Bmp4 in Zebrafish Enhances Antiviral Innate Immunity through p38 MAPK (Mitogen-Activated Protein Kinases) Pathway
Abstract
:1. Introduction
2. Results
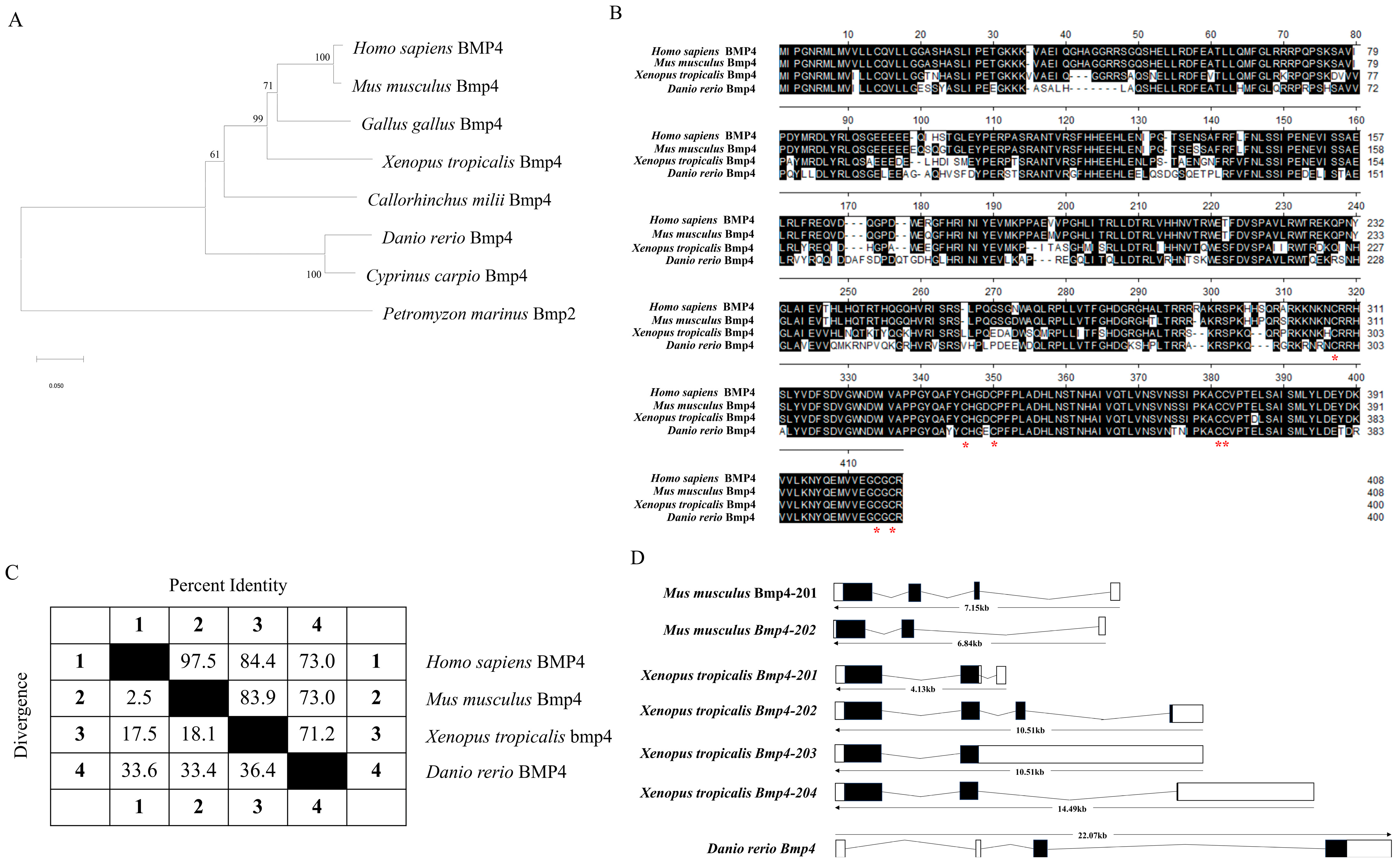
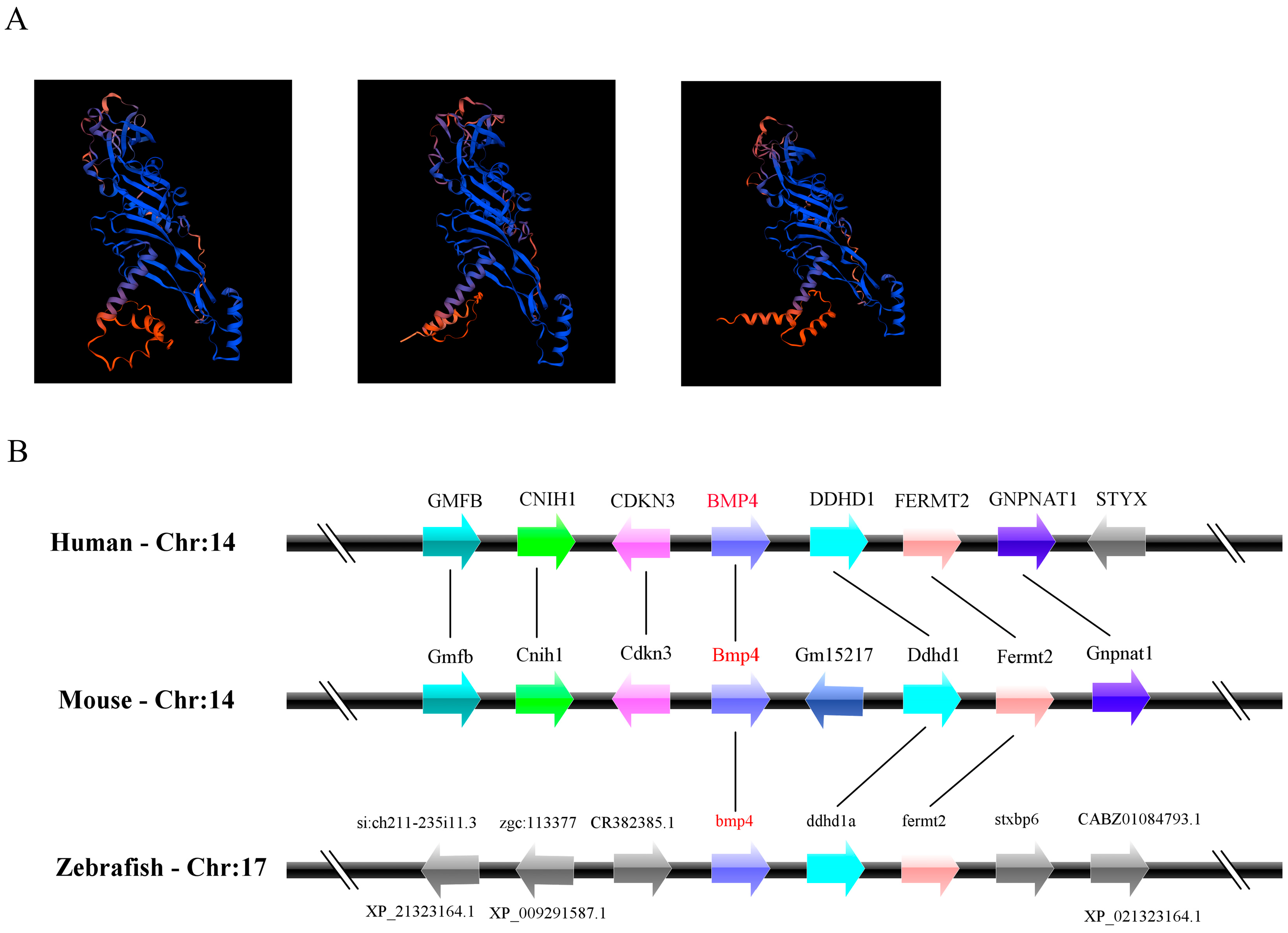
2.1. The Identification and Evolution of Zebrafish Bmp4
2.2. The Bmp4 Expression Was Increased after Virus or Poly(I:C) Challenge
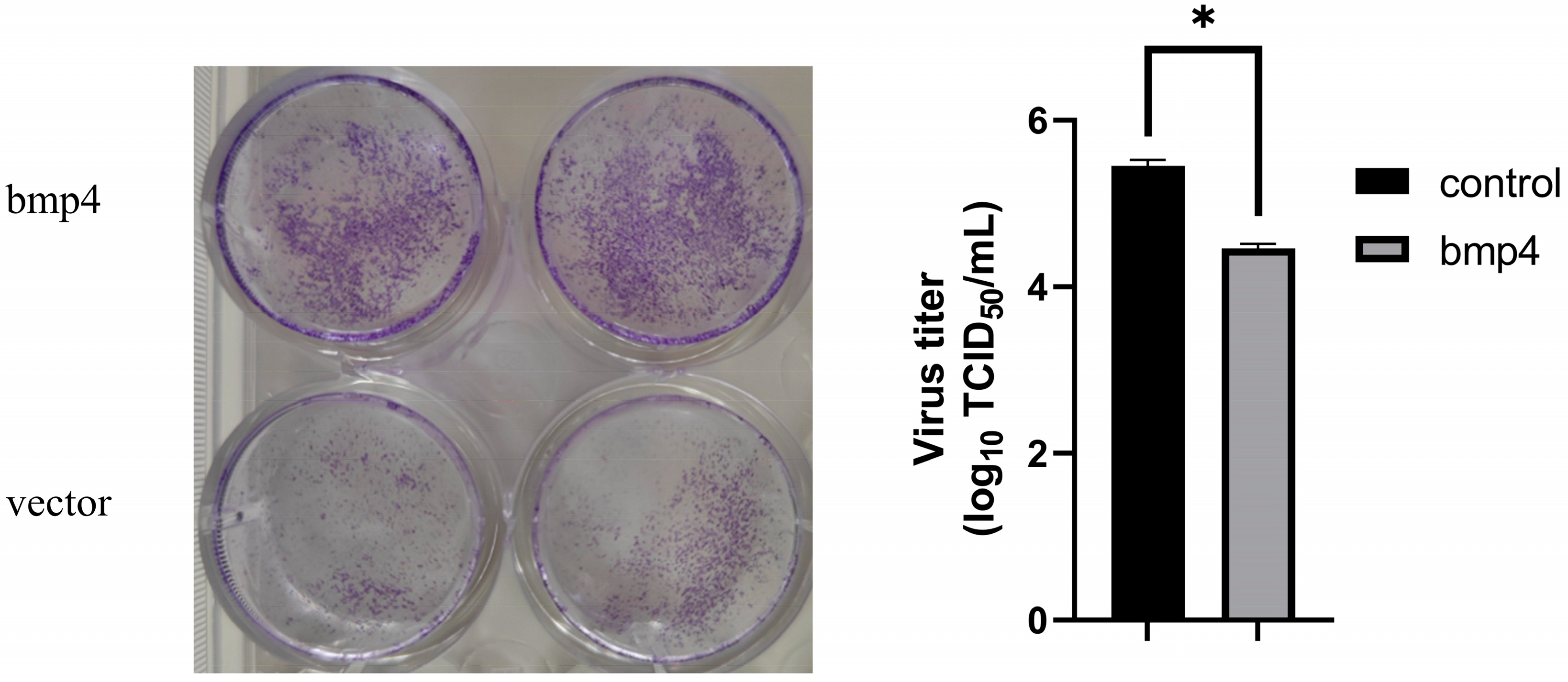
2.3. Antiviral Function of Bmp4 Both In Vitro and In Vivo
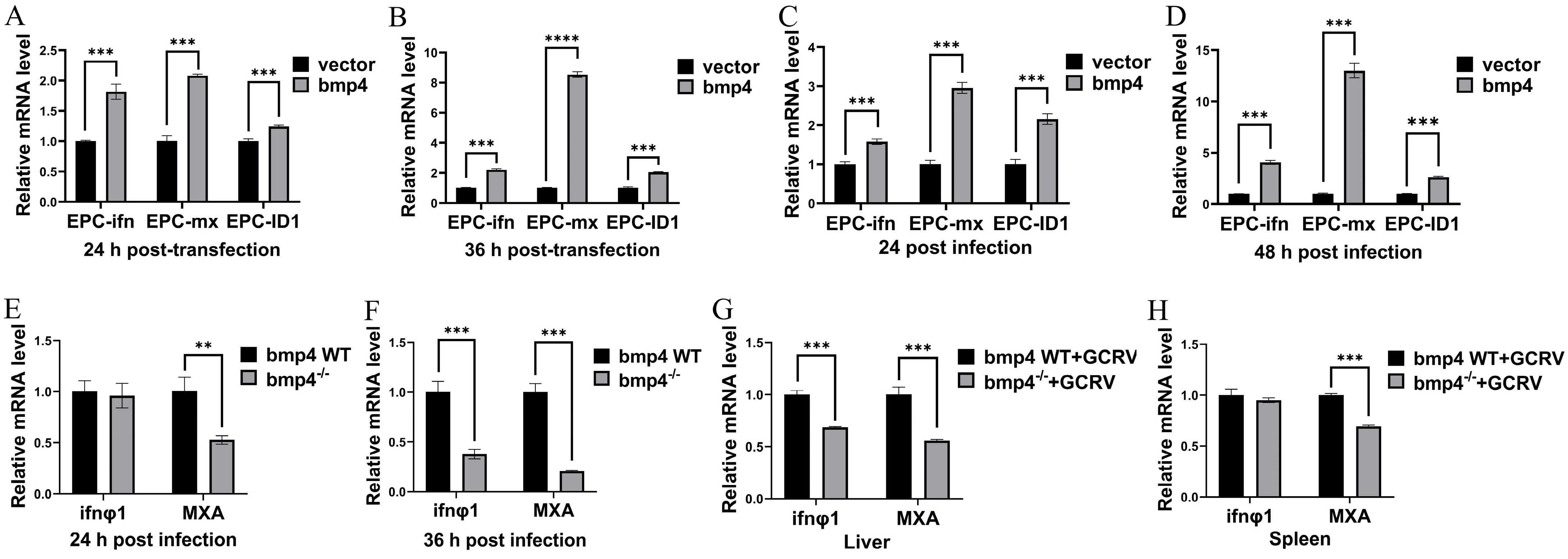
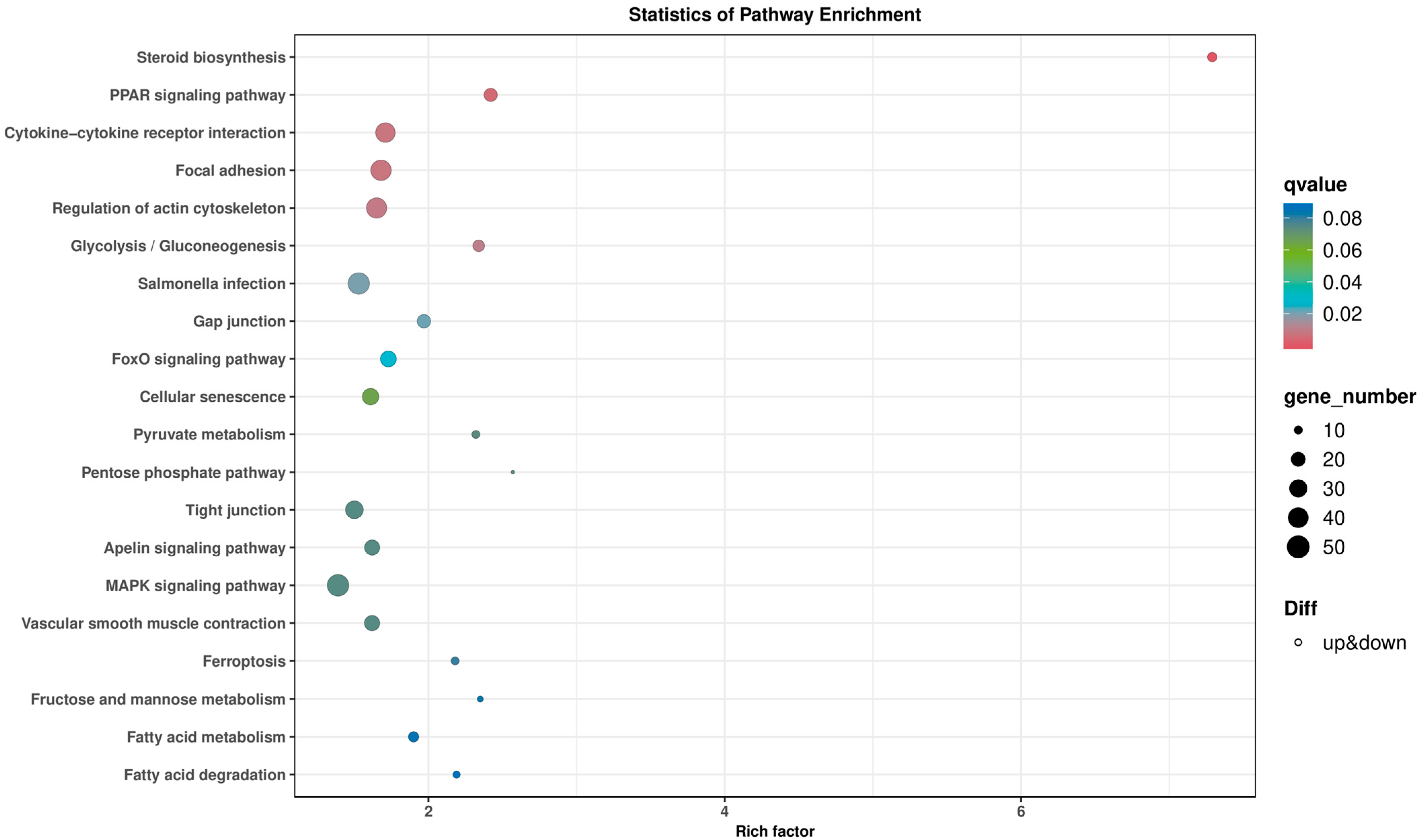
2.4. Bmp4 Increases the Expression of Antiviral Genes
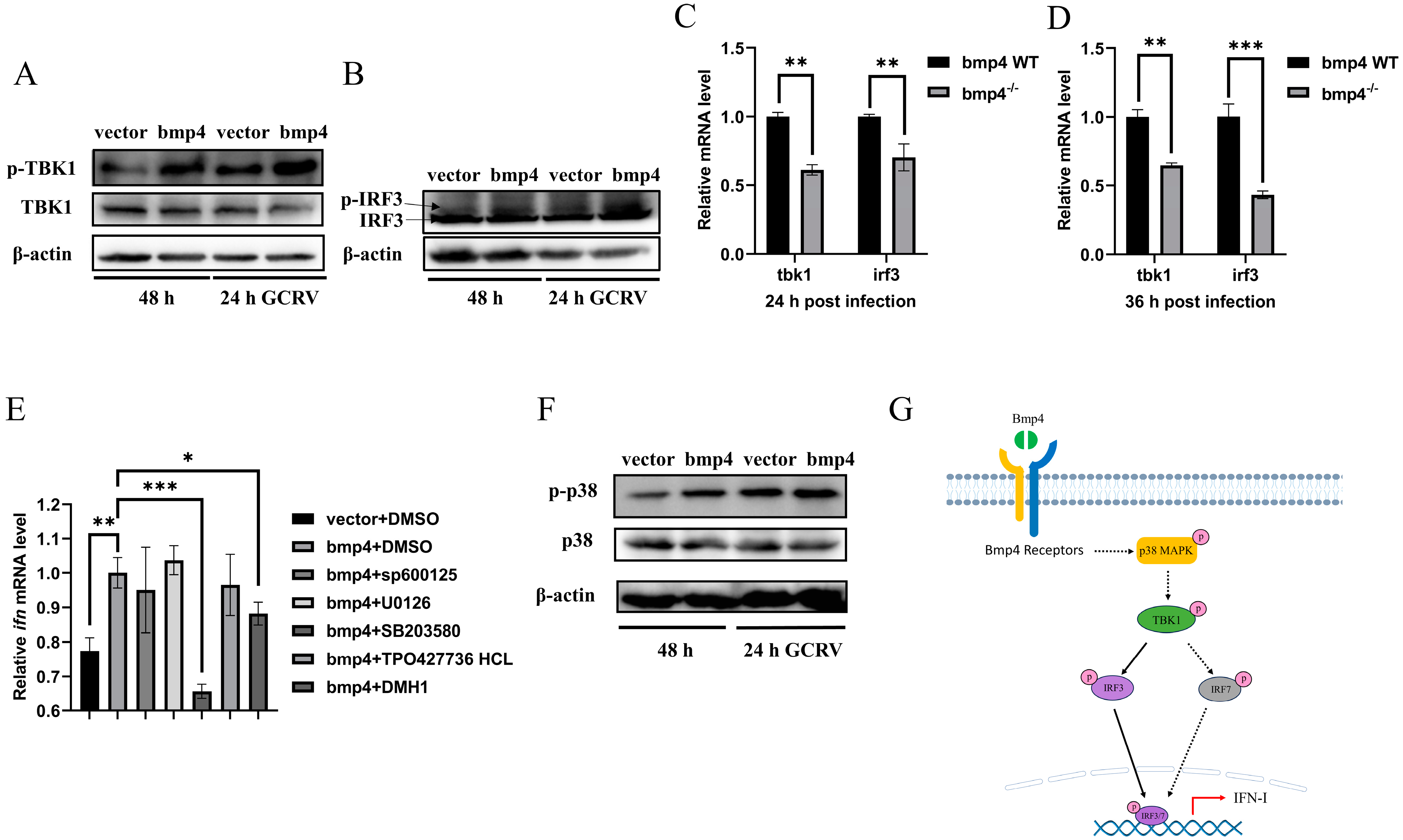
2.5. Bmp4 Activates Antiviral Signaling via p38 MAPK Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Viruses and Poly(I:C)
4.3. Sequence Comparison and Phylogenetic Analysis of BMP4 Proteins
4.4. Generation of Bmp4 Mutant Zebrafish
4.5. Plasmid Construction
4.6. Transcriptome Sequencing and Identification of DEGs
4.7. Viral Infection In Vitro
4.8. Viral Infection In Vivo
4.9. Crystal Violet Staining
4.10. Inhibitor Treatment and Analysis
4.11. Quantitative Real-Time PCR
4.12. Western Blot and Antibodies
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone Morphogenetic Proteins: A critical review. Cell. Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Kartalaei, P.S.; Vink, C.S.; Yamada-Inagawa, T.; Bollerot, K.; van Ijcken, W.; van der Linden, R.; de Sousa Lopes, S.M.C.; Monteiro, R.; Mummery, C.; et al. BMP signalling differentially regulates distinct haematopoietic stem cell types. Nat. Commun. 2015, 6, 8040. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development.; bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zheng, X.; Huang, L.; Xu, P.; Ma, Y.; Min, Z.; Tao, Q.; Tao, Y.; Meng, A. Organizer-derived Bmp2 is required for the formation of a correct Bmp activity gradient during embryonic development. Nat. Commun. 2014, 5, 3766. [Google Scholar] [CrossRef]
- Yu, Y.; Mutlu, A.S.; Liu, H.; Wang, M.C. High-throughput screens using photo-highlighting discover BMP signaling in mitochondrial lipid oxidation. Nat. Commun. 2017, 8, 865. [Google Scholar] [CrossRef]
- Shore, E.M.; Xu, M.-Q.; Shah, P.B.; Janoff, H.B.; Hahn, G.V.; Deardorff, M.A.; Sovinsky, L.; Spinner, N.B.; Zasloff, M.A.; Wozney, J.M.; et al. The human bone morphogenetic protein 4 (BMP-4) gene: Molecular structure and transcriptional regulation. Calcif. Tissue Int. 1998, 63, 221–229. [Google Scholar] [CrossRef]
- Bakrania, P.; Efthymiou, M.; Klein, J.C.; Salt, A.; Bunyan, D.J.; Wyatt, A.; Ponting, C.P.; Martin, A.; Williams, S.; Lindley, V.; et al. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: Overlap between the BMP4 and hedgehog signaling pathways. Am. J. Hum. Genet. 2008, 82, 304–319. [Google Scholar] [CrossRef]
- Dong, X.; Mao, Y.; Gao, P. The Role of Bone Morphogenetic Protein 4 in Lung Diseases. Curr. Mol. Med. 2023, 23, 324–331. [Google Scholar] [CrossRef]
- Godoy-Parejo, C.; Deng, C.; Xu, J.; Zhang, Z.; Ren, Z.; Ai, N.; Liu, W.; Ge, W.; Deng, C.; Xu, X.; et al. Protein Kinase C Modulation Determines the Mesoderm/Extraembryonic Fate Under BMP4 Induction From Human Pluripotent Stem Cells. Stem Cells 2023, 41, 578–591. [Google Scholar] [CrossRef]
- Nakatsu, D.; Kunishige, R.; Taguchi, Y.; Shinozaki-Narikawa, N.; Osaka, K.; Yokomizo, K.; Ishida, M.; Takei, S.; Yamasaki, S.; Hagiya, K.; et al. BMP4-SMAD1/5/9-RUNX2 pathway activation inhibits neurogenesis and oligodendrogenesis in Alzheimer’s patients’ iPSCs in senescence-related conditions. Stem Cell Rep. 2023, 18, 1246. [Google Scholar] [CrossRef]
- Huber, S.; Schramm, C. Role of Activin A in the Induction of Foxp3+ and Foxp3- CD4+ Regulatory T Cells. Crit. Rev. Immunol. 2011, 31, 53–60. [Google Scholar] [CrossRef]
- Martínez, V.G.; Hernández-López, C.; Valencia, J.; Hidalgo, L.; Entrena, A.; Zapata, A.G.; Vicente, A.; Sacedón, R.; Varas, A. The canonical BMP signaling pathway is involved in human monocyte-derived dendritic cell maturation. Immunol. Cell Biol. 2011, 89, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.G.; Sacedón, R.; Hidalgo, L.; Valencia, J.; Fernández-Sevilla, L.M.; Hernández-López, C.; Vicente, A.; Varas, A. The BMP Pathway Participates in Human Naive CD4+ T Cell Activation and Homeostasis. PLoS ONE 2015, 10, e0131453. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.J.; de Kretser, D.M.; Hedger, M.P. Activin and related proteins in inflammation: Not just interested bystanders. Cytokine Growth Factor Rev. 2009, 20, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Seeger, P.; Musso, T.; Sozzani, S. The TGF-β superfamily in dendritic cell biology. Cytokine Growth Factor Rev. 2015, 26, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, H.; Shinohara, M.; Mao, M.; Phaosawasdi, P.; El–Zaatari, M.; Zhang, M.; Ji, T.; Eaton, K.A.; Dang, D.; Kao, J.; et al. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology 2014, 147, 396–406.e7. [Google Scholar] [CrossRef]
- Eddowes, L.A.; Al-Hourani, K.; Ramamurthy, N.; Frankish, J.; Baddock, H.T.; Sandor, C.; Ryan, J.D.; Fusco, D.N.; Arezes, J.; Giannoulatou, E.; et al. Antiviral activity of bone morphogenetic proteins and activins. Nat. Microbiol. 2019, 4, 339–351. [Google Scholar] [CrossRef]
- Olsavszky, V.; Ulbrich, F.; Singh, S.; Diett, M.; Sticht, C.; Schmid, C.D.; Zierow, J.; Wohlfeil, S.A.; Schledzewski, K.; Dooley, S.; et al. GATA4 and LMO3 balance angiocrine signaling and autocrine inflammatory activation by BMP2 in liver sinusoidal endothelial cells. Gene 2017, 627, 491–499. [Google Scholar] [CrossRef]
- Zhong, S.; Li, H.; Wang, Y.-S.; Wang, Y.; Ji, G.; Li, H.-Y.; Zhang, S.; Liu, Z. Bmp8a is an essential positive regulator of antiviral immunity in zebrafish. Commun. Biol. 2021, 4, 318. [Google Scholar] [CrossRef]
- Hölttä-Vuori, M.; Salo, V.T.; Nyberg, L.; Brackmann, C.; Enejder, A.; Panula, P.; Ikonen, E. Zebrafish: Gaining popularity in lipid research. Biochem. J. 2010, 429, 235–242. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Z. Receptor, signal transduction and evolution of sweet, umami and bitter taste. Mar. Life Sci. Technol. 2020, 2, 6–15. [Google Scholar] [CrossRef]
- Schindler, C.; Plumlee, C. Inteferons pen the JAK-STAT pathway. Semin. Cell Dev. Biol. 2008, 19, 311–318. [Google Scholar] [CrossRef]
- Aggad, D.; Mazel, M.; Boudinot, P.; Mogensen, K.E.; Hamming, O.J.; Hartmann, R.; Kotenko, S.; Herbomel, P.; Lutfalla, G.; Levraud, J.-P. The two groups of zebrafish virus-induced interferons signal via distinct receptors with specific and shared chains. J. Immunol. 2009, 183, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Robertsen, B. The interferon system of teleost fish. Fish Shellfish. Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Jiang, J.; Chen, Y.-D.; Zhu, R.; Shi, Y.; Zhang, Q.-Y.; Gui, J.-F. The innate immune response to grass carp hemorrhagic virus (GCHV) in cultured Carassius auratus blastulae (CAB) cells. Dev. Comp. Immunol. 2007, 31, 232–243. [Google Scholar] [CrossRef]
- Tu, E.; Chia, P.Z.C.; Chen, W. TGFβ in T cell biology and tumor immunity: Angel or devil? Cytokine Growth Factor Rev. 2014, 25, 423–435. [Google Scholar] [CrossRef]
- Azadian, S.; Doustmohammadi, A.; Naseri, M.; Khodarahmi, M.; Arab, S.S.; Yazdanifar, M.; Zahiri, J.; Lewis, N.E. Reconstructing the cell–cell interaction network among mouse immune cells. Biotechnol. Bioeng. 2023, 120, 2756–2764. [Google Scholar] [CrossRef]
- Bleul, C.C.; Boehm, T. BMP Signaling Is Required for Normal Thymus Development. J. Immunol. 2005, 175, 5213–5221. [Google Scholar] [CrossRef]
- Lee, M.K.; Pardoux, C.; Hall, M.C.; Lee, P.S.; Warburton, D.; Qing, J.; Smith, S.M.; Derynck, R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007, 26, 3957–3967. [Google Scholar] [CrossRef]
- Gordon, J.; Patel, S.R.; Mishina, Y.; Manley, N.R. Evidence for an early role for BMP4 signaling in thymus and parathyroid morphogenesis. Dev. Biol. 2010, 339, 141–154. [Google Scholar] [CrossRef]
- Aleman-Muench, G.R.; Soldevila, G. When versatility matters: Activins/inhibins as key regulators of immunity. Immunol. Cell Biol. 2012, 90, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Fenton, T.M.; Czajkowska, B.I.; Klementowicz, J.E.; Travis, M.A. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012, 217, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Yanai, H.; Negishi, H.; Asagiri, M.; Sato, M.; Mizutani, T.; Shimada, N.; Ohba, Y.; Takaoka, A.; Yoshida, N.; et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005, 434, 772–777. [Google Scholar] [CrossRef]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF Family Transcription Factors in Immunity and Oncogenesis. Annu. Rev. Immunol. 2008, 26, 535–584. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hofer, M.J.; Jung, S.R.; Lim, S.-L.; Campbell, I.L. IRF7-dependent type I interferon production induces lethal immune-mediated disease in STAT1 knockout mice infected with lymphocytic choriomeningitis virus. J. Virol. 2014, 88, 7578–7588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Ding, X.; Wan, X.; Liu, L.; Yuan, X.; Zhang, W.; Hui, X.; Meng, G.; Xiao, H.; Li, B.; et al. MLL5 suppresses antiviral innate immune response by facilitating STUB1-mediated RIG-I degradation. Nat. Commun. 2018, 9, 1243. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Oh, S.P.; Yeo, C.-Y.; Lee, Y.; Schrewe, H.; Whitman, M.; Li, E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002, 16, 2749–2754. [Google Scholar] [CrossRef]
- Drummond, A.E. TGFbeta signalling in the development of ovarian function. Cell Tissue Res. 2005, 322, 107–115. [Google Scholar] [CrossRef]
- Trempolec, N.; Dave-Coll, N.; Nebreda, A.R. SnapShot: p38 MAPK Signaling. Cell 2013, 152, 656–656.e1. [Google Scholar] [CrossRef]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38MAPK: Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Shi, L.Z.; Chi, H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine 2009, 48, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Du, L.; Lv, D.; Li, H.; Shang, J.; Lu, J.; Zhou, L.; Bai, L.; Tang, H. Exosomal Interferon-Induced Transmembrane Protein 2 Transmitted to Dendritic Cells Inhibits Interferon Alpha Pathway Activation and Blocks Anti–Hepatitis B Virus Efficacy of Exogenous Interferon Alpha. Hepatology 2019, 69, 2396–2413. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Ruan, B.Y.; Chen, S.N.; Hou, J.; Huang, B.; Laghari, Z.A.; Li, L.; Nie, P. Two type II IFN members, IFN-γ and IFN-γ related (rel), regulate differentially IRF1 and IRF11 in zebrafish. Fish Shellfish Immunol. 2017, 65, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Zhang, Y.; Lin, S.; Shi, D.-L.; Shao, M. Highly efficient genome editing using oocyte-specific zcas9 transgenic zebrafish. J. Genet. Genom. 2018, 45, 509–512. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′—3′) | Application |
|---|---|---|
| EPC-bmp4-F | GTAGGCTGGAACGACTGGATTG | |
| EPC-bmp4-R | GCGTGATTGGTGGAGTTGAGA | |
| zebrafish-bmp4-F | CGCAGCCCTAAACAAAGAG | |
| zebrafish-bmp4-R | TGATTGGTGGAGTTGAGATGAT | |
| GCRV-vp5-F | CTCCCCGTGAGCGTATTT | |
| GCRV-vp5-R | GTTAGCAGCGGTAGTGACTTG | |
| EPC-ifn-F | ATAGACAACGCTAAGGTGGAGG | |
| EPC-ifn-R | TTCCGACGACTGCCTGTTC | |
| EPC-mx-F | GGGAGAAGGGATCAGTCATG | |
| EPC-mx-R | GGTTTAGTCAGAATACCGAGGG | |
| EPC-ID1-F | GATGTTGTCCGCTGCCTCT | qRT-PCR |
| EPC-ID1-R | CATGGTCATTTGCTCGTCC | |
| EPC-tbk1-F | TCAGAAGTTTGAGAACGGGAAGA | |
| EPC-tbk1-R | CGTAGACCACGATGCGGTGTAAG | |
| EPC-irf3-F | AACAAGAATGACACTGCGGA | |
| EPC-irf3-R | AACTCGGGAGGGACTTTCAT | |
| ZFL-ifnφ1-F | GTGGAGGACCAGGTGAAGTT | |
| ZFL-ifnφ1-R | GATTGACCCTTGCGTTGC | |
| ZFL-MXA-F | ATGGCTGGAGCAGGTGTT | |
| ZFL-MXA-R | TCTGTGGTGGCGATGTCA | |
| EPC-actin-F | TGTTCCAGCCATCCTTCTTG | |
| EPC-actin-R | TGATTTTCATTGTGCTGGGG | |
| ZFL-actin-F | GGTATTGTGATGGACTCTGGTGAT | |
| ZFL-actin-R | TCGGCTGTGGTGGTGAAG | |
| zebrafish-bmp4-F | ATGATTCCTGGTAATCGAATGCTG | cDNA cloning |
| zebrafish-bmp4-R | TTAGCGGCAGCCACACCC |
| Antibodies | Company | Product Code |
|---|---|---|
| Phospho-TBK1/NAK (Ser172) Rabbit antibody | Cell Signaling Technology | #5483T |
| TBK1/NAK Rabbit antibody | Cell Signaling Technology | #3504T |
| IRF3 Rabbit antibody | Bioss | #bs-2993R |
| Phospho-IRF3 (Ser386) Rabbit antibody | Bioss | #bsm-52170R |
| p38 MAPK Rabbit antibody | Bioss | #bs-0637R |
| Phospho-p38MAPK (Thr180 + Tyr182) Rabbit antibody | Bioss | #bs-2210R |
| β-Actin Rabbit antibody | Bioss | #bs-0061R |
| goat anti-rabbit IgG HRP secondary antibody | CWBIO | #CW0103S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhong, S.; Wang, Y.; Wang, X.; Liu, Z.; Hu, G. Bmp4 in Zebrafish Enhances Antiviral Innate Immunity through p38 MAPK (Mitogen-Activated Protein Kinases) Pathway. Int. J. Mol. Sci. 2023, 24, 14444. https://doi.org/10.3390/ijms241914444
Chen L, Zhong S, Wang Y, Wang X, Liu Z, Hu G. Bmp4 in Zebrafish Enhances Antiviral Innate Immunity through p38 MAPK (Mitogen-Activated Protein Kinases) Pathway. International Journal of Molecular Sciences. 2023; 24(19):14444. https://doi.org/10.3390/ijms241914444
Chicago/Turabian StyleChen, Lihui, Shenjie Zhong, Yajun Wang, Xinyuan Wang, Zhenhui Liu, and Guobin Hu. 2023. "Bmp4 in Zebrafish Enhances Antiviral Innate Immunity through p38 MAPK (Mitogen-Activated Protein Kinases) Pathway" International Journal of Molecular Sciences 24, no. 19: 14444. https://doi.org/10.3390/ijms241914444





