Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation
Abstract
:1. Introduction
2. Results
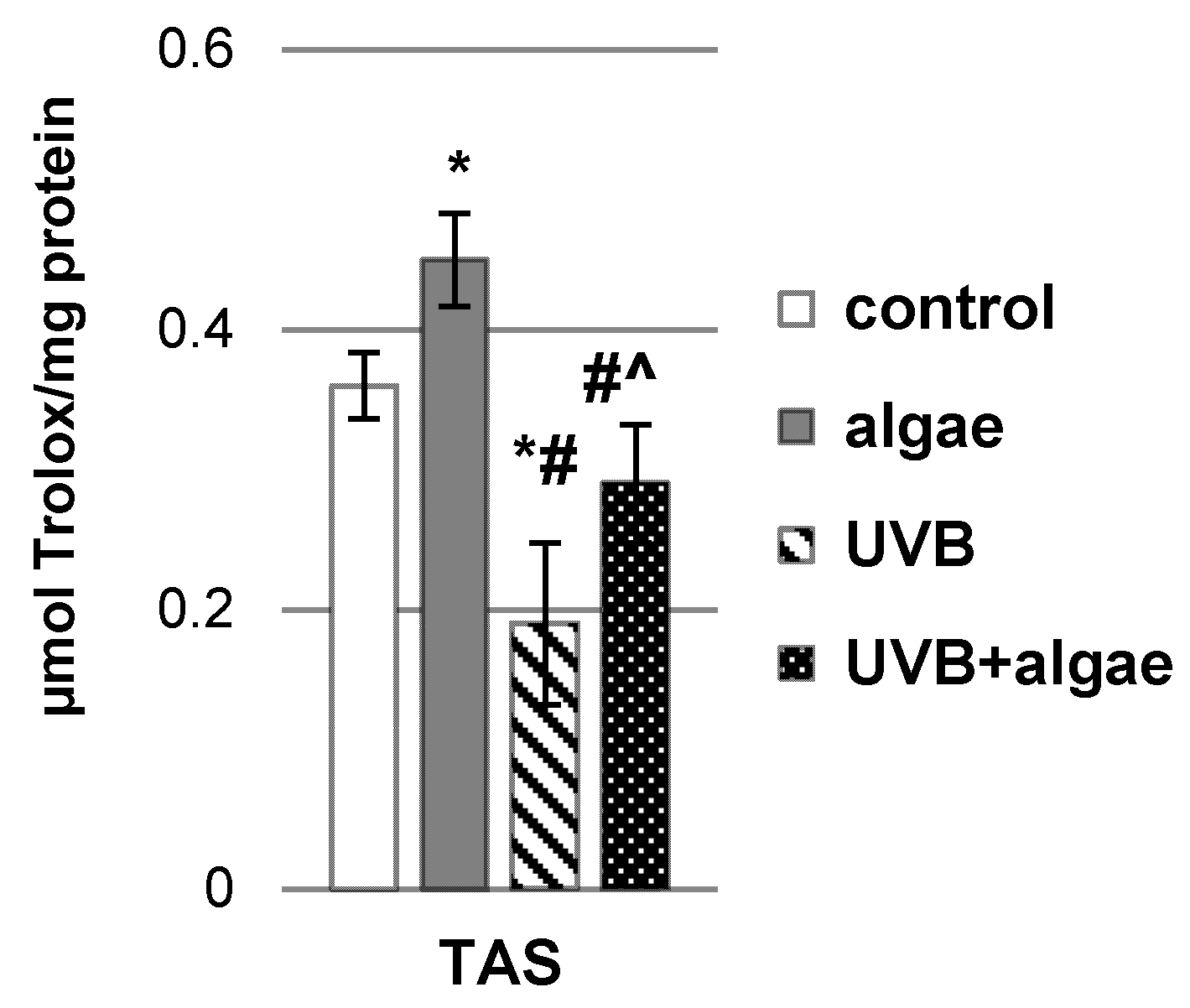
2.1. Impact of the Lipid Extract Derived from the Microalgae Nannochloropsis oceanica on the Antioxidant Status of Keratinocytes
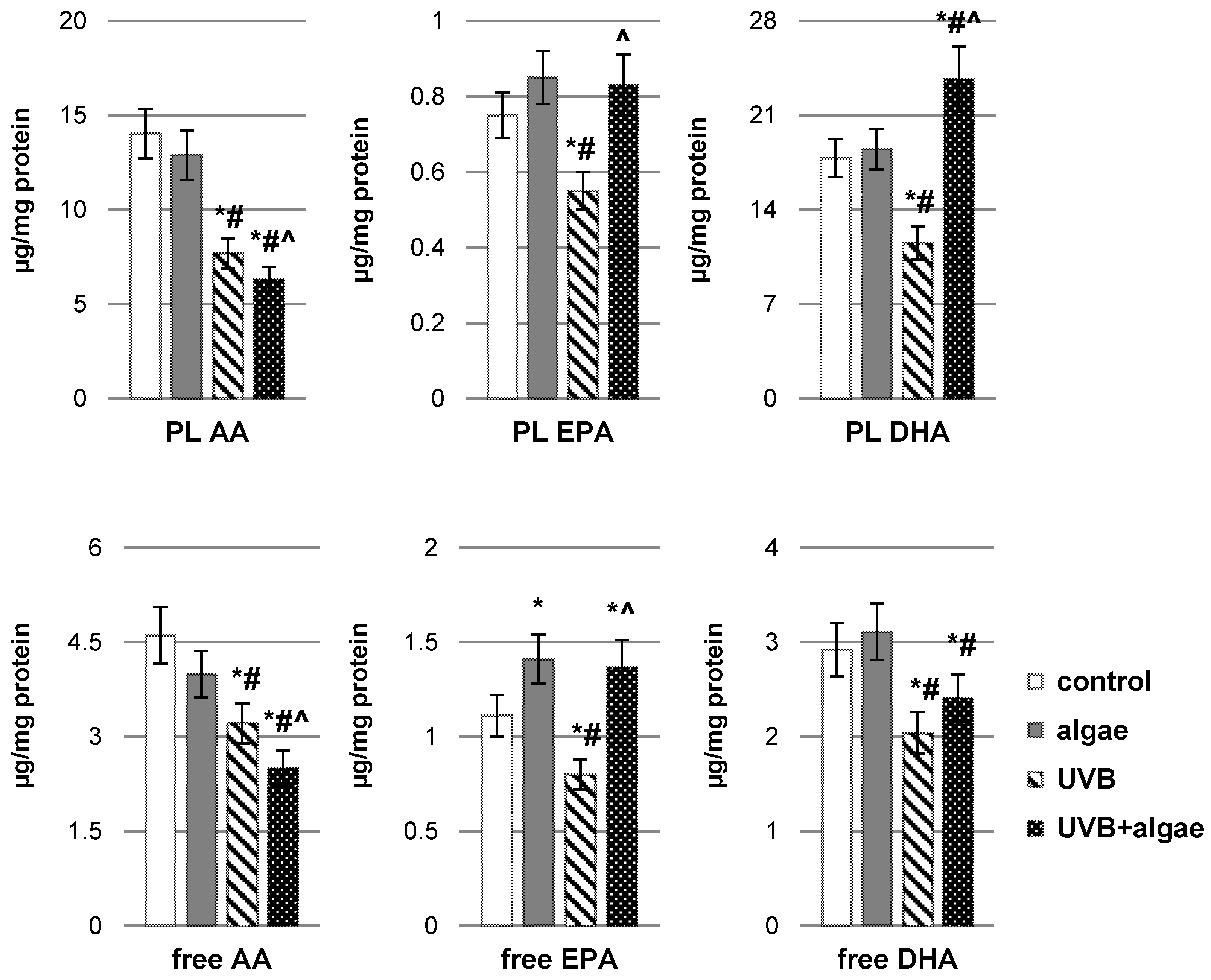
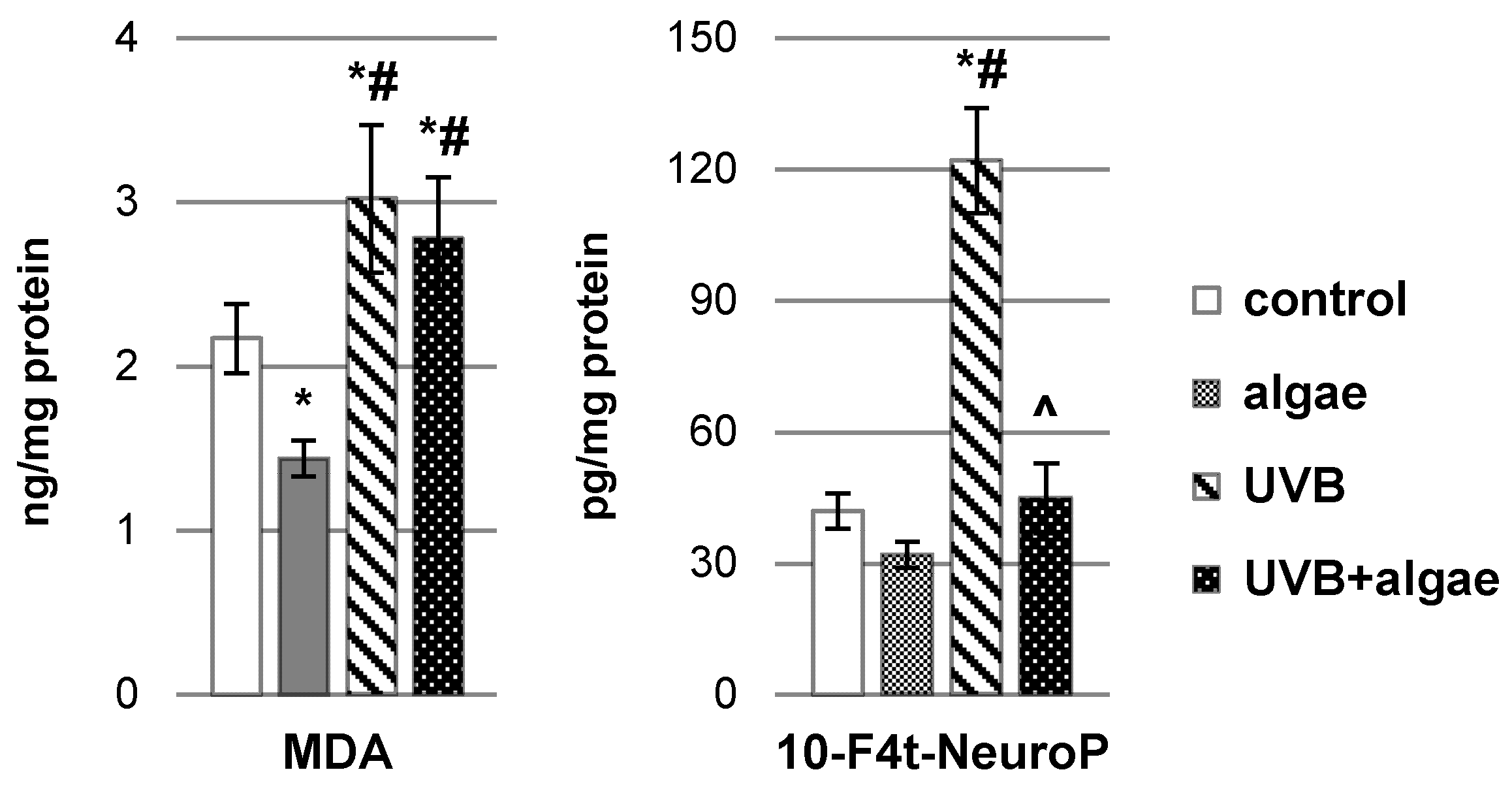
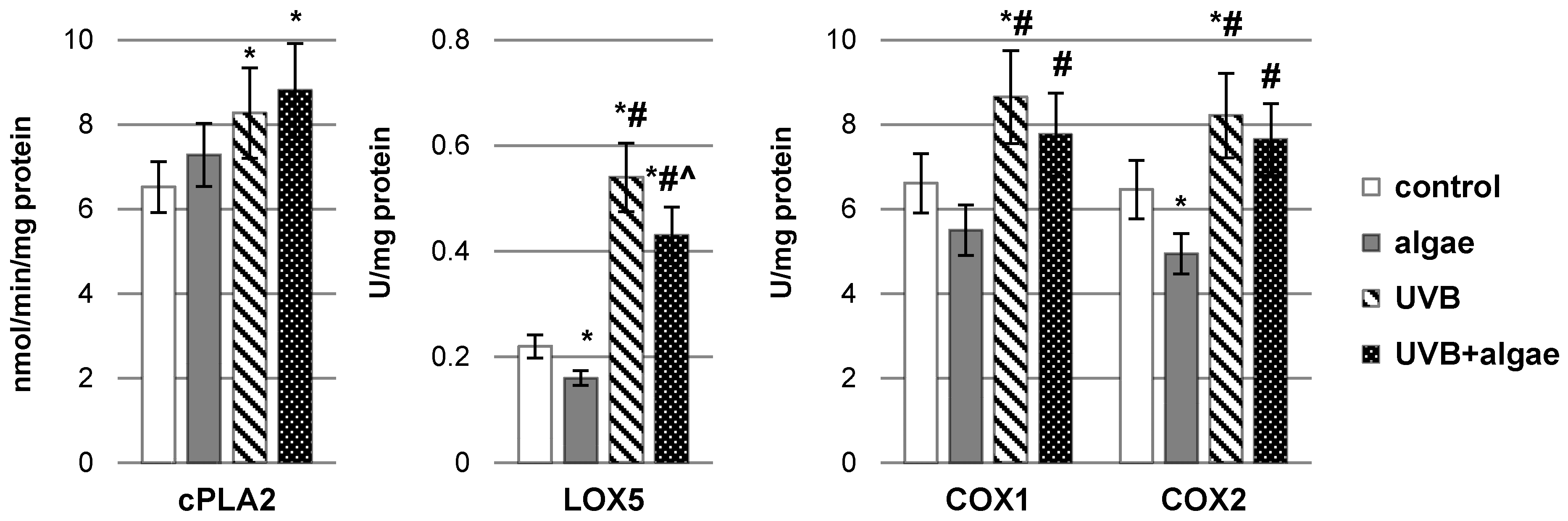
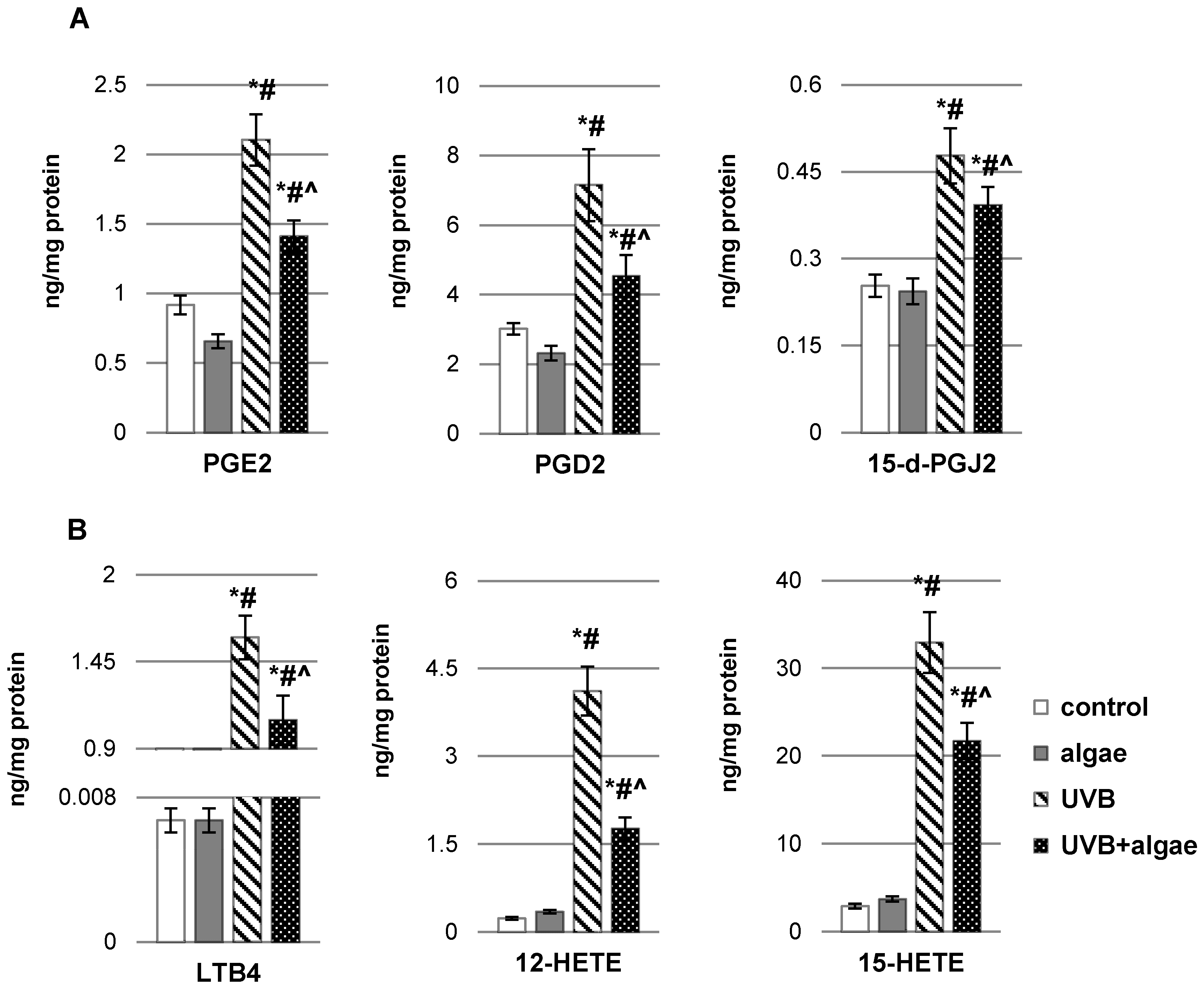
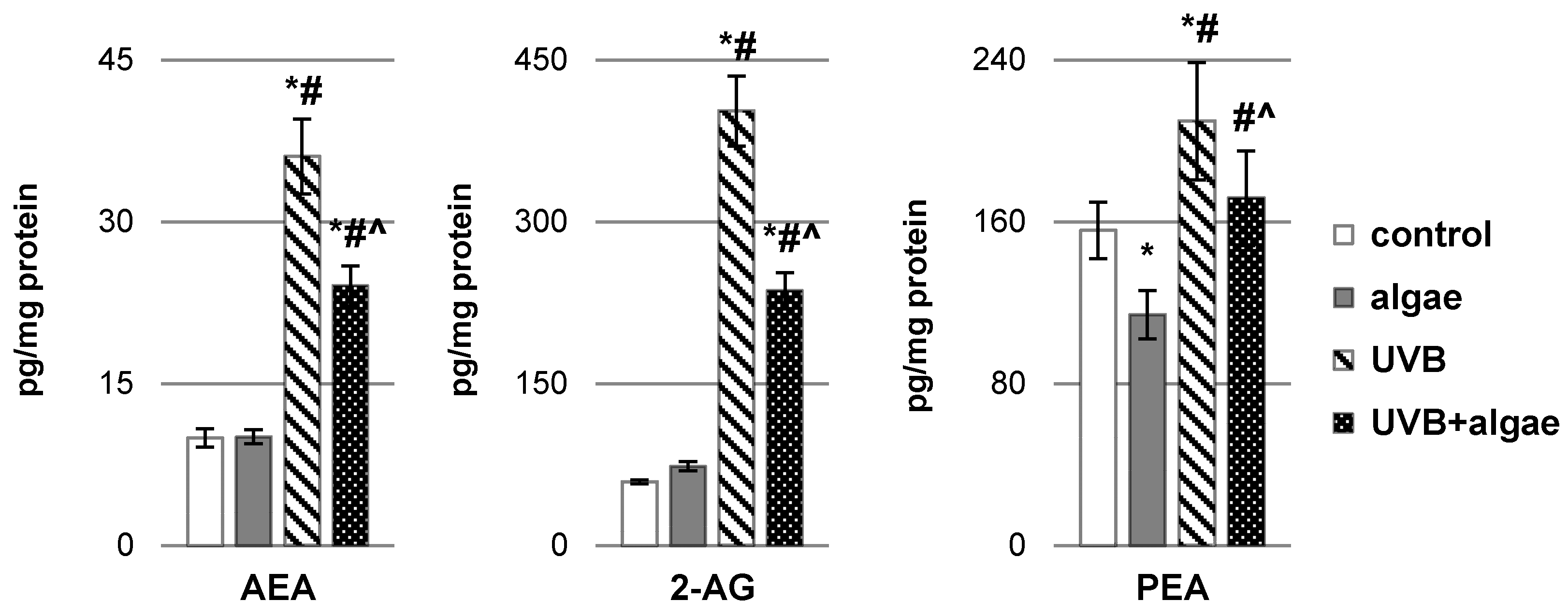
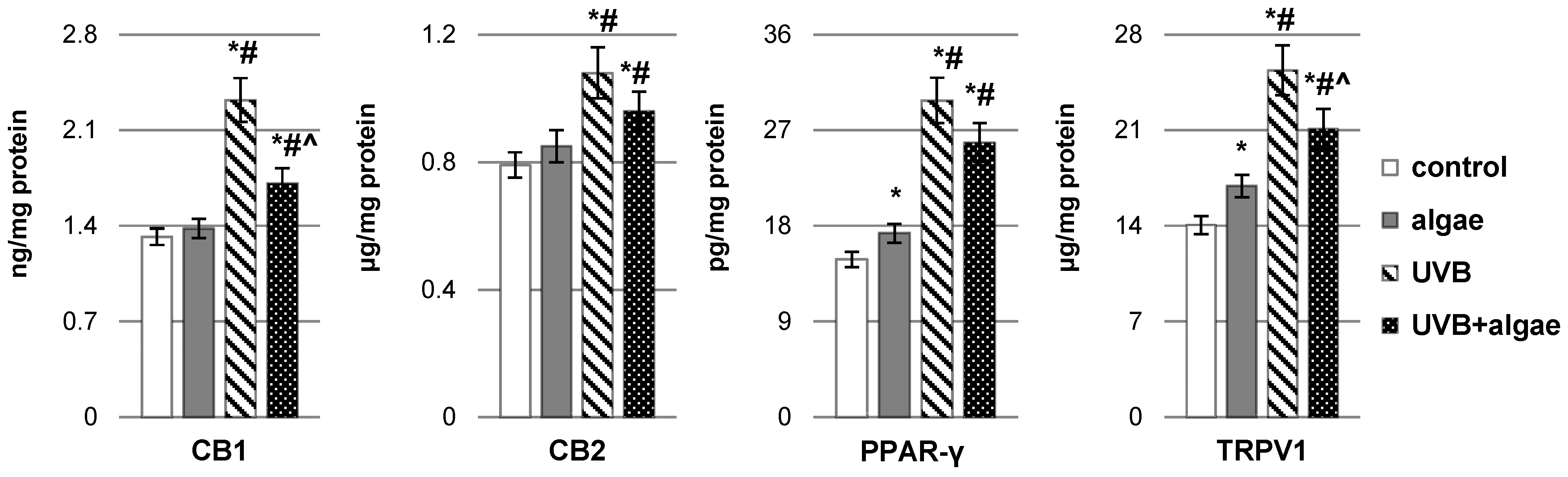
2.2. Impact of Nannochloropsis oceanica Microalgae Lipid Extract on Keratinocyte Lipid Metabolism
3. Discussion
4. Materials and Methods
4.1. Materials and Cell Culture Treatment
4.1.1. Preparation of Microalgae Lipid Extracts
4.1.2. Cell Culture
4.2. Methods
4.2.1. Estimation of Total Antioxidant Status (TAS)
4.2.2. Determination of Phospholipid and Free PUFA Levels
4.2.3. Determination of the Level of Lipid Peroxidation Products
4.2.4. Determination of the Activity of Lipolytic Enzymes
4.2.5. Determination of the Level of Endocannabinoids
4.2.6. Determination of the Level of Eicosanoids
4.2.7. Determination of Expression of Membrane Receptors
4.2.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, Y.; Liu, N.; Yang, M.; Hu, Y.; Li, X.; Fu, Y.; Luo, M.; Sun, J.; Yang, X. Potential Skin Protective Effects after UVB Irradiation Afforded by an Antioxidant Peptide from Odorrana Andersonii. Biomed. Pharmacother. 2019, 120, 109535. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Protects Keratinocyte Cell Membranes Following Exposure to UVB and Hydrogen Peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guo, K.; Chen, Y.; Zhao, J.; Jing, R.; Wang, L.; Li, X.; Hu, Z.; Xu, N.; Li, X. Keratinocyte Growth Factor 2 Ameliorates UVB-Induced Skin Damage via Activating the AhR/Nrf2 Signaling Pathway. Front. Pharmacol. 2021, 12, 655281. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and Ascorbic Acid Cooperation in Antioxidant and Antiapoptotic Effect on Human Skin Keratinocytes and Fibroblasts Exposed to UVA and UVB Radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Ryšavá, A.; Vostálová, J.; Rajnochová Svobodová, A. Effect of Ultraviolet Radiation on the Nrf2 Signaling Pathway in Skin Cells. Int. J. Radiat. Biol. 2021, 97, 1383–1403. [Google Scholar] [CrossRef]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the Study of Skin Wound Healing. The Role of Nrf2 and NF-κB. Biomedical. Pap. 2017, 161, 1–13. [Google Scholar] [CrossRef]
- Conde, T.; Lopes, D.; Łuczaj, W.; Neves, B.; Pinto, B.; Maurício, T.; Domingues, P.; Skrzydlewska, E.; Domingues, M.R. Algal Lipids as Modulators of Skin Disease: A Critical Review. Metabolites 2022, 12, 96. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Nannochloropsis Oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats. Nutrients 2021, 13, 3991. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jia, J. Photoprotection Mechanisms of Nannochloropsis Oceanica in Response to Light Stress. Algal Res. 2020, 46, 101784. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Scott, J.A.; Ross, G.M. Management of Oxidative Stress by Microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.-T.; Teoh, M.-L.; Phang, S.-M.; Convey, P.; Yap, W.-H.; Goh, B.-H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Jung, J.H.; Kim, J.Y.; Heo, J.; Cho, D.-H.; Kim, H.-S.; An, S.; An, I.-S.; Bae, S. The Protective Effect of Violaxanthin from Nannochloropsis Oceanica against Ultraviolet B-Induced Damage in Normal Human Dermal Fibroblasts. Photochem. Photobiol. 2019, 95, 595–604. [Google Scholar] [CrossRef]
- Sá, M.; Ferrer-Ledo, N.; Wijffels, R.; Crespo, J.G.; Barbosa, M.; Galinha, C.F. Monitoring of Eicosapentaenoic Acid (EPA) Production in the Microalgae Nannochloropsis Oceanica. Algal Res. 2020, 45, 101766. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Rhodes, L.E.; Al-Aasswad, N.M.I.; Massey, K.A.; Nicolaou, A. Impact of EPA Ingestion on COX- and LOX-Mediated Eicosanoid Synthesis in Skin with and without a pro-Inflammatory UVR Challenge—Report of a Randomised Controlled Study in Humans. Mol. Nutr. Food Res. 2014, 58, 580–590. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6 and Omega-3 Fatty Acids: Endocannabinoids, Genetics and Obesity. OCL 2020, 27, 7. [Google Scholar] [CrossRef]
- Guesmi, A.; Boumaiza, M.; Boudabous, A. Microbiological Quality and Safety of Commercialized Thalassotherapy Products Based on Marine Mud and Algae Extracts in Tunisia. Arch. Microbiol. 2020, 202, 2437–2451. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.-P.; Renimel, I.; Filaire, E. Marine Algae as Attractive Source to Skin Care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Cunha, P.; Guerra, I.; Correia, N.; Silva, J.T.; Pereira, H.; et al. Effects of Outdoor and Indoor Cultivation on the Polar Lipid Composition and Antioxidant Activity of Nannochloropsis Oceanica and Nannochloropsis Limnetica: A Lipidomics Perspective. Algal Res. 2022, 64, 102718. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate Immune Cells in Atopic Dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Tong, X.; Huang, J.; Liu, L.; Wang, D.; Yang, S. Research Progress of Keratinocyte-Programmed Cell Death in UV-Induced Skin Photodamage. Photodermatol. Photoimmunol. Photomed. 2021, 37, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Mathew, S. Merkel Cells: A Collective Review of Current Concepts. Int. J. Appl. Basic Med. Res. 2019, 9, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Song, B.; Chen, H.-D.; Gao, X.-H. Melanocytes and Skin Immunity. J. Investig. Dermatol. Symp. Proc. 2015, 17, 37–39. [Google Scholar] [CrossRef]
- Speeckaert, R.; Belpaire, A.; Speeckaert, M.; van Geel, N. The Delicate Relation between Melanocytes and Skin Immunity: A Game of Hide and Seek. Pigment. Cell Melanoma Res. 2022, 35, 392–407. [Google Scholar] [CrossRef]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The Cross-Talk between Electrophiles, Antioxidant Defence and the Endocannabinoid System in Fibroblasts and Keratinocytes after UVA and UVB Irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Skrzydlewska, E. Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes. Antioxidants 2021, 10, 402. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Lee, K.; Cheong, S.H.; Han, Y.S.; Park, S.R.; et al. Human Keratinocyte UVB-Protective Effects of a Low Molecular Weight Fucoidan from Sargassum Horneri Purified by Step Gradient Ethanol Precipitation. Antioxidants 2020, 9, 340. [Google Scholar] [CrossRef]
- Li, Y.; Hao, D.; Wei, D.; Xiao, Y.; Liu, L.; Li, X.; Wang, L.; Gan, Y.; Yan, W.; Ke, B.; et al. Photoprotective Effects of Cannabidiol against Ultraviolet-B-Induced DNA Damage and Autophagy in Human Keratinocyte Cells and Mouse Skin Tissue. Molecules 2022, 27, 6740. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Pilkington, S.M.; Murphy, S.A.; Del Carratore, F.; Sunarwidhi, A.L.; Kiezel-Tsugunova, M.; Urquhart, P.; Watson, R.E.B.; Breitling, R.; Rhodes, L.E.; et al. Dynamics of the Human Skin Mediator Lipidome in Response to Dietary ω-3 Fatty Acid Supplementation. FASEB J. 2019, 33, 13014–13027. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Biernacki, M.; Domian, N.; Žarković, N.; Skrzydlewska, E. Influence of Inhibition of COX-2-Dependent Lipid Metabolism on Regulation of UVB-Induced Keratinocytes Apoptosis by Cannabinoids. Biomolecules 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Stasiewicz, A.; Conde, T.; Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis Oceanica. Int. J. Mol. Sci. 2023, 24, 11302. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(Ut)Annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Jang, A.J.; Chang, S.S.; Park, C.; Lee, C.-M.; Benza, R.L.; Passineau, M.J.; Ma, J.; Archer, D.R.; Sutliff, R.L.; Hart, C.M.; et al. PPARγ Increases HUWE1 to Attenuate NF-κB/P65 and Sickle Cell Disease with Pulmonary Hypertension. Blood Adv. 2021, 5, 399–413. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Savaghebi, D.; Barzegar, M.; Mozafari, M.R. Manufacturing of Nanoliposomal Extract from Sargassum Boveanum Algae and Investigating Its Release Behavior and Antioxidant Activity. Food Sci. Nutr. 2020, 8, 299–310. [Google Scholar] [CrossRef]
- Picciotto, S.; Santonicola, P.; Paterna, A.; Rao, E.; Raccosta, S.; Romancino, D.P.; Noto, R.; Touzet, N.; Manno, M.; Di Schiavi, E.; et al. Extracellular Vesicles From Microalgae: Uptake Studies in Human Cells and Caenorhabditis Elegans. Front. Bioeng. Biotechnol. 2022, 10, 830189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, L.; Zhu, L.; Liu, Y.; Yang, S.; Zhao, M. Lipid Droplets, the Central Hub Integrating Cell Metabolism and the Immune System. Front. Physiol. 2021, 12, 746749. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kim, S.-K. Biosynthesis of Nanoparticles Using Marine Algae: A Review. In Marine Algae Extracts; John Wiley & Sons, Ltd.: New York, NY, USA, 2015; pp. 295–304. ISBN 978-3-527-67957-7. [Google Scholar]
- Hussein, H.A.; Abdullah, M.A. Novel Drug Delivery Systems Based on Silver Nanoparticles, Hyaluronic Acid, Lipid Nanoparticles and Liposomes for Cancer Treatment. Appl. Nanosci. 2022, 12, 3071–3096. [Google Scholar] [CrossRef]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and Skin Function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Holleran, W.M.; Takagi, Y.; Uchida, Y. Epidermal Sphingolipids: Metabolism, Function, and Roles in Skin Disorders. FEBS Lett. 2006, 580, 5456–5466. [Google Scholar] [CrossRef] [PubMed]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar Lipidomic Profile Shows Chlorococcum Amblystomatis as a Promising Source of Value-Added Lipids. Sci. Rep. 2021, 11, 4355. [Google Scholar] [CrossRef]
- Gao, Q.-M.; Yu, K.; Xia, Y.; Shine, M.B.; Wang, C.; Navarre, D.; Kachroo, A.; Kachroo, P. Mono- and Digalactosyldiacylglycerol Lipids Function Nonredundantly to Regulate Systemic Acquired Resistance in Plants. Cell Rep. 2014, 9, 1681–1691. [Google Scholar] [CrossRef]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as Sources of Omega-3 Polyunsaturated Fatty Acids: Biotechnological Aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Zheng, J.; Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Han, X.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Ko, C.S.; et al. Photoprotective Effect of Carpomitra Costata Extract against Ultraviolet B-Induced Oxidative Damage in Human Keratinocytes. J. Env. Pathol. Toxicol. Oncol. 2016, 35, 11–28. [Google Scholar] [CrossRef]
- Lee, J.-J.; An, S.; Kim, K.B.; Heo, J.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S.; Bae, S. Extract of Ettlia Sp. YC001 Exerts Photoprotective Effects against UVB Irradiation in Normal Human Dermal Fibroblasts. J. Microbiol. Biotechnol. 2016, 26, 775–783. [Google Scholar] [CrossRef]
- Mukerjee, S.; Saeedan, A.S.; Ansari, M.N.; Singh, M. Polyunsaturated Fatty Acids Mediated Regulation of Membrane Biochemistry and Tumor Cell Membrane Integrity. Membranes 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Nagarajan, D.; Cheah, W.Y. Eicosapentaenoic Acid Production from Nannochloropsis Oceanica CY2 Using Deep Sea Water in Outdoor Plastic-Bag Type Photobioreactors. Bioresour. Technol. 2018, 253, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Ma, Y.; Wei, W.; Liu, W.; Ding, Y.; Balamurugan, S. Sequential Treatment with Bicarbonate and Low-Temperature to Potentiate Both Biomass and Lipid Productivity in Nannochloropsis Oceanica. J. Chem. Technol. Biotechnol. 2019, 94, 3413–3419. [Google Scholar] [CrossRef]
- Leung, K.S.; Chan, H.F.; Leung, H.H.; Galano, J.-M.; Oger, C.; Durand, T.; Lee, J.C.-Y. Short-Time UVA Exposure to Human Keratinocytes Instigated Polyunsaturated Fatty Acid without Inducing Lipid Peroxidation. Free Radic. Res. 2017, 51, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Sisignano, M.; Angioni, C.; Ferreiros, N.; Schuh, C.-D.; Suo, J.; Schreiber, Y.; Dawes, J.M.; Antunes-Martins, A.; Bennett, D.L.H.; McMahon, S.B.; et al. Synthesis of Lipid Mediators during UVB-Induced Inflammatory Hyperalgesia in Rats and Mice. PLoS ONE 2013, 8, e81228. [Google Scholar] [CrossRef]
- Neumann, U.; Louis, S.; Gille, A.; Derwenskus, F.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Anti-Inflammatory Effects of Phaeodactylum Tricornutum Extracts on Human Blood Mononuclear Cells and Murine Macrophages. J. Appl. Phycol. 2018, 30, 2837–2846. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Liu, M.; Qin, X.; Yu, X.; Zhao, H.; Li, X.; Li, W. COX-2 Is Required to Mediate Crosstalk of ROS-Dependent Activation of MAPK/NF-κB Signaling with pro-Inflammatory Response and Defense-Related NO Enhancement during Challenge of Macrophage-like Cell Line with Giardia Duodenalis. PLoS Negl. Trop. Dis. 2022, 16, e0010402. [Google Scholar] [CrossRef]
- Park, N.-Y.; Im, S.; Jiang, Q. Different Forms of Vitamin E and Metabolite 13′-Carboxychromanols Inhibit Cyclooxygenase-1 and Its Catalyzed Thromboxane in Platelets, and Tocotrienols and 13′-Carboxychromanols Are Competitive Inhibitors of 5-Lipoxygenase. J. Nutr. Biochem. 2022, 100, 108884. [Google Scholar] [CrossRef]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. Gamma-Tocopherol and Its Major Metabolite, in Contrast to Alpha-Tocopherol, Inhibit Cyclooxygenase Activity in Macrophages and Epithelial Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-Chain Carboxychromanols, Metabolites of Vitamin E, Are Potent Inhibitors of Cyclooxygenases. Proc. Natl. Acad. Sci. USA 2008, 105, 20464–20469. [Google Scholar] [CrossRef]
- Konieczka, P.; Barszcz, M.; Chmielewska, N.; Cieślak, M.; Szlis, M.; Smulikowska, S. Interactive Effects of Dietary Lipids and Vitamin E Level on Performance, Blood Eicosanoids, and Response to Mitogen Stimulation in Broiler Chickens of Different Ages. Poult. Sci. 2017, 96, 359–369. [Google Scholar] [CrossRef]
- Ued, F.V.; Mathias, M.G.; Toffano, R.B.D.; Barros, T.T.; Almada, M.O.R.V.; Salomão, R.G.; Coelho-Landell, C.A.; Hillesheim, E.; Camarneiro, J.M.; Camelo-Junior, J.S.; et al. Vitamin B2 and Folate Concentrations Are Associated with ARA, EPA and DHA Fatty Acids in Red Blood Cells of Brazilian Children and Adolescents. Nutrients 2019, 11, 2918. [Google Scholar] [CrossRef]
- Bhosale, P.; Bernstein, P.S. Synergistic Effects of Zeaxanthin and Its Binding Protein in the Prevention of Lipid Membrane Oxidation. Biochim. Biophys. Acta 2005, 1740, 116–121. [Google Scholar] [CrossRef]
- Sauer, L.; Li, B.; Bernstein, P.S. Ocular Carotenoid Status in Health and Disease. Annu. Rev. Nutr. 2019, 39, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha, S.; Vallikannan, B. Fatty Acids Modulate the Efficacy of Lutein in Cataract Prevention: Assessment of Oxidative and Inflammatory Parameters in Rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef]
- Karen-Ng, L.P.; Ahmad, U.S.; Gomes, L.; Hunter, K.D.; Wan, H.; Hagi-Pavli, E.; Parkinson, E.K. Extracellular Prostaglandins E1 and E2 and Inflammatory Cytokines Are Regulated by the Senescence Program in Potentially Premalignant Oral Keratinocytes. Cancers 2022, 14, 2636. [Google Scholar] [CrossRef]
- Prasad, R.; Katiyar, S.K. Prostaglandin E2 Promotes UV Radiation-Induced Immune Suppression through DNA Hypermethylation. Neoplasia 2013, 15, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H. Prostaglandin E2 Induces Skin Aging via E-Prostanoid 1 in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, C.; Wu, J. 15-Deoxy-∆-12,14-Prostaglandin J2 (15d-PGJ2), an Endogenous Ligand of PPAR-γ: Function and Mechanism. PPAR Res. 2019, 2019, 7242030. [Google Scholar] [CrossRef]
- Jung, W.-K.; Park, I.-S.; Park, S.-J.; Yea, S.S.; Choi, Y.H.; Oh, S.; Park, S.-G.; Choi, I.-W. The 15-Deoxy-Delta12,14-Prostaglandin J2 Inhibits LPS-Stimulated AKT and NF-kappaB Activation and Suppresses Interleukin-6 in Osteoblast-like Cells MC3T3E-1. Life Sci. 2009, 85, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-K.; Seo, D.-I.; Park, W.S.; Jung, W.-K.; Lee, D.-S.; Park, S.-G.; Choi, J.S.; Kang, M.-S.; Choi, Y.H.; Choi, I.; et al. Attenuation of IFN-γ-Induced B7-H1 Expression by 15-Deoxy-Delta(12,14)-Prostaglandin J2 via Downregulation of the Jak/STAT/IRF-1 Signaling Pathway. Life Sci. 2014, 112, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Lipoxins and Aspirin-Triggered 15-Epi-Lipoxin Biosynthesis: An Update and Role in Anti-Inflammation and pro-Resolution. Prostaglandins Other Lipid Mediat. 2002, 68–69, 433–455. [Google Scholar] [CrossRef]
- Ryu, H.-C.; Kim, C.; Kim, J.-Y.; Chung, J.-H.; Kim, J.-H. UVB Radiation Induces Apoptosis in Keratinocytes by Activating a Pathway Linked to “BLT2-Reactive Oxygen Species”. J. Investig. Dermatol. 2010, 130, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, F.R.; Sales-Campos, H.; Nardini, V.; da Costa, T.A.; Fonseca, M.T.C.; Júnior, V.R.; Sorgi, C.A.; da Silva, J.S.; Chica, J.E.L.; Faccioli, L.H.; et al. The Inhibition of 5-Lipoxygenase (5-LO) Products Leukotriene B4 (LTB4) and Cysteinyl Leukotrienes (cysLTs) Modulates the Inflammatory Response and Improves Cutaneous Wound Healing. Clin. Immunol. 2018, 190, 74–83. [Google Scholar] [CrossRef]
- Hutchins, H.L.; Li, Y.; Hannon, K.; Watkins, B.A. Eicosapentaenoic Acid Decreases Expression of Anandamide Synthesis Enzyme and Cannabinoid Receptor 2 in Osteoblast-like Cells. J. Nutr. Biochem. 2011, 22, 195–200. [Google Scholar] [CrossRef]
- Selvaraj, R.K.; Klasing, K.C. Lutein and Eicosapentaenoic Acid Interact to Modify iNOS mRNA Levels through the PPARgamma/RXR Pathway in Chickens and HD11 Cell Lines. J. Nutr. 2006, 136, 1610–1616. [Google Scholar] [CrossRef]
- Koskela, H.; Purokivi, M.; Nieminen, R.; Moilanen, E. The Cough Receptor TRPV1 Agonists 15(S)-HETE and LTB4 in the Cough Response to Hypertonicity. Inflamm. Allergy Drug Targets 2012, 11, 102–108. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty Acids, Eicosanoids and PPAR Gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Couto, D.; Melo, T.; Conde, T.A.; Costa, M.; Silva, J.; Domingues, M.R.M.; Domingues, P. Chemoplasticity of the Polar Lipid Profile of the Microalgae Chlorella Vulgaris Grown under Heterotrophic and Autotrophic Conditions. Algal Res. 2021, 53, 102128. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In Vitro Cytotoxicity Assays: Comparison of LDH, Neutral Red, MTT and Protein Assay in Hepatoma Cell Lines Following Exposure to Cadmium Chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Pour Nouroozi, R. Determination of Protein Concentration Using Bradford Microplate Protein Quantification Assay. Iejm 2015, 4, 158. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Xue, Y.; Chen, G.; Wu, Z.; Fang, H. Serum Levels of Total Antioxidant Status, Nitric Oxide and Nitric Oxide Synthase in Minor Recurrent Aphthous Stomatitis Patients. Medicine 2019, 98, e14039. [Google Scholar] [CrossRef]
- Christie, W. Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv. Lipid Methodol. 1993, 2, e111. [Google Scholar]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Gutzki, F.-M.; Beckmann, B.; Frölich, J.C. Simultaneous GC-MS/MS Measurement of Malondialdehyde and 4-Hydroxy-2-Nonenal in Human Plasma: Effects of Long-Term L-Arginine Administration. Anal. Biochem. 2017, 524, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.; Le Faouder, P.; Vigor, C.; Oger, C.; Galano, J.-M.; Dray, C.; Lee, J.C.-Y.; Valet, P.; Gladine, C.; Durand, T.; et al. Simultaneous Quantitative Profiling of 20 Isoprostanoids from Omega-3 and Omega-6 Polyunsaturated Fatty Acids by LC-MS/MS in Various Biological Samples. Anal. Chim. Acta 2016, 921, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Jia, X.; Zhang, Y.; Gapter, L.A.; Lim, Y.; Agarwal, R.; Ng, K. Pachymic Acid Inhibits Cell Growth and Modulates Arachidonic Acid Metabolism in Nonsmall Cell Lung Cancer A549 Cells. Mol. Carcinog. 2010, 49, 271–282. [Google Scholar] [CrossRef]
- Petrovic, N.; Murray, M. Using N,N,N′,N′-Tetramethyl-p-Phenylenediamine (TMPD) to Assay Cyclooxygenase Activity in Vitro. Methods Mol. Biol. 2010, 594, 129–140. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Study of Sample Preparation for Determination of Endocannabinoids and Analogous Compounds in Human Serum by LC-MS/MS in MRM Mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef]
- Watkins, B.A.; Kim, J.; Kenny, A.; Pedersen, T.L.; Pappan, K.L.; Newman, J.W. Circulating Levels of Endocannabinoids and Oxylipins Altered by Dietary Lipids in Older Women Are Likely Associated with Previously Identified Gene Targets. Biochim. Biophys. Acta 2016, 1861, 1693–1704. [Google Scholar] [CrossRef]
- Hnasko, R.; Lin, A.; McGarvey, J.A.; Stanker, L.H. A Rapid Method to Improve Protein Detection by Indirect ELISA. Biochem. Biophys. Res. Commun. 2011, 410, 726–731. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biernacki, M.; Conde, T.; Stasiewicz, A.; Surażyński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation. Int. J. Mol. Sci. 2023, 24, 14323. https://doi.org/10.3390/ijms241814323
Biernacki M, Conde T, Stasiewicz A, Surażyński A, Domingues MR, Domingues P, Skrzydlewska E. Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation. International Journal of Molecular Sciences. 2023; 24(18):14323. https://doi.org/10.3390/ijms241814323
Chicago/Turabian StyleBiernacki, Michał, Tiago Conde, Anna Stasiewicz, Arkadiusz Surażyński, Maria Rosário Domingues, Pedro Domingues, and Elżbieta Skrzydlewska. 2023. "Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation" International Journal of Molecular Sciences 24, no. 18: 14323. https://doi.org/10.3390/ijms241814323
APA StyleBiernacki, M., Conde, T., Stasiewicz, A., Surażyński, A., Domingues, M. R., Domingues, P., & Skrzydlewska, E. (2023). Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation. International Journal of Molecular Sciences, 24(18), 14323. https://doi.org/10.3390/ijms241814323







