017, 32, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Zhang, L.; Kajiwara, H.; Kuboyama, N.; Abiko, Y. Reduction of CXCR4 expression in Rheumatoid Arthritis Rat Joints by low level diode laser irradiation. Laser Ther. 2011, 20, 53–58. [Google Scholar] [CrossRef]
- Pap, T.; Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615. [Google Scholar] [CrossRef]
- Carlos, F.P.; de Paula Alves da Silva, M.; de Lemos Vasconcelos Silva Melo, E.; Costa, M.S.; Zamuner, S.R. Protective effect of low-level laser therapy (LLLT) on acute zymosan-induced arthritis. Lasers Med. Sci. 2014, 29, 757–763. [Google Scholar] [CrossRef]
- Karu, T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Pakozdi, A.; Szentpetery, A.; Besenyei, T.; Koch, A.E. Chemokines and angiogenesis in rheumatoid arthritis. Front. Biosci. (Elite Ed.) 2009, 1, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Kishimoto, T. Interleukin 6: From bench to bedside. Nat. Clin. Pr. Rheum. 2006, 2, 619–626. [Google Scholar] [CrossRef]
- Souza, N.H.C.; Mesquita-Ferrari, R.A.; Rodrigues, M.; da Silva, D.F.T.; Ribeiro, B.G.; Alves, A.N.; Garcia, M.P.; Nunes, F.D.; da Silva Junior, E.M.; França, C.M.; et al. Photobiomodulation and different macrophages phenotypes during muscle tissue repair. J. Cell. Mol. Med. 2018, 22, 4922–4934. [Google Scholar] [CrossRef] [PubMed]
- Maddur, M.S.; Miossec, P.; Kaveri, S.V.; Bayry, J. Th17 cells: Biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am. J. Pathol. 2012, 181, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Byng-Maddick, R.; Ehrenstein, M.R. The impact of biological therapy on regulatory T cells in rheumatoid arthritis. Rheumatology 2015, 54, 768–775. [Google Scholar] [CrossRef]
- Cooles, F.A.; Isaacs, J.D.; Anderson, A.E. Treg cells in rheumatoid arthritis: An update. Curr. Rheumatol. Rep. 2013, 15, 352. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. MECHANISMS OF DISEASE The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, V3–V11. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, H.; da Silva, J.A.P.; Souto-Carneiro, M.M. Potential roles for CD8(+) T cells in rheumatoid arthritis. Autoimmun. Rev. 2013, 12, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, M.; Yao, M.; Yaroslavsky, I.; Cohen, R.; Smotrich, M.; Kochevar, I.E. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg. Med. 2009, 41, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Li, K.; Liang, Z.W.; Dai, C.; Shen, X.F.; Gong, Y.Z.; Wang, S.; Hu, X.Y.; Wang, Z. Low-level laser facilitates alternatively activated macrophage/microglia polarization and promotes functional recovery after crush spinal cord injury in rats. Sci. Rep. 2017, 7, 620. [Google Scholar] [CrossRef]
- Souza, N.H.; Ferrari, R.A.; Silva, D.F.; Nunes, F.D.; Bussadori, S.K.; Fernandes, K.P. Effect of low-level laser therapy on the modulation of the mitochondrial activity of macrophages. Braz. J. Phys. Ther. 2014, 18, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Fahimipour, F.; Mahdian, M.; Houshmand, B.; Asnaashari, M.; Sadrabadi, A.N.; Farashah, S.E.N.; Mousavifard, S.M.; Khojasteh, A. The effect of He-Ne and Ga-Al-As laser light on the healing of hard palate mucosa of mice. Lasers Med. Sci. 2013, 28, 93–100. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Cheng, Y.J.; Huang, F.C.; Yang, C.C. The Fluence Effects of Low-Level Laser Therapy on Inflammation, Fibroblast-Like Synoviocytes, and Synovial Apoptosis in Rats with Adjuvant-Induced Arthritis. Photomed. Laser Surg. 2014, 32, 669–677. [Google Scholar] [CrossRef]
- Burger, E.; Mendes, A.C.S.C.; Bani, G.M.A.C.; Brigagao, M.R.P.L.; Santos, G.B.; Malaquias, L.C.C.; Chavasco, J.K.; Verinaud, L.M.; de Camargo, Z.P.; Hamblin, M.R.; et al. Low-level Laser Therapy to the Mouse Femur Enhances the Fungicidal Response of Neutrophils against Paracoccidioides brasiliensis. PLoS Neglected Trop. Dis. 2015, 9, e0003541. [Google Scholar] [CrossRef]
- Cerdeira, C.D.; Pereira Lima Brigagao, M.R.; de Carli, M.L.; Ferreira, C.d.S.; Isac Moraes, G.d.O.; Hadad, H.; Costa Hanemann, J.A.; Hamblin, M.R.; Sperandio, F.F. Low-level laser therapy stimulates the oxidative burst in human neutrophils and increases their fungicidal capacity. J. Biophotonics 2016, 9, 1180–1188. [Google Scholar] [CrossRef]
- Fahimipour, F.; Houshmand, B.; Alemi, P.; Asnaashari, M.; Tafti, M.A.; Akhoundikharanagh, F.; Farashah, S.E.N.; Aminisharifabad, M.; Korani, A.S.; Mahdian, M.; et al. The effect of He-Ne and Ga-Al-As lasers on the healing of oral mucosa in diabetic mice. J. Photochem. Photobiol. B-Biol. 2016, 159, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Trentham, D.E.; Townes, A.S.; Kang, A.H. Autoimmunity to type II collagen an experimental model of arthritis. J. Exp. Med. 1977, 146, 857–868. [Google Scholar] [CrossRef]
- Trentham, D.E. Collagen arthritis in rats, arthritogenic lymphokines and other aspects. Int. Rev. Immunol. 1988, 4, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.M.; Wood, F.D.; McDaniel, E.G.; Daft, F.S. Adjuvant Arthritis Induced In Germ-Free Rats. Proc. Soc. Exp. Biol. Med. 1963, 112, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Karumuthil-Melethil, S.; Sofi, M.H.; Gudi, R.; Johnson, B.M.; Perez, N.; Vasu, C. TLR2-and Dectin 1-Associated Innate Immune Response Modulates T-Cell Response to Pancreatic beta-Cell Antigen and Prevents Type 1 Diabetes. Diabetes 2015, 64, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, M.E.; Tarussio, D.; Chobaz-Peclat, V.; Busso, N.; So, A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res. Ther. 2005, 7, R370–R379. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Schorlemmer, H.U.; Pope, C.; Allison, A.C. Zymosan-induced arthritis: A model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977, 20, 1396–1401. [Google Scholar] [CrossRef]
- Rocha, F.A.; Aragão, A.G., Jr.; Oliveira, R.C.; Pompeu, M.M.; Vale, M.R.; Ribeiro, R.A. Periarthritis promotes gait disturbance in zymosan-induced arthritis in rats. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 1999, 48, 485–490. [Google Scholar] [CrossRef]
- Lin, Y.S.; Huang, M.H.; Chai, C.Y. Effects of helium–neon laser on the mucopolysaccharide induction in experimental osteoarthritic cartilage. Osteoarthr. Cartil. 2006, 14, 377–383. [Google Scholar] [CrossRef][Green Version]
- da Rosa, A.S.; dos Santos, A.F.; da Silva, M.M.; Facco, G.G.; Perreira, D.M.; Alves, A.C.A.; Leal Junior, E.C.P.; de Carvalho, P.d.T.C. Effects of Low-level Laser Therapy at Wavelengths of 660 and 808 nm in Experimental Model of Osteoarthritis. Photochem. Photobiol. 2012, 88, 161–166. [Google Scholar] [CrossRef]
- Murray, D.G. Experimentally induced arthritis using intra-articular papain. Arthritis Rheum. 1964, 7, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ramonda, R.; Frallonardo, P.; Oliviero, F.; Lorenzin, M.; Ortolan, A.; Scanu, A.; Punzi, L. Pain and microcrystalline arthritis. Reumatismo 2014, 66, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Soriano, F.; Campana, V.; Moya, M.; Gavotto, A.; Simes, J.; Soriano, M.; Soriano, R.; Spitale, L.; Palma, J. Photobiomodulation of pain and inflammation in microcrystalline arthropathies: Experimental and clinical results. Photomed. Laser Surg. 2006, 24, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Bossini, P.S.; Renno, A.C.M.; Ribeiro, D.A.; Fangel, R.; Ribeiro, A.C.; Lahoz, M.D.; Parizotto, N.A. Low level laser therapy (830 nm) improves bone repair in osteoporotic rats: Similar outcomes at two different dosages. Exp. Gerontol. 2012, 47, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.H.; Wen, C.Y.; Jia, X.F.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.Z.; Yao, J.; et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Bradley, E.W.; Weivoda, M.M.; Hwang, S.M.; Pirtskhalava, T.; Decklever, T.; Curran, G.L.; Ogrodnik, M.; Jurk, D.; Johnson, K.O.; et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2017, 72, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Takao, K.; Miyakawa, T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 1167–1172. [Google Scholar] [CrossRef]
- Smith, C.L.; Blake, J.A.; Kadin, J.A.; Richardson, J.E.; Bult, C.J.; Mouse Genome Database, G. Mouse Genome Database (MGD)-2018: Knowledgebase for the laboratory mouse. Nucleic Acids Res. 2018, 46, D836–D842. [Google Scholar] [CrossRef]
- Kuboyama, N.; Ohta, M.; Sato, Y.; Abiko, Y. Anti-inflammatory Activities of Light Emitting Diode Irradiation on Collagen-induced Arthritis in Mice. Nippon. Laser Igakkaishi 2012, 33, 19–25. [Google Scholar] [CrossRef][Green Version]
- de Morais, N.C.R.; Barbosa, A.M.; Vale, M.L.; Villaverde, A.B.; de Lima, C.J.; Cogo, J.C.; Zamuner, S.R. Anti-Inflammatory Effect of Low-Level Laser and Light-Emitting Diode in Zymosan-Induced Arthritis. Photomed. Laser Surg. 2010, 28, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Peimani, A.; Sardary, F. Effect of low-level laser on healing of temporomandibular joint osteoarthritis in rats. J. Dent. (Tehran Iran) 2014, 11, 319–327. [Google Scholar]
- Mangueira, N.M.; Xavier, M.; de Souza, R.A.; Salgado, M.A.; Silveira, L., Jr.; Villaverde, A.B. Effect of low-level laser therapy in an experimental model of osteoarthritis in rats evaluated through Raman spectroscopy. Photomed. Laser Surg. 2015, 33, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Son, T.; Lee, A.; Youn, I.; Seo, D.H.; Kim, H.S.; Jung, B. The effects of a minimally invasive laser needle system on complete Freund’s adjuvant-induced arthritis. Lasers Med. Sci. 2014, 29, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, J.; Kuboyama, N.; Abiko, Y. Low-level laser irradiation treatment reduces CCL2 expression in rat rheumatoid synovia via a chemokine signaling pathway. Lasers Med. Sci. 2011, 26, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Dai, T.H.; Yaroslavsky, I.; Cohen, R.; Apruzzese, W.A.; Smotrich, M.H.; Hamblin, M.R. Low-level laser therapy for zymosan-induced arthritis in rats: Importance of illumination time. Lasers Surg. Med. 2007, 39, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; de Carvalho, P.T.; Parente, M.; Xavier, M.; Frigo, L.; Aimbire, F.; Leal Junior, E.C.; Albertini, R. Low-level laser therapy in different stages of rheumatoid arthritis: A histological study. Lasers Med. Sci. 2013, 28, 529–536. [Google Scholar] [CrossRef]
- Lemos, G.A.; Rissi, R.; de Souza Pires, I.L.; de Oliveira, L.P.; de Aro, A.A.; Pimentel, E.R.; Palomari, E.T. Low-level laser therapy stimulates tissue repair and reduces the extracellular matrix degradation in rats with induced arthritis in the temporomandibular joint. Lasers Med. Sci. 2016, 31, 1051–1059. [Google Scholar] [CrossRef]
- Neves, M.; Retameiro, A.C.B.; Tavares, A.L.F.; Reginato, A.; Menolli, R.A.; Leal, T.; Ribeiro, L.F.C.; Bertolini, G.R.F. Physical exercise and low-level laser therapy on the nociception and leukocyte migration of Wistar rats submitted to a model of rheumatoid arthritis. Lasers Med. Sci. 2020, 35, 1277–1287. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Mazulo-Neto, J.C.R.; Souza, L.G.; Mazulo, F.R.d.S.; Filgueiras, M.d.C. Effects of photobiomodulation in pain and articular degeneration in mice arthritis model. BrJP 2021, 4, 104–107. [Google Scholar] [CrossRef]
- Capaldi, R.A. structure and function of cytochrome c oxidase. Annu. Rev. Biochem. 1990, 59, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]
- Sommer, A.P.; Bieschke, J.; Friedrich, R.P.; Zhu, D.; Wanker, E.E.; Fecht, H.J.; Mereles, D.; Hunstein, W. 670 nm laser light and EGCG complementarily reduce amyloid-β aggregates in human neuroblastoma cells: Basis for treatment of Alzheimer’s disease? Photomed. Laser Surg. 2012, 30, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, N.; Ohta, M.; Sato, Y.; Abiko, Y. Anti-inflammatory activities of light emitting diode irradiation on collagen-induced arthritis in mice (a secondary publication). Laser Ther. 2014, 23, 191–199. [Google Scholar] [CrossRef]
- Algarni, A.D.; Alrabai, H.M.; Al-Ahaideb, A.; Kachanathu, S.J.; AlShammari, S.A. Arabic translation, cultural adaptation, and validation study of Knee Outcome Survey: Activities of Daily Living Scale (KOS-ADLS). Rheumatol. Int. 2017, 37, 1585–1589. [Google Scholar] [CrossRef]
- Ip, D. Does addition of low-level laser therapy (LLLT) in conservative care of knee arthritis successfully postpone the need for joint replacement? Lasers Med. Sci. 2015, 30, 2335–2339. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Hung, M.; Keener, J.D.; Bowen, R.C.; McAllister, J.; Chen, W.; Ebersole, G.; Granger, E.K.; Chamberlain, A.M. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 144–148. [Google Scholar] [CrossRef]
- Dasgupta, B.; Cimmino, M.A.; Maradit-Kremers, H.; Schmidt, W.A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S.; et al. 2012 provisional classification criteria for polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann. Rheum. Dis. 2012, 71, 484–492. [Google Scholar] [CrossRef]
- Kelly, C.A.; Saravanan, V.; Nisar, M.; Arthanari, S.; Woodhead, F.A.; Price-Forbes, A.N.; Dawson, J.; Sathi, N.; Ahmad, Y.; Koduri, G.; et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics-a large multicentre UK study. Rheumatology 2014, 53, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Gerlag, D.M.; Safy, M.; Maijer, K.I.; Tang, M.W.; Tas, S.W.; Starmans-Kool, M.J.F.; van Tubergen, A.; Janssen, M.; de Hair, M.; Hansson, M.; et al. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: The PRAIRI study. Ann. Rheum. Dis. 2019, 78, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Sokka, T.; Pincus, T. Erythrocyte Sedimentation Rate, C-Reactive Protein, or Rheumatoid Factor Are Normal at Presentation in 35%-45% of Patients with Rheumatoid Arthritis Seen Between 1980 and 2004: Analyses from Finland and the United States. J. Rheumatol. 2009, 36, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- S, G.N.; Kamal, W.; George, J.; Manssor, E. Radiological and biochemical effects (CTX-II, MMP-3, 8, and 13) of low-level laser therapy (LLLT) in chronic osteoarthritis in Al-Kharj, Saudi Arabia. Lasers Med. Sci. 2017, 32, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, B.; Viharos, L.; Gervain, M.; Gálfi, M. The effect of low-level laser in knee osteoarthritis: A double-blind, randomized, placebo-controlled trial. Photomed. Laser Surg. 2009, 27, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.; Omar, M.T.; Al-Askar, A.B.; Al-Muteri, N.K. Effect of low-level laser therapy in patients with chronic knee osteoarthritis: A single-blinded randomized clinical study. Lasers Med. Sci. 2014, 29, 749–755. [Google Scholar] [CrossRef]
- Gur, A.; Cosut, A.; Sarac, A.J.; Cevik, R.; Nas, K.; Uyar, A. Efficacy of different therapy regimes of low-power laser in painful osteoarthritis of the knee: A double-blind and randomized-controlled trial. Lasers Surg. Med. 2003, 33, 330–338. [Google Scholar] [CrossRef]
- Alfredo, P.P.; Bjordal, J.M.; Dreyer, S.H.; Meneses, S.R.; Zaguetti, G.; Ovanessian, V.; Fukuda, T.Y.; Junior, W.S.; Lopes Martins, R.; Casarotto, R.A.; et al. Efficacy of low level laser therapy associated with exercises in knee osteoarthritis: A randomized double-blind study. Clin. Rehabil. 2012, 26, 523–533. [Google Scholar] [CrossRef]
- Al Rashoud, A.S.; Abboud, R.J.; Wang, W.; Wigderowitz, C. Efficacy of low-level laser therapy applied at acupuncture points in knee osteoarthritis: A randomised double-blind comparative trial. Physiotherapy 2014, 100, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kheshie, A.R.; Alayat, M.S.; Ali, M.M. High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: A randomized controlled trial. Lasers Med. Sci. 2014, 29, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, A.W.; Ostapczuk, M.S.; Stosch, D. Positive effects of low level laser therapy (LLLT) on Bouchard’s and Heberden’s osteoarthritis. Lasers Surg. Med. 2016, 48, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, V.O.; Fukuda, T.Y.; Guimarães, M.; Shiwa, S.; de Lima Bdel, C.; Martins, R.; Casarotto, R.A.; Alfredo, P.P.; Bjordal, J.M.; Fucs, P.M. Short-Term Efficacy Of Low-Level Laser Therapy In Patients With Knee Osteoarthritis: A Randomized Placebo-Controlled, Double-Blind Clinical Trial. Rev. Bras. De Ortop. 2011, 46, 526–533. [Google Scholar] [CrossRef]
- Stelian, J.; Gil, I.; Habot, B.; Rosenthal, M.; Abramovici, I.; Kutok, N.; Khahil, A. Improvement of pain and disability in elderly patients with degenerative osteoarthritis of the knee treated with narrow-band light therapy. J. Am. Geriatr. Soc. 1992, 40, 23–26. [Google Scholar] [CrossRef]
- Djavid, G.E.; Mehrdad, R.; Ghasemi, M.; Hasan-Zadeh, H.; Sotoodeh-Manesh, A.; Pouryaghoub, G. In chronic low back pain, low level laser therapy combined with exercise is more beneficial than exercise alone in the long term: A randomised trial. Aust. J. Physiother. 2007, 53, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Heussler, J.K.; Hinchey, G.; Margiotta, E.; Quinn, R.; Butler, P.; Martin, J.; Sturgess, A.D. A double blind randomised trial of low power laser treatment in rheumatoid arthritis. Ann. Rheum. Dis. 1993, 52, 703–706. [Google Scholar] [CrossRef]
- Bliddal, H.; Hellesen, C.; Ditlevsen, P.; Asselberghs, J.; Lyager, L. Soft-laser therapy of rheumatoid arthritis. Scand. J. Rheumatol. 1987, 16, 225–228. [Google Scholar] [CrossRef]
- Paolillo, A.R.; Paolillo, F.R.; João, J.P.; João, H.A.; Bagnato, V.S. Synergic effects of ultrasound and laser on the pain relief in women with hand osteoarthritis. Lasers Med. Sci. 2015, 30, 279–286. [Google Scholar] [CrossRef]
- Ferreira de Meneses, S.R.; Hunter, D.J.; Young Docko, E.; Pasqual Marques, A. Effect of low-level laser therapy (904 nm) and static stretching in patients with knee osteoarthritis: A protocol of randomised controlled trial. BMC Musculoskelet. Disord. 2015, 16, 252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).