Fluorescent Molecules That Help Reveal Previously Unidentified Neural Connections in Adult, Neonatal and Peripubertal Mammals
Abstract
1. Introduction
2. A Brief History of the Discovery of Neuronal Connections (Contiguity vs. Continuity)
- (1)
- The tracer should not influence the transport system of the neuron.
- (2)
- The tracer should be minimally neurotoxic.
- (3)
- The tracer should be present in large enough concentrations for identification.
3. Classification of Neuronal Tracers
- At the beginning, tritiated amino acids (leucin or proline) were used to explore the terminal field of neuronal connections. These tracers were injected into a given area of the brain and then they were taken up by the injured neuronal cell bodies and transported along the axons. Autoradiography was used to visualize the transported amino acids. This method was introduced in the 1960s [9].
- Later, many other tracers were introduced which meet the above-mentioned criteria. The number of molecules is abundant (plant lectins: wheat germ agglutinin (WGA) and phaseolus vulgaris leucoagglutinin (PHA-L), dextran amine DAs, choleratoxin, etc.). In this review, we focus on the ever-increasing number of fluorescent tracers.
- Some tracers can be picked up from the blood, where the blood brain barrier is missing (circum ventricular organs: median eminence, area postrema, neurohypophysis, organum vasculosum of the lamina terminalis, pineal body, subcommissural organ, and subfornical organ), but do not pass through synapses. These tracers may be administered intravenously, intraperitoneally or somewhere peripherally as well. For example, intraperitoneal injection of FluoroGold (FG) was able to label hypophyseotropic neurons in the hypothalamus [10,11].
- (B) Some tracers are able to pass gap junctions (electrical synapsis). Mills and Massey [12] characterized the gap junctions in the retina using several types of tracers constructed from neurobiotin (biotin ethylenediamine). These tracers had low molecular weight (MW) of 244–555 kDa; therefore, they were suitable to demonstrate the diversity of electrical synapses. Other small molecules, such as lucifer yellow [13] and biotinylated dextran amine (BDA) with 3000 or lower MW, were also shown to pass gap junctions. Trementozzi and co-workers [14] investigated the transport of dextran chains through gap junctions in the range of 10–70 kDa. It was found that not the free dextran but dextran chains of up to 40 kDa can diffuse through at least five cell layers in a gap junction-dependent manner within 30 min in cell cultures.
- (C) Transsynaptic tracers can pass synapses. These are the neurotropic viruses. When a virus enters a neuron, it replicates and then it enters the next member of the neuronal chain and replicates again [15]. Finally, the virus infection becomes widespread and the animal dies. It is important to choose a suitable survival time for the infected animal.
4. Fluorescent Molecules as Non-Transsynaptic Tracers
5. Transsynaptic Tracers
5.1. Attenuated Pseudorabies Virus Bartha (PRV-Ba)
5.2. Herpes Simplex Virus (HSV)
5.3. Vesicular Stomatitis Virus (VSV)
6. Discovery of Green Fluorescent Protein (GFP) and Other Fluorescent Proteins
7. Fluorescent Protein Expressing Transgenic Animals in the Research of Neuronal Networks
8. The Mechanism of Action of Tracers
- Weak alkaline tracers may cross the cell membrane and may be trapped by lysosomes and endosomes which are acid cellular components.
- Tracers may be taken up by non-specific passive endocytosis.
- Tracers may bind to the neuron by sugar-mediated uptake.
- Other tracers enter the neurons at the terminals and are carried by vesicles to the cell body.
- Latex microspheres can enter the damaged membrane surface, but there is a minimal entry through undamaged fiber membrane surfaces as well.
- Several tracers enter injured neurons at the injection site and spread bidirectionally with diffusion.
- Static viral tracers enter the neuron via interaction between glucans or protein receptors expressed on the cell membrane and components of a viral vector. If the receptor is present on the terminals, it results in retrograde spreading. If the receptor is present on the soma, it results in anterograde spreading. Researchers can manipulate the direction of spread.
9. Applications of GFP-Labeled Virus Tracer in Our Study in Adult Rats
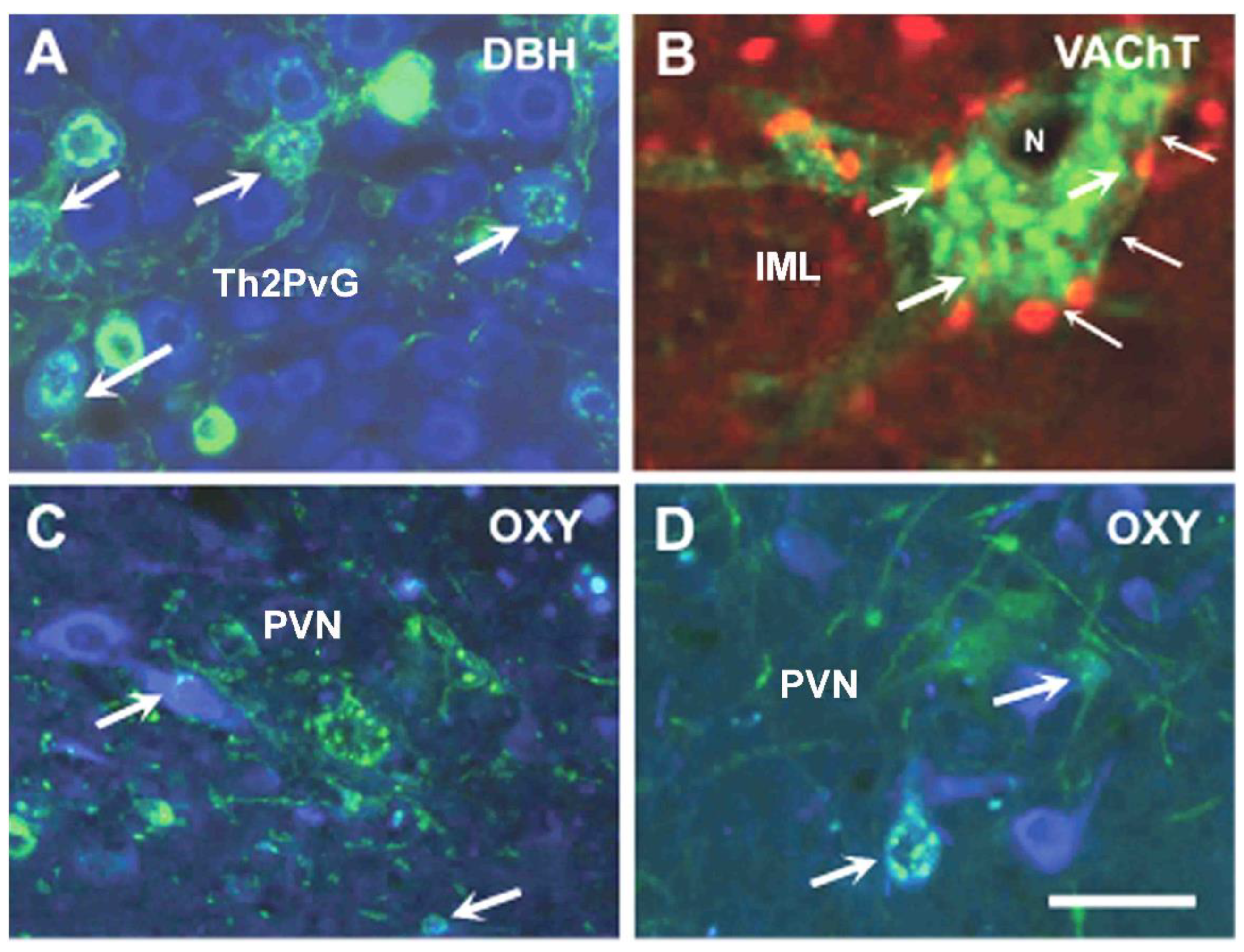
9.1. Autonomic Innervation of Mandibular Gingiva and Lower Lip
9.2. Autonomic and Sensory Innervation of the Mammary Gland in Lactating Rats
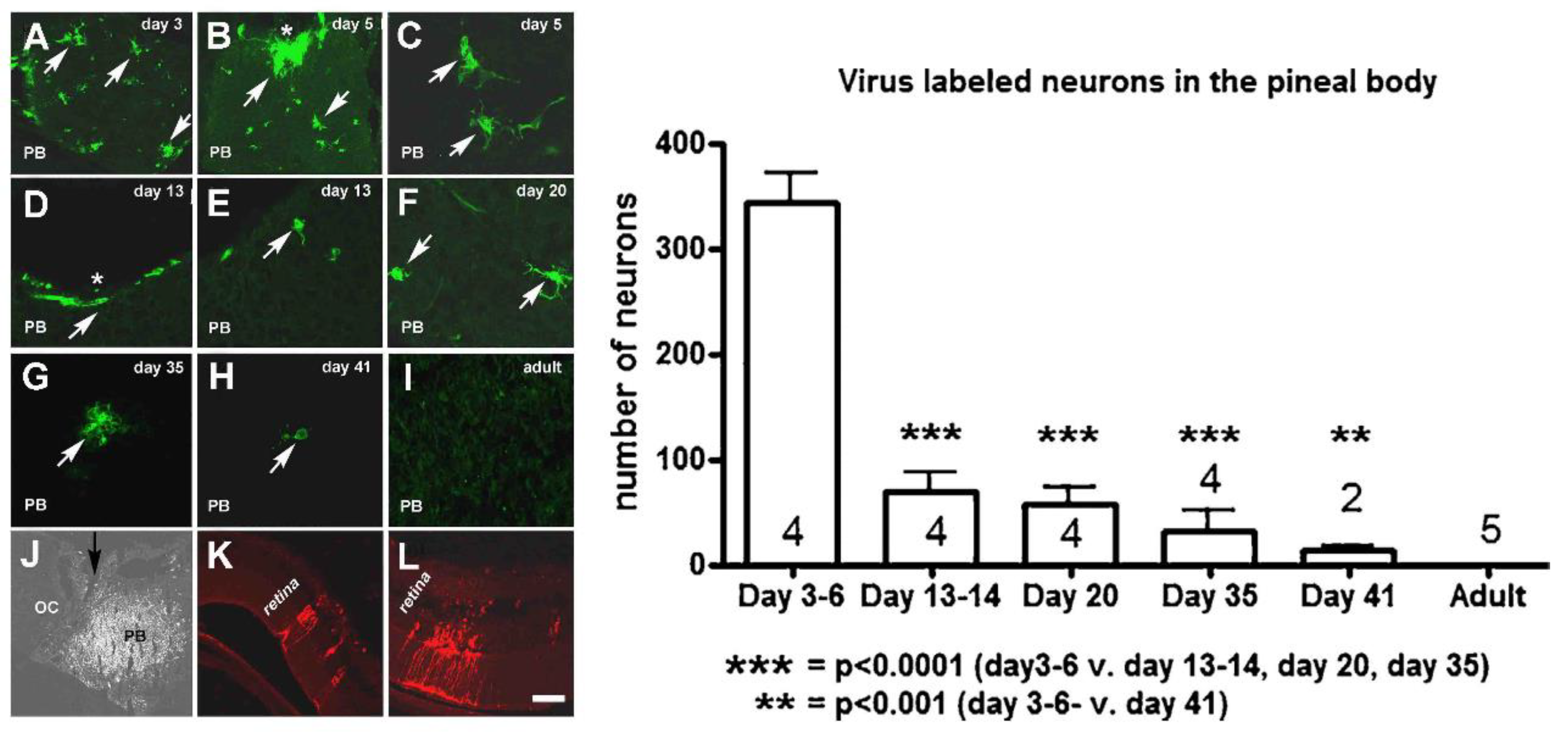
9.3. Usefulness of Fluorescence Virus Tracers in the Study of Neural Pathways at Different Ages
10. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Apáthy, S. The Leading Element of the Nervous System and Its Topographical Reviews on the Cells. Messages from the Zoological Station at Naples; Stazione Zoologica Anton Dohrn: Napoli, Italy, 1888. [Google Scholar]
- Bethe, A. A new proof of the function of neurofibrils. Folia Neurobiol. 1907, 1, 100–101. [Google Scholar]
- Ramon y Cajal, S. Nobel Lecture. Neurons and Synapses: The History of Its Discovery; 1906, Nobel Lectures, Physiology or Medicine 1901–1921; Elsevier Publishing Co.: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Ruska, E.; Knoll, M. Die magnetische Sammelspule für schnelle Elektronenstrahlen. (The magnetic concentrating coil for fast electron beams.). Z. techn. Physik 1931, 12, 389–400. [Google Scholar]
- Luse, S.A. Electron microscopic observations of the central nervous system. J. Biophysic. Biochemic. Cytol. 1956, 2, 531–541. [Google Scholar] [CrossRef]
- Loewi, O. The Ferrier Lecture: On problems connected with the principle of humoral transmission of nervous impulses. Proc. R. Soc. 1935, 118B, 299–316. [Google Scholar]
- Weidmann, S. The electrical constants of Purkinje fibres. J. Physiol. 1952, 118, 348–360. [Google Scholar] [CrossRef]
- Saleeba, C.; Dempsey, B.; Le, S.; Goodchild, A.; McMullan, S. A Student’s Guide to Neural Circuit Tracing. Front. Neurosci. 2019, 13, 2019. [Google Scholar] [CrossRef] [PubMed]
- Lasek, R.; Joseph, B.; Whitlock, D.G. Evaluation of a radioautographic neuroanatomical tracing method. Brain Res. 1968, 8, 319–336. [Google Scholar] [CrossRef]
- Merchenthaler, I. Enkephalin-immunoreactive neurons in the parvicellular subdivisions of the paraventricular nucleus project to the external zone of the median eminence. J. Comp. Neurol. 1992, 326, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Köves, K.; Vereczki, V.; Kausz, M.; Kántor, O.; Molnár, J.; Nemeskéri, Á.; Heinzlmann, A.; Szabó, E.; Szabó, F.; Fógel, K.; et al. PACAP and VIP in the photoneuroendocrine system (PNES). Med. Sci. Mon. 2002, 8, SR5–SR20. [Google Scholar]
- Mills, S.L.; Massey, S.C. A series of biotinylated tracers distinguishes three types of gap junction in retina. J. Neurosci. 2000, 20, 8629–8636. [Google Scholar] [CrossRef] [PubMed]
- Opsahl, H.; Rivedal, E. Quantitative determination of Ggp junction intercellular communication by scrape loading and image analysis. Cell Adhes. Commun. 2000, 7, 367–375. [Google Scholar]
- Trementozzi, A.N.; Zhao, C.; Smyth, H.; Cui, Z.; Stachowiak, J.C. Gap junction-mediated delivery of polymeric macromolecules. ACS Biomater. Sci. Eng. 2022, 8, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, G. Advances in viral transneuronal tracing. J. Neurosci. Methods 2010, 194, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Kristensson, K. Transport of fluorescent protein tracer in peripheral nerves. Acta Neuropath. 1970, 16, 293–300. [Google Scholar] [CrossRef]
- Kuypers, H.G.J.M.; Huisman, A.M. Fluorescent Neuronal Tracers. In Advances in Cellular Neurobiology; Fedoroff, S., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 5, pp. 307–340. ISBN 9781483266879. [Google Scholar]
- Evans, H.M.; Schulemann, W. The action of vital stains belonging to the benzidine group. Science 1914, 39, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xue, X.; Yu, P.; Ni, Y.; Chen, F. Evans Blue Dye: A Revisit of Its Applications in Biomedicine. Contrast Media Mol. Imaging 2018, 2018, 7628037. [Google Scholar] [CrossRef]
- Steinwall, O.; Klatzo, I. Selective vulnerability of the blood-brain barrier in chemically induced lesions. J. Neuropathol. Exper. Neurol. 1966, 25, 542–559. [Google Scholar] [CrossRef]
- Steward, O.; Scoville, S.A. Retrograde labeling of central nervous pathways with tritiated or Evans blue-labeled bovine serum albumin. Neurosci. Lett. 1976, 3, 191–196. [Google Scholar] [CrossRef]
- Van der Kooy, D.; Kuypers, H.G.J.M.; Catsman-Berrevoets, C.E. Single mammillary body cells with divergent axon collaterals. Demonstration by a simple fluorescent retrograde double labeling technique in the rat. Brain Res. 1978, 158, 189–196. [Google Scholar] [CrossRef]
- Bentivoglio, M.; Kuypers, H.G.; Catsman-Berrevoets, C.E.; Loewe, H.; Dann, O. Two new fluorescent retrograde neuronal tracers which are transported over long distances. Neurosci. Lett. 1980, 18, 25–30. [Google Scholar] [CrossRef]
- Kuypers, H.G.; Bentivoglio, M.; Catsman-Berrevoets, C.E.; Bharos, A.T. Double retrograde neuronal labeling through divergent axon collaterals, using two fluorescent tracers with the same excitation wavelength which label different features of the cell. Exp. Brain Res. 1980, 40, 383–392. [Google Scholar] [CrossRef]
- Keizer, K.; Kuypers, H.G.; Huisman, A.M.; Dann, O. Diamidino yellow dihydrochloride (DY.2HCl); a new fluorescent retrograde neuronal tracer, which migrates only very slowly out of the cell. Exp. Brain Res. 1983, 51, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Swanson, L.W. SITS: A covalently bound fluorescent retrograde tracer that does not appear to be taken up by fibers-of-passage. Brain Res. 1982, 249, 137–141. [Google Scholar] [CrossRef]
- Schmued, L.C.; Fallon, J.H. Fluoro-gold: A new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986, 377, 147–154. [Google Scholar] [CrossRef]
- Katz, L.; Burkhalter, A.; Dreyer, W. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature 1984, 310, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Dong, Y.; Kondoh, K.; Lu, Z. Trans-synaptic Neural Circuit-Tracing with Neurotropic Viruses. Neurosci. Bull. 2019, 35, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Bartha, A. Experimental reduction of virulence of Aujeszky’s disease virus. Magy. Állatorv. Lapja 1961, 16, 42–45. [Google Scholar]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 9, 462–500. [Google Scholar] [CrossRef]
- Mettenleiter, T.C.; Zsak, L.; Kaplan, A.S.; Ben-Porat, T.; Lomniczi, B. Role of a structural glycoprotein of pseudorabies in virus virulence. J. Virol. 1987, 61, 4030–4032. [Google Scholar] [CrossRef]
- Kratchmarov, R.; Kramer, T.; Greco, T.M.; Taylor, M.P.; Ch’ng, T.H.; Cristea, I.M.; Enquist, L.W. Glycoproteins gE and gI are required for efficient KIF1A-dependent anterograde axonal transport of alphaherpesvirus particles in neurons. J. Virol. 2013, 87, 9431–9440. [Google Scholar] [CrossRef]
- Boldogkői, Z.; Reichart, A.; Tóth, I.E.; Sik, A.; Erdélyi, F.; Medveczky, I.; Llorens-Cortes, C.; Palkovits, M.; Lenkei, Z. Construction of recombinant pseudorabies viruses optimized for labeling and neurochemical characterization of neural circuitry. Brain Res. Mol. Brain Res. 2002, 109, 105–118. [Google Scholar] [CrossRef]
- Ho, D.Y.; Mocarski, E.S. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology 1988, 167, 279–283. [Google Scholar] [CrossRef]
- Beier, K.V.; Saunders, A.; Oldenburg, I.A.; Miyamichi, K.; Akhtar, N.; Luo, L.; Whelan, S.P.; Sabatini, B.; Cepko, C.L. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. PNAS 2011, 108, 15414–15419. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Chalfie, M.; Tsien, R.J. GFP: Lighting Up Life. Nobel Lect. 2008, 106, 10073–10080. [Google Scholar]
- Shimomura, O.; Johnson, F.H. Properties of the bioluminescent protein aequorin. Biochemistry 1969, 8, 3991–3997. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green fluorescent protein as a marker for gene expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, C.; Chalfie, M. Combinatorial marking of cells and organelles with reconstituted fluorescent proteins. Cell 2004, 119, 137–144. [Google Scholar] [CrossRef]
- Delagrave, S.; Hawtin, R.E.; Silva, C.M.; Yang, M.M.; Youvan, D.C. Red-shifted excitation mutants of the green fluorescent protein. Biotechnology 1995, 13, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef]
- Tramier, M.; Zahid, M.; Mevel, J.C.; Masse, M.J.; Coppey-Moisan, M. Sensitivity of CFP/YFP and GFP/mCherry pairs to donor photobleaching on FRET determination by fluorescence lifetime imaging microscopy in living cells. Microsc. Res. Tech. 2006, 69, 933–939. [Google Scholar] [CrossRef]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar] [CrossRef]
- Merzlyak, E.M.; Goedhart, J.; Shcherbo, D.; Bulina, M.E.; Shcheglov, A.S.; Arkady, F.; Gaintzeva, A.; Lukyanov, K.A.; Lukyanov, S.; Gadella, T.W.J.; et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods 2007, 4, 555–557. [Google Scholar] [CrossRef]
- Jöns, A.; Mettenleiter, T.C. Green fluorescent protein expressed by recombinant pseudorabies virus as an in vivo marker for viral replication. J. Virol. Method. 1997, 66, 283–292. [Google Scholar] [CrossRef]
- Boldogkői, Z.; Bálint, K.; Awatramani, G.B.; Balya, D.; Busskamp, V.; Viney, T.J.; Lagali, P.S.; Duebel, J.; Pásti, E.; Tombácz, D.; et al. Genetically timed, activity-sensor and rainbow transsynaptic viral tools. Nat. Methods 2009, 6, 127–130. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N.; Ghosh, P.K. Selective neuronal expression of green fluorescent protein with cytomegalovirus promoter reveals entire neuronal arbor in transgenic mice. J. Neurosci. 1998, 18, 10640–10651. [Google Scholar] [CrossRef]
- Martin, E.I.; Ressler, K.J.; Jasnow, A.M.; Dabrowska, J.; Hazra, R.; Rainnie, D.G.; Nemeroff, C.B.; Owens, M.J. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol. Psychiatry 2010, 67, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, J.; Martinon, D.; Moaddab, M.; Rainnie, D.G. Targeting corticotropin-releasing factor (CRF) projections from the oval nucleus of the BNST using cell-type specific neuronal tracing studies in mouse and rat brain. J. Neuroendocrinol. 2016, 28, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Prönneke, A.; Scheuer, B.; Wagener, R.J.; Mock, M.; Witte, M.; Staiger, J.F. Characterizing VIP Neurons in the Barrel Cortex of VIPcre/tdTomato Mice Reveals Layer-Specific Differences. Cerebral Cortex 2015, 25, 4854–4868. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.A.; Jiang, M.; Lam, T.; Smith, K.L.; Swann, J.W. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J. Neurosci. 2000, 20, 3354–3368. [Google Scholar] [CrossRef]
- Xu, X.; Roby, K.D.; Callaway, E.M. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010, 518, 389–404. [Google Scholar] [CrossRef]
- Chiu, C.-S.; Jensen, K.; Sokolova, I.; Wang, D.; Li, M.; Deshpande, P. Number, Density, and Surface/Cytoplasmic Distribution of GABA Transporters at Presynaptic Structures of Knock-In Mice Carrying GABA Transporter Subtype 1-Green Fluorescent Protein Fusions. J. Neurosci. 2002, 22, 10251–10266. [Google Scholar] [CrossRef]
- Porrero, C.; Rubio-Garrido, P.; Avendaño, C.; Clascá, F. Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res. 2010, 1345, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Mellor, R.H.; Bernstein, M.; Keller-Peck, C.; Nguyen, Q.T.; Wallace, M.; Nerbonne, J.M.; Lichtman, J.W.; Sanes, J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 2000, 28, 41–51. [Google Scholar] [CrossRef]
- Spergel, D.J.; Krüth, U.; Hanley, D.F.; Sprengel, R.; Seeburg, P.H. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J. Neurosci. 1999, 19, 2037–2050. [Google Scholar] [CrossRef]
- Liu, H.; Kishi, T.; Roseberry, A.G.; Cai, X.; Lee, C.E.; Montez, J.M.; Friedman, J.M.; Elmquist, J.K. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J. Neurosci. 2003, 23, 7143–7154. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Saito, H.; Suzuki, M.; Mori, K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 2000, 11, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Maskos, U.; Kissa, K.S.; Cloment, C.; Brûlet, P. Retrograde trans-synaptic transfer of green fluorescent protein allows the genetic mapping of neuronal circuits in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 10120–10125. [Google Scholar] [CrossRef]
- Tallini, Y.N.; Shui, B.; Greene, K.S.; Deng, K.Y.; Doran, R.; Fisher, P.J.; Zipfel, W.; Kotlikoff, M.I. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol. Genom. 2006, 27, 391–397. [Google Scholar] [CrossRef]
- Card, J.P.; Sved, A.F. Central autonomic pathways. In Central Regulation of Autonomic Functions, 2nd ed.; Llewellyn-Smith, I.J., Verberne, A.J.M., Eds.; Oxford University Press: Oxford, GB, USA, 2011; Chapter 1; pp. 3–22. [Google Scholar]
- Szabó, E.; Csáki, Á.; Boldogkői, Z.; Tóth, Z.; Köves, K. Identification of autonomic neuronal chains innervating gingiva and lip. Auton. Neurosci. 2015, 190, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Köves, K.; Boldogkői, Z.; Csáki, A.; Lohinai, Z.; Tóth, Z.; Ciofi, P. Hypothalamic neuropeptides in the autonomic innervation of gingiva and lip. J. Trans. Neurosci. 2016, 1, 7–19. [Google Scholar]
- Köves, K.; Györgyi, Z.; Szabó, F.K.; Boldogkői, Z. Characterization of the autonomic innervation of mammary gland in lactating rats studied by retrograde transynaptic virus labeling and immunohistochemistry. Acta Physiol. Hung. 2012, 99, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Vígh-Teichmann, I.; Petter, H.; Vigh, B. GABA-immunoreactive intrinsic and immunonegative secondary neurons in the cat pineal organ. J. Pineal Res. 1991, 10, 18–29. [Google Scholar] [CrossRef]
- Sakai, Y.; Hira, Y.; Matsushima, S. Central GABAergic innervation of the mammalian pineal gland: A light and electron microscopic immunocytochemical investigation in rodent and nonrodent species. J. Comp. Neurol. 2001, 430, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sakai, Y.; Hira, Y.; Oomori, Y.; Daikoku, S. Immunohistochemical studies on sympathetic and non-sympathetic nerve fibers and neuronal cell bodies in the pineal gland of cotton rats, Sigmodon hispidus. Arch. Histol. Cytol. 1994, 57, 47–58. [Google Scholar] [PubMed]
- Ichimura, T.; Arikuni, T.; Hashimoto, P.H. Fine-structural study of the pinealbody of 3 monkey (Macaca fuscata) with special reference to synapticformations. Cell Tissue Res. 1986, 244, 569–576. [Google Scholar] [CrossRef]
- Moore, R.Y.; Lenn, N.J. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972, 146, 1–14. [Google Scholar] [CrossRef]
- Teclemariam-Mesbah, R.; Ter Horst, G.J.; Postema, F.; Wortel, J.; Buijs, R.M. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J. Comp. Neurol. 1999, 406, 171–182. [Google Scholar] [CrossRef]
- Csáki, Á.; Vígh, B.; Boldogkői, Z.; Vereczki, V.; Szél, Á.; Köves, K. Is a neuronal chain between the pineal body and the retina in rats and hamsters? Transneural tracing studies. Neurosci. Lett. 2015, 588, 1–6. [Google Scholar] [CrossRef]
- Csáki, Á.; Vereczki, V.; Lukáts, Á.; Boldogkői, Z.; Sebestyén, A.; Puskár, Z.; Köves, K. Ontogenesis of the pinealo-retinal neuronal connection in albino rats. Neurosci. Lett. 2018, 665, 189–194. [Google Scholar] [CrossRef]
- Csáki, Á.; Köves, K.L.; Kiss, A.; Röhlich, P.; Boldogkői, Z.; Vereczki, V.; Puskár, Z.; Tombácz, D.; Csabai, Z. Pinealocytes cannot transport neurotropic viruses. Pinealo-to-retinal connection in prepubertal rats originates from pineal neurons: Light and electron microscopic immunohistochemical studies. Neurosci. Lett. 2021, 744, 135517. [Google Scholar] [CrossRef]
- Tsutsui, K.; Saigoh, E.; Ukena, K.; Teranishi, H.; Fujisawa, Y.; Kikuchi, M.; Ishii, S.; Sharp, P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000, 275, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Ubuka, T.; Bentley, G.E.; Kriegsfeld, L.J. Gonadotropin-inhibitory hormone (GnIH): Discovery, progress and prospect. Gen. Comp. Endocrinol. 2012, 177, 305–314. [Google Scholar] [CrossRef]
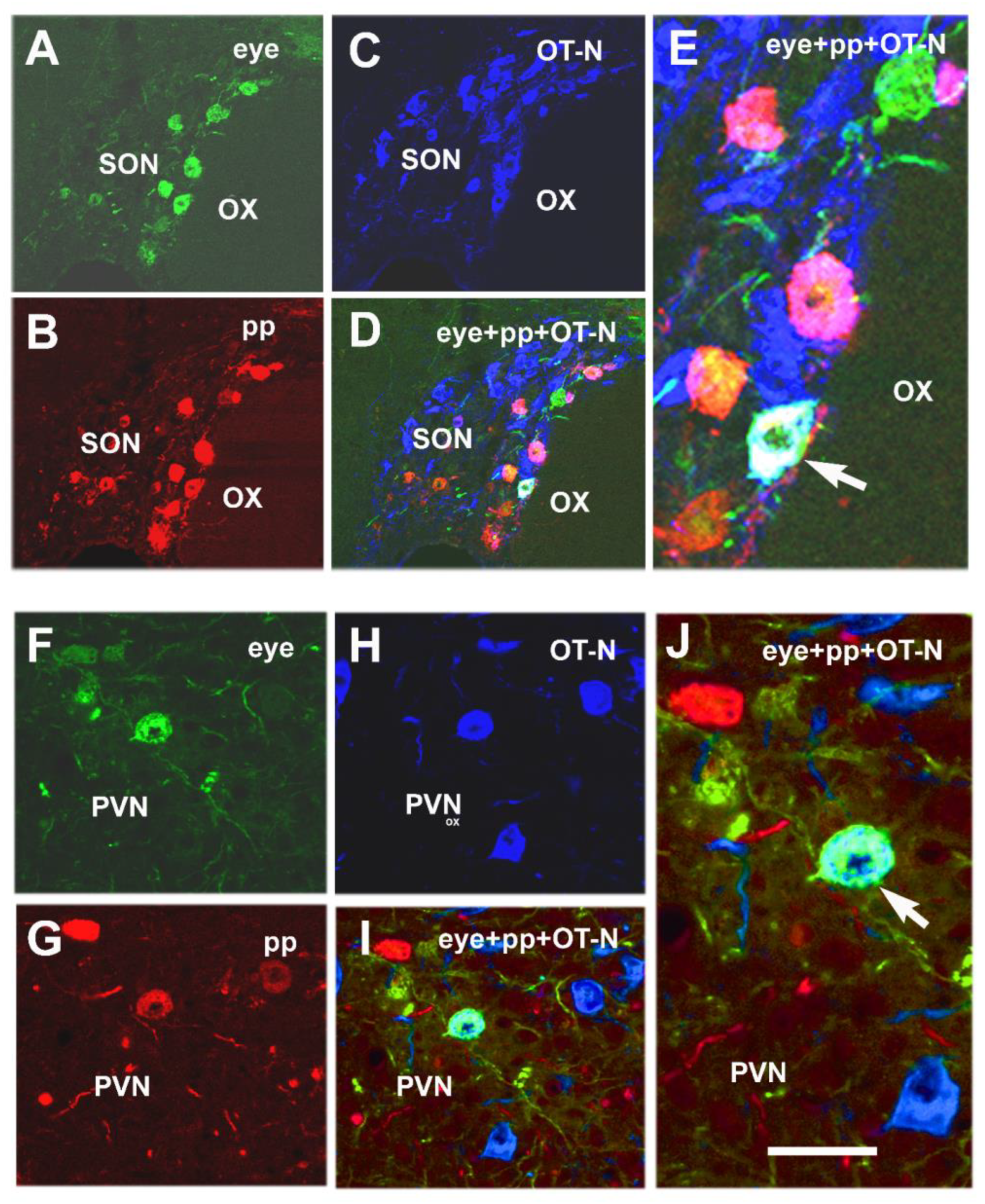
- Csáki, Á.; Köves, K.; Boldogkői, Z.S.; Tombácz, D.; Tóth, Z.E. The same magnocellular neurons send axon collaterals to the posterior pituitary and retina or to the posterior pituitary and autonomic preganglionic centers of the eye in rats. NeuroSci 2021, 2, 27–44. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Sawchenko, P.E. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: Immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 1984, 290, 219–238. [Google Scholar] [CrossRef]
- Conte, W.L.; Kamishina, H.; Reep, R.L. Multiple neuroanatomical tract-tracing using fluorescent Alexa Fluor conjugates of cholera toxin subunit B in rats. Nat. Protoc. 2009, 4, 1157–1166. [Google Scholar] [CrossRef]
- Vaney, D.I. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. J. Neurosci. Lett. 1991, 125, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhou, D.; DiFiglia, M. Neurobiotin, a useful neuroanatomical tracer for in vivo anterograde, retrograde and transneuronal tract-tracing and for in vitro labeling of neurons. J. Neurosci. Methods 1992, 41, 31–43. [Google Scholar] [CrossRef]
- Mills, S.L.; Massey, S.C. The kinetics of tracer movement through homologous gap junctions in the rabbit retina. Vis. Neurosci. 1998, 15, 765–777. [Google Scholar] [CrossRef]
| Type of tracers | Direction of Transportation | Visualization | References |
|---|---|---|---|
| Non-fluorescent non-transsynaptic tracers | |||
| Triciated amino acids | Anterograde | Autoradiography | Lasek et al. [9] |
| WGA (wheat germ agglutinin) | Ante-/retrograde | Avidin-biotinylated HRP (ABC) | Gerfen and Sawchenko [78] |
| PHA-L (phaseolus vulgaris leucoagglutinin) | Ante-/retrograde | Avidin-biotinylated HRP (ABC) | Gerfen and Sawchenko [78] |
| CTXB (choleratoxin subunit B) | Retrogade | ABC and alexafluor | Cont et al. [79] |
| Fluorescent non-transsynaptic tracers | |||
| Evans Blue | Retrograde | Fluorescence microscopy | Kuypers and Huisman [24] |
| NY (Nuclear Yellow) | Retrograde | Fluorescence microscopy | Bentivoglio et al. [23] |
| Bp (Bisbenzimide) | Retrograde | Fluorescence microscopy | Bentivoglio et al. [23] |
| Fb (Fast blue) | Retrograde | Fluorescence microscopy | Kuypers et al. [24] |
| Tb (True blue) | Retrograde | Fluorescence microscopy | Kuypers et al. [24] |
| DY (Diamidino Yellow dihydrochloride) | Retrograde | Fluorescence microscopy | Keizer et al. [25] |
| SITS (Stilbene compound) | Retrograde | Fluorescence microscopy | Schmued and Swanson [26] |
| SG (Stilbene Gold) | Retrograde | Fluorescence microscopy | Schmued and Fallon [27] |
| FG (Fluorogold) | Retrograde | Fluorescence microscopy | Schmued and Fallon [27] |
| Latex microspheres Rhodamine labeled | Retrograde | Fluorescence microscopy | Katz et al. [28] |
| Tracers passing gap junctions | |||
| Neurobiotin 286Da | Versatile | Avidin-biotinylated HRP (ABC), Streptavidin-Cy3 | Vaney [80], Huang et al. [81] |
| Mills and Massey [12] | |||
| Biotin-X cadaverine 442Da | Versatile | Avidin-biotinylated HRP (ABC), Streptavidin-Cy3 | Mills and Massey [12,82] |
| Lucifer yellow | Versatile | Fluorescence microscopy | Mills and Massey [82] |
| Non-fluorescent transsynaptic tracers | |||
| Pseudorabies virus (PRV) Ba- DupLac | Ante-/retrograde | Immunostaining hrp or fluorescence microscopy | Boldogkői et al. [34] |
| Herpes simplex virus (HSV) expressing LacZ | Anterograde | Immunostaining hrp or fluorescence microscopy | Ho and Mocarski [35] |
| Vesicular stomatitis virus (VSV) | Ante-/retrograde | Fluorescence microscopy | Beier and co-workers [36] |
| Fluorescent transsynaptic tracers | |||
| KA-GEI-memGFP-RV | Retrograde | Fluorescence microscopy | Boldogkői et al. [47] |
| KA-GEI-memTomato-A-RV | Ante-/retrograde | Fluorescence microscopy | Csáki et al. [72,74] |
| VHS-mCherry-A-RV | Ante-/retrograde | Fluorescence microscopy | Csáki et al. [73,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasziné Szabó, E.; Köves, K.; Csáki, Á. Fluorescent Molecules That Help Reveal Previously Unidentified Neural Connections in Adult, Neonatal and Peripubertal Mammals. Int. J. Mol. Sci. 2023, 24, 14478. https://doi.org/10.3390/ijms241914478
Vasziné Szabó E, Köves K, Csáki Á. Fluorescent Molecules That Help Reveal Previously Unidentified Neural Connections in Adult, Neonatal and Peripubertal Mammals. International Journal of Molecular Sciences. 2023; 24(19):14478. https://doi.org/10.3390/ijms241914478
Chicago/Turabian StyleVasziné Szabó, Enikő, Katalin Köves, and Ágnes Csáki. 2023. "Fluorescent Molecules That Help Reveal Previously Unidentified Neural Connections in Adult, Neonatal and Peripubertal Mammals" International Journal of Molecular Sciences 24, no. 19: 14478. https://doi.org/10.3390/ijms241914478
APA StyleVasziné Szabó, E., Köves, K., & Csáki, Á. (2023). Fluorescent Molecules That Help Reveal Previously Unidentified Neural Connections in Adult, Neonatal and Peripubertal Mammals. International Journal of Molecular Sciences, 24(19), 14478. https://doi.org/10.3390/ijms241914478





