Clinical Potential of Cellular Material Sources in the Generation of iPSC-Based Products for the Regeneration of Articular Cartilage
Abstract
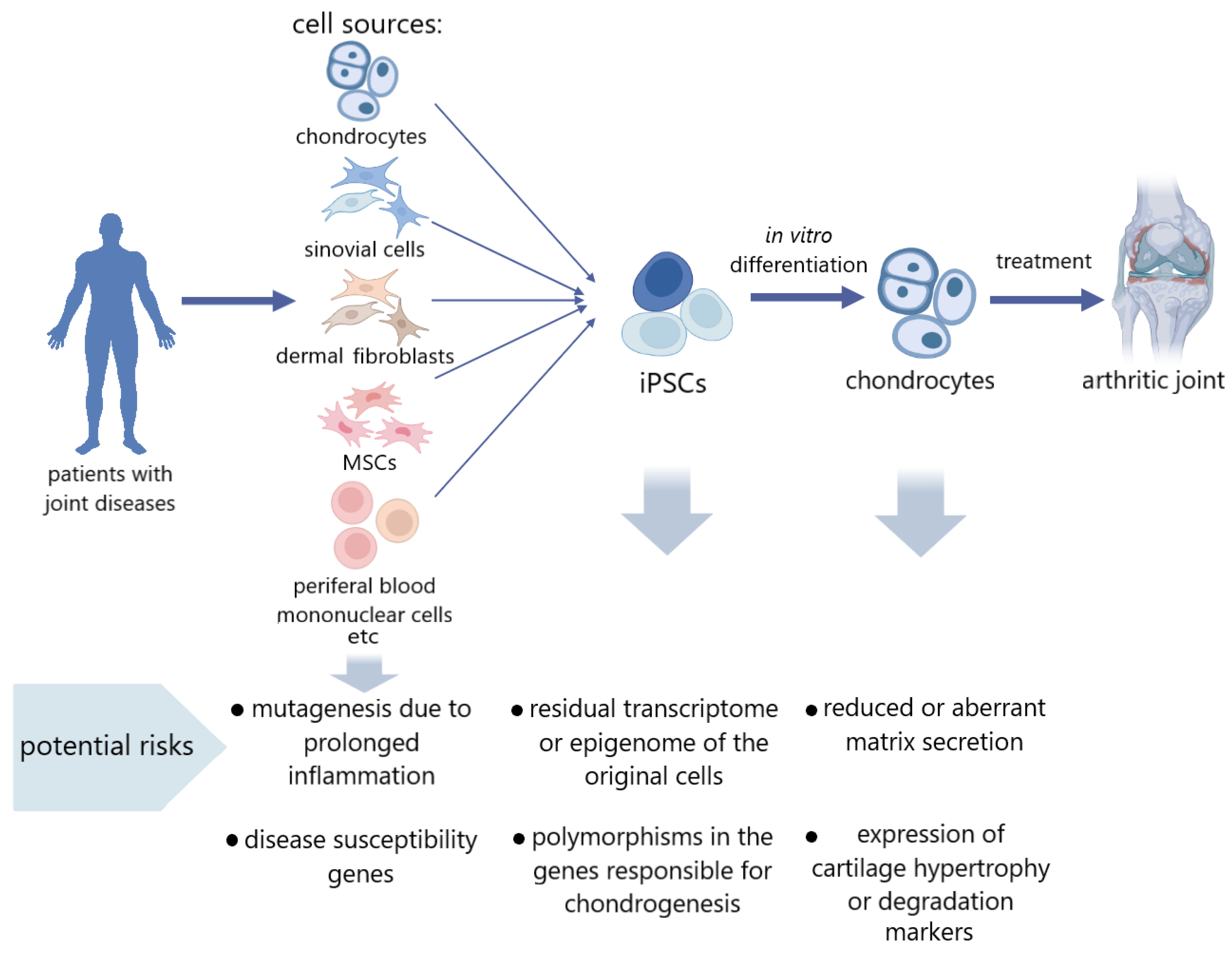
:1. Introduction
2. Differentiation Potential and Epigenetics of IPSC
3. iPSCs Derived from the Tissues of Patients with Articular Cartilage Pathology
4. Risk Factors in iPSCs Obtained from Articular Cartilage Disease Patients
5. Approaches to Work with iPSCs Obtained from Cells of Cartilage Disease Patients, as Well as Their Differentiated Derivatives
| Reprogramming Method | Type of Reprogrammed Cells | Method of Chondrogenic Differentiation | Matrix | Characteristics of Chondrogenic Derivatives | Model In Vivo, Procedure | Transplantation Results | Link |
|---|---|---|---|---|---|---|---|
| Minicircle vector | Human fibroblasts, adipose-derived stem cells | Differentiation through the stage of MSC-like precursors using dexamethasone, ascorbic acid and TGFβ3 in 3D high-density pellets culture. | Polyethylene glycol (PEG) and chondroitin sulfate methacrylate (CSM) based scaffold. | On the 14th day of differentiation, cartilage markers COL2A1, COL9A1, COL11A1, SOX9, and ACAN were expressed in derivatives. The expression of a marker of hypertrophy COL10A1 and a marker of fibrosis COL1A2 was also recorded. On day 21 of differentiation, alcian blue staining revealed the presence of proteoglycans and there was also positive immunostaining for type II collagen. | Athymic nude Sprague Dawley rats, transplantation of 21-day 3D-pellets into osteochondral defects of knee joints | At 6 weeks after transplantation, a significant decrease in the relaxation time T2 of grafts was observed, which indicates their dehydration and matrix production. Hematoxylin and eosin staining showed engraftment of cell grafts. Positive staining with alcian blue and immunochemical staining for type II collagen demonstrated remodeling of the defect, whereas the control group of empty scaffolds had no effect. No tumors or teratomas were found. | [120] |
| Episomal vectors | Human dermal fibroblast | Chondrocyte-specific iPSC reporter lines were created by introducing the COL11A2-EGFP human transgene. Differentiation through the stage of MSC-like precursors using Wnt3a and Activin A. Then, differentiation was carried out using ascorbic acid, BMP2, TGFβ1, and GDF5; after 14 days of cultivation, there was a transfer to a suspension culture. | - | In adherent culture, cells formed nodules that specifically showed COL11A2-EGFP fluorescence on day 14 of differentiation and almost all cells expressed COL11A2-EGFP on day 56 in suspension culture. The expression levels of chondrogenic markers SOX9, COL2A1, COL11A2 increased with differentiation. The proportion of SOX9-positive cells by the 42nd day of cultivation reached 91.8% ± 0.91%. On the 28th day of differentiation, slight staining with safranin O was observed but by day 42 it became intense. Immunohistochemistry showed the presence of both type I and type II collagen. Expression of collagen type I was reduced by manipulating the composition of the medium. IHH and COL10A1 mRNA expression levels were lower than in native cartilage, indicating low hypertrophy. | SCID mice, subcutaneous transplantation of 42-day cell constructs SCID rats, transplantation of 28-day cell constructs into osteochondral defects of knee joints Mini-pigs, transplantation of 56-day cell constructs into osteochondral defects of knee joints | Hyaline-like cartilage formation after subcutaneous transplantation with high collagen type II expression and intense safranin O staining and low expression of collagen types I and X. Twelve months after transplantation, collagen X expression and epiphyseal-like cartilage were observed in some areas, suggesting hypertrophy. After transplantation into defects of the knee joint of both rats and minipigs, extensive integration into the cartilage, positive staining for safranin O. In the case of rats, also intense staining with toluidine blue and the presence of type II collagen were observed. Cell clusters did not cause the formation of tumors and ectopic tissue damage as a result of transplantation. | [121] |
| (not specified) | Dermal fibroblasts of patient with knee OA | Directed differentiation in EBs using ascorbic acid, dexamethasone, TGFβ1 for 2 days, then the EBs were transferred onto cultural plastic coated with gelatin and differentiation continued in the same medium. | - | After 14 days of differentiation, intense toluidine blue staining and expression of chondrogenic markers COL2A1, ACAN, and SOX9 were observed. | Sprague Dawley rats, transplantation of cell suspension into osteochondral defects of knee joints | Fifteen weeks after transplantation, an increase in the content of proteoglycans, type 2 collagen, as well as proliferation of chondrocytes was recorded. However, the amount of cartilage matrix in the damaged area did not reach that in the healthy joint. The improvement in joint function reduced lameness in rats, but the cartilage was not completely restored. No tumors or teratomas were found | [57] |
| Sendai virus | Human cord blood mononuclear cells (CBMCs) | Directed differentiation in EBs seeded on gelatin-coated plastic, using ascorbic acid, dexamethasone, BMP2, and TGFβ3. After 4 days of cultivation the culture was transferred to 3D pellet conditions. | After 30 days of differentiation high levels of expression of chondrogenic markers SOX9, ACAN, and COL2A1 in pellets were observed; however, the levels of hypertrophic marker COL10A1 and fibrosis marker COL1A1 were also high. At the same time, the level of expression of type I collagen was higher than that of type II collagen, whereas the data for protein production were opposite. The pellets were also positively stained with toluidine blue. | Sprague-Dawley rats, transplantation of 30-day 3D pellets into osteochondral defects trochlear groove of the distal femur | At 8 weeks after transplantation, intense staining with toluidine blue and safranin O was observed in the area of the defect, demonstrating proteoglycan production and normally organized cartilage morphology. Cells inside the pellet formed lacunae. Compared with the introduction of a suspension of the obtained differentiated derivatives, the pellets showed a better therapeutic effect, although the suspension also contributed to the restoration of the cartilage. No tumors or teratomas were found. | [58] | |
| Episomal plasmid vectors without transgenes | Mouse embryonic fibroblasts | Differentiation through the stage of MSC-like precursors using fetal bovine serum (FBS) and bFGF. Then, differentiation was carried out in high-density micromass culture or alginate gel using BMP2. | Ultra-purified alginate gel | After 28 days of differentiation, alcian blue staining was intense, both in the culture within alginate gel and in micromass culture. Expression levels of the chondrogenic markers SOX9, COL2A1, and ACAN were high in both gel culture and micromass culture and increased during differentiation. Expression of the osteogenic markers Runx2, ALP, COL10A1 and adipogenic marker PPARγ increased only in high-density micromass culture. | Nude mice BALB/cScl- nu/nu, transplantation of cell suspension into gel into dorsal flanks | On the 28th day after transplantation, intense alcian blue staining was observed, as well as immunostaining for type II collagen. Additionally, over time, the expression of COL2A1 and ACAN mRNAs increased, whereas the expression of SOX9 remained almost constant. No tumors or teratomas were found. | [122] |
| Sendai virus | Normal human epidermal keratinocytes (NHEK) | Differentiation through the stage of MSC-like precursors. Then, differentiation was carried out using TGFβ1 and ascorbic acid. | - | After 26 days of differentiation, staining of micromasses with hematoxylin and eosin showed cartilaginous morphology, intense staining with safranin O and immunostaining for aggrecan and type II collagen were also recorded. | New Zealand white rabbits, transplantation of cell suspension into knee osteochondral defect | Twelve weeks after transplantation, intense safranin O staining and aggrecan immunostaining were observed. Histological evaluation of ICRS scores demonstrated a significant superiority for cartilage histology after transplantation compared with untreated controls. A decrease in the expression of markers of inflammation and catabolism IL-1β, TNF- α, and MMP13 was also observed. No tumors or teratomas were found. | [123] |
| (not specified) | Mouse gingival fibroblasts | Directed differentiation via 3D pellet formation with BMP-4, then with BMP4, dexamethasone, and TGFβ3 on a 3D orbital shaker. | - | After 28 days of differentiation in a rotational suspension culture the pellets acquired the appearance of a hyaline-like cartilage and were positively stained with safranin O. High levels of expression of the chondrogenic markers SOX9, ACAN, and COL2A1 were also recorded. Immunostaining for type I collagen was slight. | Sprague-Dawley rats, transplantation of 28-day pellets into the superficial osteochondral defects | Four weeks after transplantation, the filling of the defect with tissue similar to cartilage was observed and microCT images showed complete repair of the tissue and full integration of pellets. The healing area was stained intensely with safranin O and showed high production of type II collagen and low levels of type I and X. Signs of the tumor formation of pellets were not detected. | [124] |
| (not specified) | Human CBMCs | Directed differentiation through the stage of EBs that were resuspended and seeded on gelatin-covered plastic. Dexamethasone, BMP2, and TGFβ3 were used. | - | After 14 days of differentiation, staining with safranin O, toluidine blue, and alcian blue showed accumulation of cartilage matrix. High levels of expression of chondrogenic markers SOX9, ACAN, and COL2 were also recorded, comparable with those in primary chondrocytes. Large amounts of type I and II collagens and fibronectin were recorded in the decellularized ECM. | Sprague-Dawley rats, transplantation of decellularized ECM into osteochondral defect on the articular cartilage of the trochlear sulcus of the distal femur | Twelve weeks after transplantation, high accumulation of cartilage matrix, in particular, collagen type II after treatment, as well as low levels of expression of collagen types I and X were observed in the defect area, whereas in the control group without treatment, the results were opposite. | [125] |
| (not specified) | Cynomolgus monkey cells (not specified) | Chondrocyte-specific iPSC reporter lines were created by introducing COL11A2-EGFP human transgene. Differentiation through the stage of MSC-like precursors using Wnt3a and Activin A. Then, differentiation was carried out using ascorbic acid, BMP2, TGFβ1, and GDF5; after 14 days of cultivation, there was a transfer to a suspension culture. | - | The organoids stained positively for safranin O, and immunostaining detected the presence of large amounts of type II collagen. Type I collagen was found only on the periphery of the organoid. | Cynomolgus monkey, transplantation of cell organoids into chondral defects in the femoral trochlear crest of the right knee joints | Four weeks after transplantation, the defect was filled with transparent hyaline-like tissue, and at week 17, white cartilaginous tissue was observed. Allogeneic organoid transplantation did not elicit an immune response in primates. Positive safranin O staining was observed at both 4- and 17-weeks post-transplant, indicating cartilage matrix production. scRNA-seq showed that almost all cells in transplanted organoids expressed COL2A1 but not COL1A1. Cells in post-transplant organoids were identical to native chondrocytes by cluster analysis, excluding cells associated with integrin signaling. | [59] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adkar, S.; Brunger, J.M.; Willard, V.P.; Wu, C.L.; Gersbach, C.; Guilak, F. Genome Engineering for Personalized Arthritis Therapeutics. Trends Mol. Med. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Choi, J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Application of Induced Pluripotent Stem Cells for Disease Modeling and 3d Model Construction: Focus on Osteoarthritis. Cells 2021, 10, 3032. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and Osteoarthritis: Central Role of the Extracellular Matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.B.P.; Filiberti, A.; Husain, S.A.; Humphrey, M.B. Immune Contributions to Osteoarthritis. Curr. Osteoporos. Rep. 2017, 15, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Luyten, F.P.; Facchini, A. Biological Aspects of Early Osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 407–422. [Google Scholar] [CrossRef]
- Ministry of Health of Russian Federation; Russian Association of Traumatologists and Orthopedists; Russian Association of Rheumatologists. Clinical Guidelines—Gonarthrosis, [ID: 667]. 2021. Available online: https://legalacts.ru/doc/klinicheskie-rekomendatsii-gonartroz-utv-minzdravom-rossii (accessed on 3 September 2021).
- Coleman, S.; Briffa, N.K.; Carroll, G.; Inderjeeth, C.; Cook, N.; McQuade, J. A Randomised Controlled Trial of a Self-Management Education Program for Osteoarthritis of the Knee Delivered by Health Care Professionals. Arthritis Res. Ther. 2012, 14, R21. [Google Scholar] [CrossRef]
- Jan, M.H.; Lin, C.H.; Lin, Y.F.; Lin, J.J.; Lin, D.H. Effects of Weight-Bearing Versus Nonweight-Bearing Exercise on Function, Walking Speed, and Position Sense in Participants With Knee Osteoarthritis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2009, 90, 897–904. [Google Scholar] [CrossRef]
- Hussain, S.M.; Neilly, D.W.; Baliga, S.; Patil, S.; Meek, R.M.D. Knee Osteoarthritis: A Review of Management Options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef]
- Lee, C.; Brodke, D.; Perdue, P.W.; Patel, T. Talus Fractures: Evaluation and Treatment. J. Am. Acad. Orthop. Surg. 2020, 28, E878–E887. [Google Scholar] [CrossRef]
- Adams, J.E. Surgical management of osteoarthritis of the hand and wrist. J. Hand Ther. 2022, 35, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.A.; Komilov, N.N.; Kuliaba, T.A. Povrezhdeniya i zabolevaniya kolennogo sustava [Injures and diseases of a knee joint]. In Travmatologia i Ortopedia, 1st ed.; Komilov, N.V., Ed.; Gippokrat: Saint Petersburg, Russia, 2006; Volume 3, pp. 213–438. (In Russian) [Google Scholar]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.Y. Non-Surgical Management of Knee Osteoarthritis: Comparison of ESCEO and OARSI 2019 Guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sang, L.; Wu, D.; Rong, J.; Jiang, L. Effectiveness and Safety of Glucosamine and Chondroitin for the Treatment of Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. J. Orthop. Surg. Res. 2018, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.S. Systemic Effects of Intra-Articular Corticosteroids. Clin. Rheumatol. 2009, 28, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, M.; Halberg, P. Intraartikulaer injektion af glukokortikoider ved ledsygdomme [Intra-articular glucocorticoid injections in joint diseases]. Ugeskr. Laeger 1999, 161, 582–586. [Google Scholar]
- Farr, J.; Cole, B.; Dhawan, A.; Kercher, J.; Sherman, S. Clinical Cartilage Restoration: Evolution and Overview. Clin. Orthop. Relat. Res. 2011, 469, 2696–2705. [Google Scholar] [CrossRef]
- Fujii, Y.; Liu, L.; Yagasaki, L.; Inotsume, M.; Chiba, T.; Asahara, H. Cartilage Homeostasis and Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 6316. [Google Scholar] [CrossRef]
- Redondo, M.L.; Beer, A.J.; Yanke, A.B. Cartilage Restoration: Microfracture and Osteochondral Autograft Transplantation. J. Knee Surg. 2018, 31, 231–238. [Google Scholar] [CrossRef]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous Chondrocyte Implantation in the Knee: Systematic Review and Economic Evaluation. Health Technol. Assess. (Rockv.) 2017, 21, 1–294. [Google Scholar] [CrossRef]
- Biant, L.C.; McNicholas, M.J.; Sprowson, A.P.; Spalding, T. The Surgical Management of Symptomatic Articular Cartilage Defects of the Knee: Consensus Statements from United Kingdom Knee Surgeons. Knee 2015, 22, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Pérez, M.; Valderrabano, V.; Godoy-Santos, A.L.; de César Netto, C.; González-Martín, D.; Tejero, S. Ankle Osteoarthritis: Comprehensive Review and Treatment Algorithm Proposal. EFORT Open Rev. 2022, 7, 448–459. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, M.H.; Saris, D.; Bulstra, S.K.; Buma, P. Behandeling van kraakbeendefecten in de knie: Advies van de Nederlandse Orthopaedische Vereniging [Treatment of cartilaginous defects in the knee: Recommendations from the Dutch Orthopaedic Association]. Ned. Tijdschr. Geneeskd. 2013, 157, A5719. [Google Scholar]
- Gomoll, A.H.; Gillogly, S.D.; Cole, B.J.; Farr, J.; Arnold, R.; Hussey, K.; Minas, T. Autologous chondrocyte implantation in the patella: A multicenter experience. Am. J. Sports Med. 2014, 42, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Heir, S.; Nerhus, T.K.; Røtterud, J.H.; Løken, S.; Ekeland, A.; Engebretsen, L.; Årøen, A. Focal Cartilage Defects in the Knee Impair Quality of Life as Much as Severe Osteoarthritis: A Comparison of Knee Injury and Osteoarthritis Outcome Score in 4 Patient Categories Scheduled for Knee Surgery. Am. J. Sports Med. 2010, 38, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.; Gui, J.; Lavigne, P. Autologous Chondrocyte Implantation: Natural History of Postimplantation Periosteal Hypertrophy and Effects of Repair-Site Debridement on Outcome. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 1318–1324. [Google Scholar] [CrossRef]
- Henderson, I.; Tuy, B.; Oakes, B. Reoperation after Autologous Chondrocyte Implantation. J. Bone Jt. Surg.—Ser. B 2004, 86, 205–211. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Kalkreuth, R.H.; Niemeyer, P.; Uhl, M.; Erggelet, C. Long-Term Clinical and MRI Results of Matrix-Assisted Autologous Chondrocyte Implantation for Articular Cartilage Defects of the Knee. Cartilage 2019, 10, 305–313. [Google Scholar] [CrossRef]
- Murray, R.; Winkler, P.W.; Shaikh, H.S.; Musahl, V. High Tibial Osteotomy for Varus Deformity of the Knee. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e2100141. [Google Scholar] [CrossRef]
- Price, A.J.; Alvand, A.; Troelsen, A.; Katz, J.N.; Hooper, G.; Gray, A.; Carr, A.; Beard, D. Knee Replacement. Lancet 2018, 392, 1672–1682. [Google Scholar] [CrossRef]
- Cameron, K.L.; Driban, J.B.; Svoboda, S.J. Osteoarthritis and the Tactical Athlete: A Systematic Review. J. Athl. Train. 2016, 51, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Hoburg, A.; Löer, I.; Körsmeier, K.; Siebold, R.; Niemeyer, P.; Fickert, S.; Ruhnau, K. Matrix-Associated Autologous Chondrocyte Implantation Is an Effective Treatment at Midterm Follow-up in Adolescents and Young Adults. Orthop. J. Sport. Med. 2019, 7, 2325967119841077. [Google Scholar] [CrossRef] [PubMed]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal Stem Cells: Amazing Remedies for Bone and Cartilage Defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal Stem Cells for Cartilage Regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, S.M.; Kim, S.H.; Tatman, P.; Gee, A.O.; Kim, D.H.; Lee, K.E.; Jung, Y.; Kim, S.J. Effect of Self-Assembled Peptide-Mesenchymal Stem Cell Complex on the Progression of Osteoarthritis in a Rat Model. Int. J. Nanomed. 2014, 9 (Suppl. S1), 141–157. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, X.; Lv, X.; Liu, V.; Zhao, G.; Zhang, X.; Cao, W.; Wang, R.; Wang, W. In Vivo Human Adipose-Derived Mesenchymal Stem Cell Tracking after Intra-Articular Delivery in a Rat Osteoarthritis Model. Stem. Cell Res. Ther. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.V.; Lorenz, S.; Nerlich, A.G.; Guehring, T.; Richter, W. Disc Cell Therapy with Bone—Marrow—Derived Autologous Mesenchymal Stromal Cells in a Large Porcine Disc Degeneration Model. Eur. Spine J. 2018, 27, 2639–2649. [Google Scholar] [CrossRef]
- Desancé, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.M.; Batho, A.; Legendre, F.; Audigié, F.; et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int. J. Mol. Sci. 2018, 19, 537. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Mardones, R.; Giai Via, A.; Pipino, G.; Jofre, C.M.; Munõz, S.; Narvaez, E.; Maffulli, N. BM-MSCs Differentiated to Chondrocytes for Treatment of Full-Thickness Cartilage Defect of the Knee. J. Orthop. Surg. Res. 2020, 15, 455. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of Mesenchymal Stem Cells in Cartilage Regeneration: From Characterization to Application. npj Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, A.; Kyriacou, H.; Seah, K.T.M.; Khan, W.S. Use of Human Induced Pluripotent Stem Cells for Cartilage Regeneration in Vitro and within Chondral Defect Models of Knee Joint Cartilage in Vivo: A Preferred Reporting Items for Systematic Reviews and Meta-Analyses Systematic Literature Review. Cytotherapy 2021, 23, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Thorp, H.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Fabrication of Hyaline-like Cartilage Constructs Using Mesenchymal Stem Cell Sheets. Sci. Rep. 2020, 10, 20869. [Google Scholar] [CrossRef]
- Krill, M.; Early, N.; Everhart, J.S.; Flanigan, D.C. Autologous Chondrocyte Implantation (ACI) for Knee Cartilage Defects: A Review of Indications, Technique, and Outcomes. JBJS Rev. 2018, 6, e5. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and Complementary Therapies in Osteoarthritis and Cartilage Repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef]
- Hinckel, B.B.; Gomoll, A.H. Autologous Chondrocytes and Next-Generation Matrix-Based Autologous Chondrocyte Implantation. Clin. Sports Med. 2017, 36, 525–548. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Peng, M.; Xie, B.; Zhang, L.; Tang, X. Autologous Nasal Chondrocytes Delivered by Injectable Hydrogel for in Vivo Articular Cartilage Regeneration. Cell Tissue Bank. 2018, 19, 35–46. [Google Scholar] [CrossRef]
- Sherman, S.L.; Thyssen, E.; Nuelle, C.W. Osteochondral Autologous Transplantation. Clin. Sports Med. 2017, 36, 489–500. [Google Scholar] [CrossRef]
- Delanois, R.E.; Etcheson, J.I.; Sodhi, N.; Henn, R.F.; Gwam, C.U.; George, N.E.; Mont, M.A. Biologic Therapies for the Treatment of Knee Osteoarthritis. J. Arthroplast. 2019, 34, 801–813. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage Tissue Engineering: Molecular Control of Chondrocyte Differentiation for Proper Cartilage Matrix Reconstruction. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Jiang, Y.; Tuan, R.S. Bioactivity of Human Adult Stem Cells and Functional Relevance of Stem Cell-Derived Extracellular Matrix in Chondrogenesis. Stem Cell Res. Ther. 2023, 14, 160. [Google Scholar] [CrossRef]
- Lietman, S.A. Induced Pluripotent Stem Cells in Cartilage Repair. World J. Orthop. 2016, 7, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, X.; Liang, Y.; Gu, H.; Song, K.; Zou, X.; Zhou, G. Repair of Cartilage Defects in Osteoarthritis Rats with Induced Pluripotent Stem Cell Derived Chondrocytes. BMC Biotechnol. 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.A.; Nam, Y.; Park, N.; Lee, J.; Park, S.H.; Ju, J.H. Repair Potential of Nonsurgically Delivered Induced Pluripotent Stem Cell-Derived Chondrocytes in a Rat Osteochondral Defect Model. J. Tissue Eng. Regen. Med. 2018, 12, 1843–1855. [Google Scholar] [CrossRef]
- Abe, K.; Yamashita, A.; Morioka, M.; Horike, N.; Takei, Y.; Koyamatsu, S.; Okita, K.; Matsuda, S.; Tsumaki, N. Engraftment of Allogeneic IPS Cell-Derived Cartilage Organoid in a Primate Model of Articular Cartilage Defect. Nat. Commun. 2023, 14, 804. [Google Scholar] [CrossRef] [PubMed]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous Induced Pluripotent Stem Cell–Based Cell Therapies: Promise, Progress, and Challenges. Curr. Protoc. 2021, 1, e88. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 850–859. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Hospital, M.G.; Song, B.; Hospital, M.; Massachusetts, B.; Herrington, T.M.; Hospital, M.G.; Park, T.; Hospital, M.; Massachusetts, B.; et al. Personalized IPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Castro-Viñuelas, R.; Sanjurjo-Rodríguez, C.; Piñeiro-Ramil, M.; Hermida-Gómez, T.; Rodríguez-Fernández, S.; Oreiro, N.; de Toro, J.; Fuentes, I.; Blanco, F.J.; Díaz-Prado, S. Generation and Characterization of Human Induced Pluripotent Stem Cells (IPSCs) from Hand Osteoarthritis Patient-Derived Fibroblasts. Sci. Rep. 2020, 10, 4272. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zeng, W.; Wan, R.; Wang, J.; Zhou, Q.; Qiu, S.; Singh, S. Chondrogenic Differentiation of Induced Pluripotent Stem Cells from Osteoarthritic Chondrocytes in Alginate Matrix. Eur. Cells Mater. 2012, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.A.; Park, N.; Nam, Y.; Ju, J.H. Generation of Induced-Pluripotent Stem Cells Using Fibroblast-like Synoviocytes Isolated from Joints of Rheumatoid Arthritis Patients. J. Vis. Exp. 2016, 2016, e54072. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Park, N.; Lee, K.; Jung, H.; Jung, S.M.; Lee, J.; Ju, J.H. Characterization of Early-Onset Finger Osteoarthritis-Like Condition Using Patient-Derived Induced Pluripotent Stem Cells. Cells 2021, 10, 317. [Google Scholar] [CrossRef]
- Saitta, B.; Passarini, J.; Sareen, D.; Ornelas, L.; Sahabian, A.; Argade, S.; Krakow, D.; Cohn, D.H.; Svendsen, C.N.; Rimoin, D.L. Patient-Derived Skeletal Dysplasia Induced Pluripotent Stem Cells Display Abnormal Chondrogenic Marker Expression and Regulation by BMP2 and TGFβ1. Stem Cells Dev. 2014, 23, 1464–1478. [Google Scholar] [CrossRef]
- Yamashita, A.; Morioka, M.; Kishi, H.; Kimura, T.; Yahara, Y.; Okada, M.; Fujita, K.; Sawai, H.; Ikegawa, S.; Tsumaki, N. Statin Treatment Rescues FGFR3 Skeletal Dysplasia Phenotypes. Nature 2014, 513, 507–511. [Google Scholar] [CrossRef]
- Yokoyama, K.; Ikeya, M.; Umeda, K.; Oda, H.; Nodomi, S.; Nasu, A.; Matsumoto, Y.; Izawa, K.; Horigome, K.; Kusaka, T.; et al. Enhanced Chondrogenesis of Induced Pluripotent Stem Cells from Patients with Neonatal-Onset Multisystem Inflammatory Disease Occurs via the Caspase 1-Independent CAMP/Protein Kinase A/CREB Pathway. Arthritis Rheumatol. 2015, 67, 302–314. [Google Scholar] [CrossRef]
- Xu, M.; Shaw, G.; Murphy, M.; Barry, F. Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Are Functionally and Genetically Different From Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells 2019, 37, 754–765. [Google Scholar] [CrossRef]
- Kim, K.; Zhao, R.; Doi, A.; Ng, K.; Unternaehrer, J.; Cahan, P.; Hongguang, H.; Loh, Y.-H.; Aryee, M. Donor Cell Type Can Influence the Epigenome and Differentiation Potential of Human Induced Pluripotent Stem Cells. Nat. Biotechnol. 2012, 176, 139–148. [Google Scholar] [CrossRef]
- Boreström, C.; Simonsson, S.; Enochson, L.; Bigdeli, N.; Brantsing, C.; Ellerström, C.; Hyllner, J.; Lindahl, A. Footprint-Free Human Induced Pluripotent Stem Cells From Articular Cartilage with Redifferentiation Capacity: A First Step Toward a Clinical-Grade Cell Source. Stem Cells Transl. Med. 2014, 3, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Doi, A.; Ng, K.; Zhao, R.; Cahan, P. Epigenetic Memory in Induced Pluripotent Stem Cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.M.; Liu, S.; Figueroa, M.E.; Kulalert, W.; Eminli, S.; Tan, K.Y.; Apostolou, E.; Stadtfeld, M.; Li, Y.; Shioda, T.; et al. Cell Type of Origin Influences the Molecular and Functional Properties of Mouse Induced Pluripotent Stem Cells. Nat. Biotechnol. 2010, 28, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Vaskova, E.A.; Stekleneva, A.E.; Medvedev, S.P.; Zakian, S.M. “Epigenetic Memory” Phenomenon in Induced Pluripotent Stem Cells. Acta Nat. 2013, 5, 15–21. [Google Scholar] [CrossRef]
- Kyttälä, A.; Moraghebi, R.; Valensisi, C.; Kettunen, J.; Andrus, C.; Pasumarthy, K.K.; Nakanishi, M.; Nishimura, K.; Ohtaka, M.; Weltner, J.; et al. Genetic Variability Overrides the Impact of Parental Cell Type and Determines IPSC Differentiation Potential. Stem Cell Rep. 2016, 6, 200–212. [Google Scholar] [CrossRef]
- Kajiwara, M.; Aoi, T.; Okita, K.; Takahashi, R.; Inoue, H.; Takayama, N.; Endo, H.; Eto, K.; Toguchida, J.; Uemoto, S.; et al. Donor-Dependent Variations in Hepatic Differentiation from Human-Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12538–12543. [Google Scholar] [CrossRef]
- Burrows, C.K.; Banovich, N.E.; Pavlovic, B.J.; Patterson, K.; Gallego Romero, I.; Pritchard, J.K.; Gilad, Y. Genetic Variation, Not Cell Type of Origin, Underlies the Majority of Identifiable Regulatory Differences in IPSCs. PLoS Genet. 2016, 12, e1005793. [Google Scholar] [CrossRef]
- Shutova, M.V.; Surdina, A.V.; Ischenko, D.S.; Naumov, V.A.; Bogomazova, A.N.; Vassina, E.M.; Alekseev, D.G.; Lagarkova, M.A.; Kiselev, S.L. An Integrative Analysis of Reprogramming in Human Isogenic System Identified a Clone Selection Criterion. Cell Cycle 2016, 15, 986–997. [Google Scholar] [CrossRef]
- Pichard, L.; Brondelo, J.M.; Becker, F.; Desprat, R.; De Ceuninck, F.; Pastoureau, P.; Noel, D.; Jorgensen, C.; Lemaitre, J.M. Generation of Human Pluripotent Stem Cell Lines (IPSCs) from Mesenchymal Stem Cells (MSCs) from Three Elderly Patients with Osteoarthritis. Stem Cell Res. 2020, 44, 101721. [Google Scholar] [CrossRef]
- Choompoo, N.; Bartley, O.J.M.; Precious, S.V.; Vinh, N.N.; Schnell, C.; Garcia, A.; Roberton, V.H.; Williams, N.M.; Kemp, P.J.; Kelly, C.M.; et al. Induced Pluripotent Stem Cells Derived from the Developing Striatum as a Potential Donor Source for Cell Replacement Therapy for Huntington Disease. Cytotherapy 2021, 23, 111–118. [Google Scholar] [CrossRef]
- Ratneswaran, A.; Kapoor, M. Osteoarthritis Year in Review: Genetics, Genomics, Epigenetics. Osteoarthr. Cartil. 2021, 29, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rubab, A.; Chan, W.C.W.; Chan, D. Osteoarthritis Year in Review 2022: Genetics, Genomics and Epigenetics. Osteoarthr. Cartil. 2023, 31, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Diaz-Hernandez, M.E.; Chihab, S.; Priyadarshani, P.; Bhattaram, P.; Mortensen, L.J.; Guzzo, R.M.; Drissi, H. Differential Chondrogenic Differentiation between IPSC Derived from Healthy and OA Cartilage Is Associated with Changes in Epigenetic Regulation and Metabolic Transcriptomic Signatures. eLife 2023, 12, e83138. [Google Scholar] [CrossRef] [PubMed]
- Nanki, K.; Fujii, M.; Shimokawa, M.; Matano, M.; Nishikori, S.; Date, S.; Takano, A.; Toshimitsu, K.; Ohta, Y.; Takahashi, S.; et al. Somatic Inflammatory Gene Mutations in Human Ulcerative Colitis Epithelium. Nature 2020, 577, 254–259. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Yi, H.; Diecke, S.; Kim, J.; Jung, H.; Rim, Y.A.; Jung, S.M.; Kim, M.; Kim, Y.G.; et al. Generation of Disease-Specific Induced Pluripotent Stem Cells from Patients with Rheumatoid Arthritis and Osteoarthritis. Arthritis Res. Ther. 2014, 16, R41. [Google Scholar] [CrossRef]
- Kim, M.J.; Son, M.J.; Son, M.Y.; Seol, B.; Kim, J.; Park, J.; Kim, J.H.; Kim, Y.H.; Park, S.A.; Lee, C.H.; et al. Generation of Human Induced Pluripotent Stem Cells from Osteoarthritis Patient-Derived Synovial Cells. Arthritis Rheum. 2011, 63, 3010–3021. [Google Scholar] [CrossRef]
- Hu, J.; Lu, C.; Zhu, W.; Jiang, Q.; Du, W.; Wu, N. Establishment of an Induced Pluripotent Stem Cell Line (SHFDi001-A) from a Patient with Ankylosing Spondylitis. Stem Cell Res. 2020, 46, 101879. [Google Scholar] [CrossRef]
- Wolnik, J.; Kubiak, G.; Skoczyńska, M.; Wiland, P.; Fearon, U.; Veale, D.; Dulak, J.; Biniecka, M. Generation of Two HiPSC Lines, (DMBi003-A and DMBi004-A), by Reprogramming Peripheral Blood Mononuclear Cells and Fibroblast-like Synoviocytes from Rheumatoid Arthritis Patients. Stem Cell Res. 2022, 64, 102886. [Google Scholar] [CrossRef]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed Differentiation of Human Embryonic Stem Cells toward Chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Yu, F. The Potential of Induced Pluripotent Stem Cells as a Tool to Study Skeletal Dysplasias and Cartilage-Related Pathologic Conditions. Osteoarthr. Cartil. 2016, 25, 616–624. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Shao, Z. Association between GDF5 Rs143383 Genetic Polymorphism and Musculoskeletal Degenerative Diseases Susceptibility: A Meta-Analysis. BMC Med. Genet. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, Y.; Li, H.; Li, H.; He, Q.; Xue, Y.; Shen, C.; Zhang, C.; Xiang, J.; Ding, J.; et al. Single Nucleotide Polymorphisms and Osteoarthritis an Overview and a Meta-Analysis. Medicine 2016, 95, e2811. [Google Scholar] [CrossRef] [PubMed]
- van Hoolwerff, M.; Ruiz, A.R.; Bouma, M.; Eka, H.; Koning, R.I.; Jost, C.R.; Mulder, A.A.; Freund, C.; Guilak, F.; Ramos, Y.F.M.; et al. High-Impact FN1 Mutation Decreases Chondrogenic Potential and Affects Cartilage Deposition via Decreased Binding to Collagen Type II. Sci. Adv. 2021, 7, eabg8583. [Google Scholar] [CrossRef] [PubMed]
- Layh-Schmitt, G.; Lu, S.; Navid, F.; Brooks, S.R.; Lazowick, E.; Davis, K.M.; Montagna, C.; Gadina, M.; Colbert, R.A. Generation and Differentiation of Induced Pluripotent Stem Cells Reveal Ankylosing Spondylitis Risk Gene Expression in Bone Progenitors. Clin. Rheumatol. 2017, 36, 143–154. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.C.; Yoon, N.E.; Kim, Y.; Choi, J.; Park, N.; Jung, H.; Jung, B.H.; Ju, J.H. Metabolomic Profiles of Induced Pluripotent Stem Cells Derived from Patients with Rheumatoid Arthritis and Osteoarthritis. Stem Cell Res. Ther. 2019, 10, 319. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Lu, L.; Liu, L.; Yu, X.; Pei, F. Cellular Senescence in Knee Osteoarthritis: Molecular Mechanisms and Therapeutic Implications. Ageing Res. Rev. 2021, 70, 101413. [Google Scholar] [CrossRef]
- Zhang, X.X.; He, S.H.; Liang, X.; Li, W.; Li, T.F.; Li, D.F. Aging, Cell Senescence, the Pathogenesis and Targeted Therapies of Osteoarthritis. Front. Pharmacol. 2021, 12, 728100. [Google Scholar] [CrossRef]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular Senescence in Osteoarthritis Pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Strässler, E.T.; Aalto-Setälä, K.; Kiamehr, M.; Landmesser, U.; Kränkel, N. Age Is Relative—Impact of Donor Age on Induced Pluripotent Stem Cell-Derived Cell Functionality. Front. Cardiovasc. Med. 2018, 5, 4. [Google Scholar] [CrossRef]
- Greene, M.A.; Loeser, R.M.D. Aging-Related Inflammation in Osteoarthritis Meredith. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lu, S.J.; Klimanskaya, I.; Gomes, I.; Kim, D.; Chung, Y.; Honig, G.R.; Kim, K.S.; Lanza, R. Hemangioblastic Derivatives from Human Induced Pluripotent Stem Cells Exhibit Limited Expansion and Early Senescence. Stem Cells 2010, 28, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Ito, S.; Nishio, N.; Xiao, H.; Zhang, R.; Suzuki, H.; Okawa, Y.; Murohara, T.; Isobe, K.I. Establishment of Induced Pluripotent Stem Cells from Aged Mice Using Bone Marrow-Derived Myeloid Cells. J. Mol. Cell Biol. 2011, 3, 91–98. [Google Scholar] [CrossRef]
- Lapasset, L.; Milhavet, O.; Prieur, A.; Besnard, E.; Babled, A.; Ät-Hamou, N.; Leschik, J.; Pellestor, F.; Ramirez, J.M.; De Vos, J.; et al. Rejuvenating Senescent and Centenarian Human Cells by Reprogramming through the Pluripotent State. Genes Dev. 2011, 25, 2248–2253. [Google Scholar] [CrossRef]
- Yagi, T.; Kosakai, A.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Nabetani, A.; Ishikawa, F.; Arai, Y.; Hirose, N.; et al. Establishment of Induced Pluripotent Stem Cells from Centenarians for Neurodegenerative Disease Research. PLoS ONE 2012, 7, e41572. [Google Scholar] [CrossRef]
- Puri, D.; Wagner, W. Epigenetic Rejuvenation by Partial Reprogramming. BioEssays 2023, 45, 2200208. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W. The Link between Epigenetic Clocks for Aging and Senescence. Front. Genet. 2019, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef]
- Colasuonno, F.; Borghi, R.; Niceforo, A.; Muzzi, M.; Bertini, E.; Di Giulio, A.; Moreno, S.; Compagnucci, C. Senescence-Associated Ultrastructural Features of Long-Term Cultures of Induced Pluripotent Stem Cells (IPSCs). Aging 2017, 9, 2206–2219. [Google Scholar] [CrossRef]
- Petrini, S.; Borghi, R.; Oria, V.D.; Restaldi, F.; Moreno, S.; Novelli, A.; Bertini, E.; Compagnucci, C. Aged Induced Pluripotent Stem Cell (IPSCs) as a New Cellular Model for Studying Premature Aging. Aging 2017, 9, 1453–1466. [Google Scholar] [CrossRef]
- Yamashita, A.; Liu, S.; Woltjen, K.; Thomas, B.; Meng, G.; Hotta, A.; Takahashi, K.; Ellis, J.; Yamanaka, S.; Rancourt, D.E. Cartilage Tissue Engineering Identifies Abnormal Human Induced Pluripotent Stem Cells. Sci. Rep. 2013, 3, 1978. [Google Scholar] [CrossRef] [PubMed]
- Mashima, H.; Zhang, R.; Kobayashi, T.; Tsukamoto, H.; Liu, T.; Iwama, T.; Hagiya, Y.; Yamamoto, M.; Fukushima, S.; Okada, S.; et al. Improved Safety of Induced Pluripotent Stem Cell-Derived Antigen-Presenting Cell-Based Cancer Immunotherapy. Mol. Ther.—Methods Clin. Dev. 2021, 21, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.D.; Tchao, J.; Wu, L.; Carman, A.J. Precision Installation of a Highly Efficient Suicide Gene Safety Switch in Human Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 2020, 9, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Hanamatsu, H.; Onodera, T.; Furukawa, J.I.; Xu, L.; Homan, K.; Baba, R.; Kawasaki, T.; Iwasaki, N. Establishment of the Removal Method of Undifferentiated Induced Pluripotent Stem Cells Coexisting with Chondrocytes Using R-17F Antibody. Regen. Med. 2022, 17, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Falcon, C.; Smith, L.; Al-Obaidi, M.; Abu Zaanona, M.; Purvis, K.; Minagawa, K.; Athar, M.; Salzman, D.; Bhatia, R.; Goldman, F.; et al. Combinatorial Suicide Gene Strategies for the Safety of Cell Therapies. Front. Immunol. 2022, 13, 975233. [Google Scholar] [CrossRef]
- Dicks, A.R.; Steward, N.; Guilak, F.; Wu, C.L. Chondrogenic Differentiation of Human-Induced Pluripotent Stem Cells. Methods Mol. Biol. 2023, 2598, 87–114. [Google Scholar] [CrossRef]
- Li, Y.; Hai, Y.; Chen, J.; Liu, T. Differentiating Chondrocytes from Peripheral Blood-Derived Human Induced Pluripotent Stem Cells. J. Vis. Exp. 2017, 2, e55722. [Google Scholar] [CrossRef]
- Nejadnik, H.; Diecke, S.; Lenkov, O.D.; Chapelin, F.; Donig, J.; Tong, X.; Derugin, N.; Chan, R.C.F.; Gaur, A.; Yang, F.; et al. Improved Approach for Chondrogenic Differentiation of Human Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2015, 11, 242–253. [Google Scholar] [CrossRef]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of Scaffoldless Hyaline Cartilaginous Tissue from Human IPSCs. Stem Cell Rep. 2015, 4, 404–418. [Google Scholar] [CrossRef]
- Hontani, K.; Onodera, T.; Terashima, M.; Momma, D.; Matsuoka, M.; Baba, R.; Joutoku, Z.; Matsubara, S.; Homan, K.; Hishimura, R.; et al. Chondrogenic Differentiation of Mouse Induced Pluripotent Stem Cells Using the Three-Dimensional Culture with Ultra-Purified Alginate Gel. J. Biomed. Mater. Res.—Part A 2019, 107, 1086–1093. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wu, K.C.; Ding, D.C. Induced Pluripotent Stem Cell-Differentiated Chondrocytes Repair Cartilage Defect in a Rabbit Osteoarthritis Model. Stem Cells Int. 2020, 2020, 8867349. [Google Scholar] [CrossRef]
- Zhang, M.; Niibe, K.; Kondo, T.; Limraksasin, P.; Okawa, H.; Miao, X.; Kamano, Y.; Yamada, M.; Jiang, X.; Egusa, H. Rapid and Efficient Generation of Cartilage Pellets from Mouse Induced Pluripotent Stem Cells by Transcriptional Activation of BMP-4 with Shaking Culture. J. Tissue Eng. 2022, 13, 20417314221114616. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, K.; Han, H.; Mo, H.; Jung, H.; Ryu, Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Prochondrogenic Effect of Decellularized Extracellular Matrix Secreted from Human Induced Pluripotent Stem Cell-Derived Chondrocytes. Acta Biomater. 2023, 167, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yamashita, A.; Ozono, K.; Tsumaki, N. Limited Immunogenicity of Human Induced Pluripotent Stem Cell-Derived Cartilages. Tissue Eng.—Part A 2016, 22, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Pigeot, S.; Klein, T.; Gullotta, F.; Dupard, S.J.; Garcia Garcia, A.; García-García, A.; Prithiviraj, S.; Lorenzo, P.; Filippi, M.; Jaquiery, C.; et al. Manufacturing of Human Tissues as Off-the-Shelf Grafts Programmed to Induce Regeneration. Adv. Mater. 2021, 33, 2103737. [Google Scholar] [CrossRef]
- Shimomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Shin-Ya, M.; Ichimaru, S.; Tsuchida, S.; Mazda, O.; et al. Hypoxia Promotes Differentiation of Pure Cartilage from Human Induced Pluripotent Stem Cells. Mol. Med. Rep. 2022, 26, 229. [Google Scholar] [CrossRef]
- Middendorf, J.M.; Diamantides, N.; Shortkroff, S.; Dugopolski, C.; Kennedy, S.; Cohen, I.; Bonassar, L.J. Multiscale Mechanics of Tissue-Engineered Cartilage Grown from Human Chondrocytes and Human-Induced Pluripotent Stem Cells. J. Orthop. Res. 2020, 38, 1965–1973. [Google Scholar] [CrossRef]
- Wu, J.Y.; Vunjak-Novakovic, G. Bioengineering Human Cartilage-Bone Tissues for Modeling of Osteoarthritis. Stem Cells Dev. 2022, 31, 399–405. [Google Scholar] [CrossRef]
- Kwon, H.; Paschos, N.; Hu, J.C. Articular Cartilage Tissue Engineering: The Role of Signaling Molecules. Cell. Mol. Life Sci. 2016, 73, 1173–1194. [Google Scholar] [CrossRef]
- Limraksasin, P.; Kosaka, Y.; Zhang, M.; Horie, N.; Kondo, T.; Okawa, H.; Yamada, M.; Egusa, H. Shaking Culture Enhances Chondrogenic Differentiation of Mouse Induced Pluripotent Stem Cell Constructs. Sci. Rep. 2020, 10, 14996. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Xie, M.; Wen, J.; Niibe, K.; Zhang, X.; Luo, J.; Yan, R.; Zhang, Z.; Egusa, H.; et al. Recapitulation of Cartilage/Bone Formation Using IPSCs via Biomimetic 3D Rotary Culture Approach for Developmental Engineering. Biomaterials 2020, 260, 120334. [Google Scholar] [CrossRef] [PubMed]
- Lach, M.S.; Rosochowicz, M.A.; Richter, M.; Jagiełło, I.; Suchorska, W.M.; Trzeciak, T. The Induced Pluripotent Stem Cells in Articular Cartilage Regeneration and Disease Modelling: Are We Ready for Their Clinical Use? Cells 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.; Gamini, R.; Alvarez-Garcia, O. Identification of Transcription Factors Responsible for Dysregulated Networks in Human Osteoarthritis Cartilage by Global Gene Expression Analysis. Osteoarthr. Cartil. 2018, 26, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
| Reprogramming Method | Type of Reprogrammed Cells, Pathology | Comparison with Healthy Donor Cells | iPSC Characteristics | Method of Chondrogenic Differentiation | Characteristics of Chondrogenic Derivatives | Link |
|---|---|---|---|---|---|---|
| Sendai virus | Fibroblasts of the skin, non-erosive OA of the hand, risarthrosis of the arm | + | Alkaline phosphatase activity; expression of OCT4, SOX2, KLF4 and C-MYC, as well as NANOG and CRYPTO. Ability to differentiate into cells of three germ leaves in embryoid bodies (EBs): expression of α-fetoprotein (endoderm), α-actin of smooth muscles (mesoderm), neuron-specific β-tubulin class III (ectoderm). After reprogramming, allelic variants of SNP in the GDF 5, SMAD3, ALDH1A2, and IL 1R1 genes observed in parental fibroblasts were preserved. There were no significant differences from the IPSC of healthy donors. | Directed differentiation in EBs using Wnt3a, Activin A, then BMP2, GDF5, TGFβ3. | Staining with Masson’s trichrome and safranin O showed a lower presence of the collagen matrix and proteoglycans, respectively, compared with chondro-derivatives of healthy donors. | [64] |
| Sendai virus | MSCs, OA | + | Alkaline phosphatase activity; expression of OCT4, SOX2, NANOG, SEA4. Analysis of teratoma formation demonstrated differentiation into three germ leaves: pulmonary epithelium (endoderm), embryonic mesenchyme, smooth muscle cells, adipose, cartilage and bone tissue (mesoderm), neural tube, horny epidermis (ectoderm). There were no significant differences from the iPSCs of healthy donors. | - | - | [81] |
| Lentivirus system | Chondrocytes, OA | + | Alkaline phosphatase activity, expression of OCT4, SOX2, NANOG, KLF4, TRA-1–60; however, klf4 expression was lower in iPSCs from both healthy and OA chondrocytes compared with other pluripotency genes. | Differentiation through the stage of MSC-like precursors using FBS and bFGF. Then differentiation was carried out in 3D pellet conditions using BMP2. | On days 4, 7, and 21 of differentiation, SOX9, COL2A1, ACAN, and PRG4 mRNA expression was significantly higher in iPSC derivatives from healthy donors compared with iPSC derivatives from OA donors. The micromass culture of iPSC derivatives from healthy donors was intensely stained with alcian blue, in contrast to iPSC derivatives from donors with OA. At the stage of MSC-like progenitors, iPSC derivatives from donors with OA showed significantly higher levels of expression of the pro-inflammatory genes CCL2, CCL3, CXCL3, and NOS2 in the aggressive environment of the IL1β or TNF-α inflammatory stimulus compared with iPSC derivatives from healthy donors. | [85] |
| Sendai virus | PBMCs, fibroblast-like synoviocytes, RA | − | Typical iPSC morphology, expression of OCT4, NANOG, TRA-1–81, and SSEA-4. Ability to differentiate into cells of three germ leaves in ET: expression of NF, NESTING, TUBB3 (ectoderm), vimentin, BRACHYURY T, NKX 2.5 (mesoderm), GATA4, SOX17, FOXA2 (endoderm). | - | - | [90] |
| Episomal plasmid vectors without transgenes | PBMCs, ankylosing spondylitis | − | Typical morphology of ESC, expression of OCT4, SEA 4, SOX2, and TRA-1-60. Analysis of teratoma formation showed the ability to differentiate into three germ leaves: nervous (ectoderm), cartilaginous (mesoderm), glandular (endoderm) tissue. | - | - | [89] |
| Lentivirus system | Fibroblast-like synoviocytes, OA, RA | − | Expression of NANOG, OCT4, SOX2, KLF4, TRA-1-80, TRA-1-60, REX, and SSEA-4; however, before reprogramming, the expression level of KLF4 was high. Analysis of teratoma formation showed the ability to differentiate into three germ leaves: skin structure (ectoderm), blood vessels and adipose tissue (mesoderm), gland (endoderm). | - | - | [87] |
| Lentivirus system | Fibroblast-like synoviocytes, RA | − | Alkaline phosphatase activity, expression of OCT3/4, SOX2, NANOG, LIN28, DPPB5, and TDGF1, as well as SEA 4, TRA-1-60, TRA-1-81, and Klf4. Analysis of teratoma formation showed the ability to differentiate into three germ leaves: the formation of glandular and adipose tissues and blood vessels. Expression of OTX2 (ectoderm), BRACHYURY (mesoderm), SOX17 (endoderm) was observed. | - | - | [66] |
| Sendai virus | Dermal fibroblast, OA fingers with early onset | + | Alkaline phosphatase activity, expression of SEA 4, TRA-1-60, TRA-1-81, LIN28, OCT4, SOX2, and KLF4. Expression of OCT4 and NANOG was higher in the iPSCs obtained from an OA patient compared with the iPSCs of a healthy donor. | Directed differentiation using TGFβ1, production of chondrogenic pellets and cultivation in a medium with the addition of dexamethasone, TGFβ3. | Pellets from the iPSC of an OA patient had a larger size and vacuum-like formations inside the structures. On both days 7 and 21, expression of the SOX9 chondrogenic marker in the iPSC derivatives of the OA patient was high, though a slight tendency to decrease expression was recorded. Expression of ASAT was low, both in the derivatives of the patient and the healthy donor on days 7 and 21. In addition, on days 7 and 21 COL2A1 expression was significantly lower than in IPSC derivatives of a healthy donor. On day 7, a higher expression of COL1A1 was recorded in the cells of an OA patient than in the cells of a healthy donor, but no difference was recorded on day 21. Differences in the expression of the COL10A1 hypertrophy marker were also not observed. On day 7, VEGF expression was higher than in the control group, but on day 21, low expression rates were observed. AQP1 was expressed significantly more than in IPSC derivatives of a healthy donor. | [67] |
| Retrovirus system | MSCs-like synovial cells, OA | +(hESCs) | iPSC-like morphology, alkaline phosphatase activity, expression of OCT-4, NANOG, SOX2, hTERT, RES 1, LIN28, TDGF, TRA-1-60, and SSEA-3. Analysis of teratoma formation in vivo and EBs in vitro demonstrated a decrease in the expression of OCT-4 and NANOG pluripotent markers, as well as the ability to differentiate into three germ sheets: expression of Pax6, Tuj1, and Nestin (ectoderm), Brachyury, GATA-2, desmin, and α-actin of smooth muscles, a number of chondrogenic markers, such as SOX9, ACAN, COL2 (mesoderm), GATA-6, SOX17, FoxA2, and α-fetoprotein. There were no significant differences from the indicators of hESC expression. The reprogramming efficiency was 0.007–0.01%. | Directed chondrogenic differentiation in EBs (protocol not specified), the production of chondrogenic granules (protocol not specified), the use of an agarose substrate, as well as a three-dimensional polycaprolactone scaffold. | Cartilage-like cell aggregates were formed that stained positively with alcian blue and safranin O. Expression of SOX9, aggrecan, and type II collagen was also observed. Expression of type X collagen in the differentiated derivatives of one of the two iPSC lines obtained was higher than in the hESC derivatives. Aggrecan expression was higher in the derivatives of both iPSC lines compared with the derivatives of hESCs. Cells cultured on a three-dimensional scaffold for 2 months showed the morphology of chondrocytes, intense expression of SOX9 and collagens of types I, II, and X, and were stained with alcian blue. | [88] |
| Lentivirus system | Chondrocytes, OA of knee joins | + | Typical morphology, alkaline phosphatase activity, expression of OCT-4, SOX-2, REX-1, NANOG, SSEA-1, SSEA-4, TRA1-60, and TRA1-81 were observed in two of the three obtained colonies. ET analysis demonstrated the ability to differentiate into derivatives of three germ leaves: Nestin expression, histological characteristics of nervous tissue (ectoderm), desmin, histological characteristics of bone and muscle tissue (mesoderm) and α-fetoprotein, histological characteristics of intestinal-like and respiratory-like epithelium (endoderm). The clones were directionally differentiated into derivatives of three germ leaves in vitro: expression of NSE, NF-M, MBP, GAD, Nestin (ectoderm), GATA-4, NKX2.5, MLC-2A and MLC-2V (mesoderm), PDX-1, PAX-6, NKX2.2, and insulin (endoderm). | Transfection with a TGFβ1-carrying lentivirus for endogenous expression, use of an alginate matrix, and co-cultivation with native mature chondrocytes. | Increased expression of TGFβ1 in the transduced iPSCs was confirmed by Western blotting. In the experimental group of TGFβ1-induced iPSCs in the co-culture alginate matrix, the expression of type II collagen, aggrecan, and COMP was significantly higher than in other experimental groups but lower than in native chondrocytes. VEGF expression was zero. | [65] |
| mRNA transfection | Chondrocytes, skin fibroblasts, foreskin fibroblasts, OA (after replacement therapy with autologous chondrocytes) | +(ESCs) | Alkaline phosphatase activity, expression of OCT4, SSEA 4, TRA-1-60, NANOG. Reprogramming efficiency is approximately 0.1%. ET analysis demonstrated the ability to differentiate into derivatives of three germ leaves: βΙΙΙ-tubulin (ectoderm), α-actin of smooth muscles (mesoderm), HNF3ß (endoderm). Teratoma analysis also demonstrated the formation of a neural epithelium (ectoderm), spontaneously contracting cardiomyocytes (mesoderm), cylindrical epithelium (endoderm). No significant differences from the ESC were recorded. | Directed differentiation in monolayer culture in the DEF-CS system using Activin-A, Wnt3a, FGF2, BMP4 at the first stage, FGF2, BMP4, follistatin, and NT4 at the second, FGF2, BMP4, NT4, and GDF5 [91]. Preparation of chondrogenic granules, directed differentiation using TGFβ1 and dexamethasone. | Expression of pluripotency markers decreased with differentiation, expression of CDH1, MLH1, and GSC showed the mesodermal direction of differentiation. High expression of PDGFR, SOX6, SOX9, ACAN, COL2A1 types A and B. Expression of type X collagen in the late differentiated derivatives was low. | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremeev, A.; Pikina, A.; Ruchko, Y.; Bogomazova, A. Clinical Potential of Cellular Material Sources in the Generation of iPSC-Based Products for the Regeneration of Articular Cartilage. Int. J. Mol. Sci. 2023, 24, 14408. https://doi.org/10.3390/ijms241914408
Eremeev A, Pikina A, Ruchko Y, Bogomazova A. Clinical Potential of Cellular Material Sources in the Generation of iPSC-Based Products for the Regeneration of Articular Cartilage. International Journal of Molecular Sciences. 2023; 24(19):14408. https://doi.org/10.3390/ijms241914408
Chicago/Turabian StyleEremeev, Artem, Arina Pikina, Yevgeny Ruchko, and Alexandra Bogomazova. 2023. "Clinical Potential of Cellular Material Sources in the Generation of iPSC-Based Products for the Regeneration of Articular Cartilage" International Journal of Molecular Sciences 24, no. 19: 14408. https://doi.org/10.3390/ijms241914408
APA StyleEremeev, A., Pikina, A., Ruchko, Y., & Bogomazova, A. (2023). Clinical Potential of Cellular Material Sources in the Generation of iPSC-Based Products for the Regeneration of Articular Cartilage. International Journal of Molecular Sciences, 24(19), 14408. https://doi.org/10.3390/ijms241914408






