Identification of Na+/K+-ATPase Inhibitor Bufalin as a Novel Pseudorabies Virus Infection Inhibitor In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Screening of the Anti-PRV Compounds Using Cell-Based ELISA
2.2. Bufalin Inhibited PRV Infection in a Dose-Dependent Manner
2.3. Bufalin Inhibited PRV Infection by Interfering with Viral Entry
2.4. Na+/K+-ATPase Inhibitor Activity of Bufalin Was Involved in PRV Replication Suppression
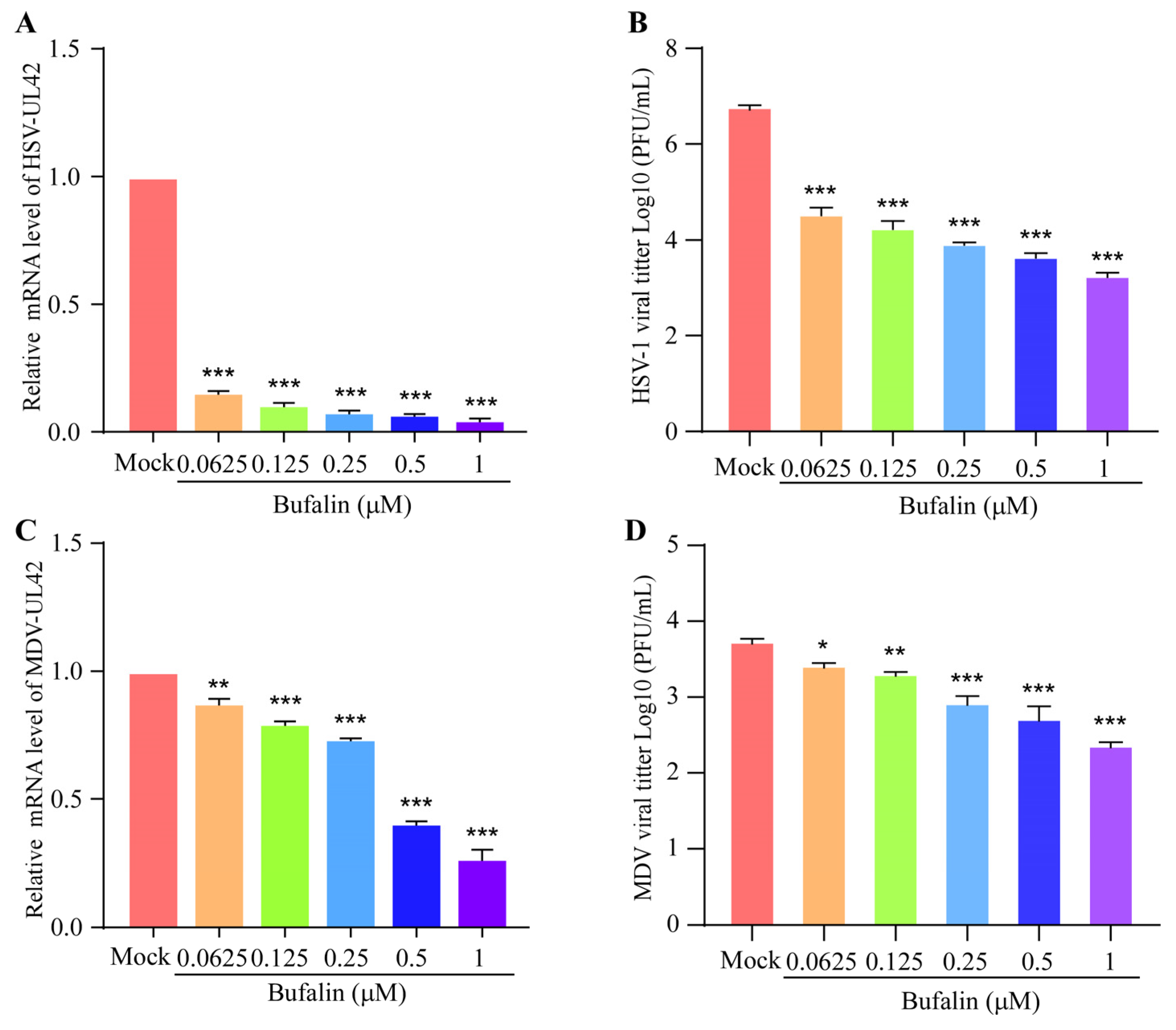
2.5. Bufalin Showed a Wide Inhibitory Spectrum to Other Herpesviruses
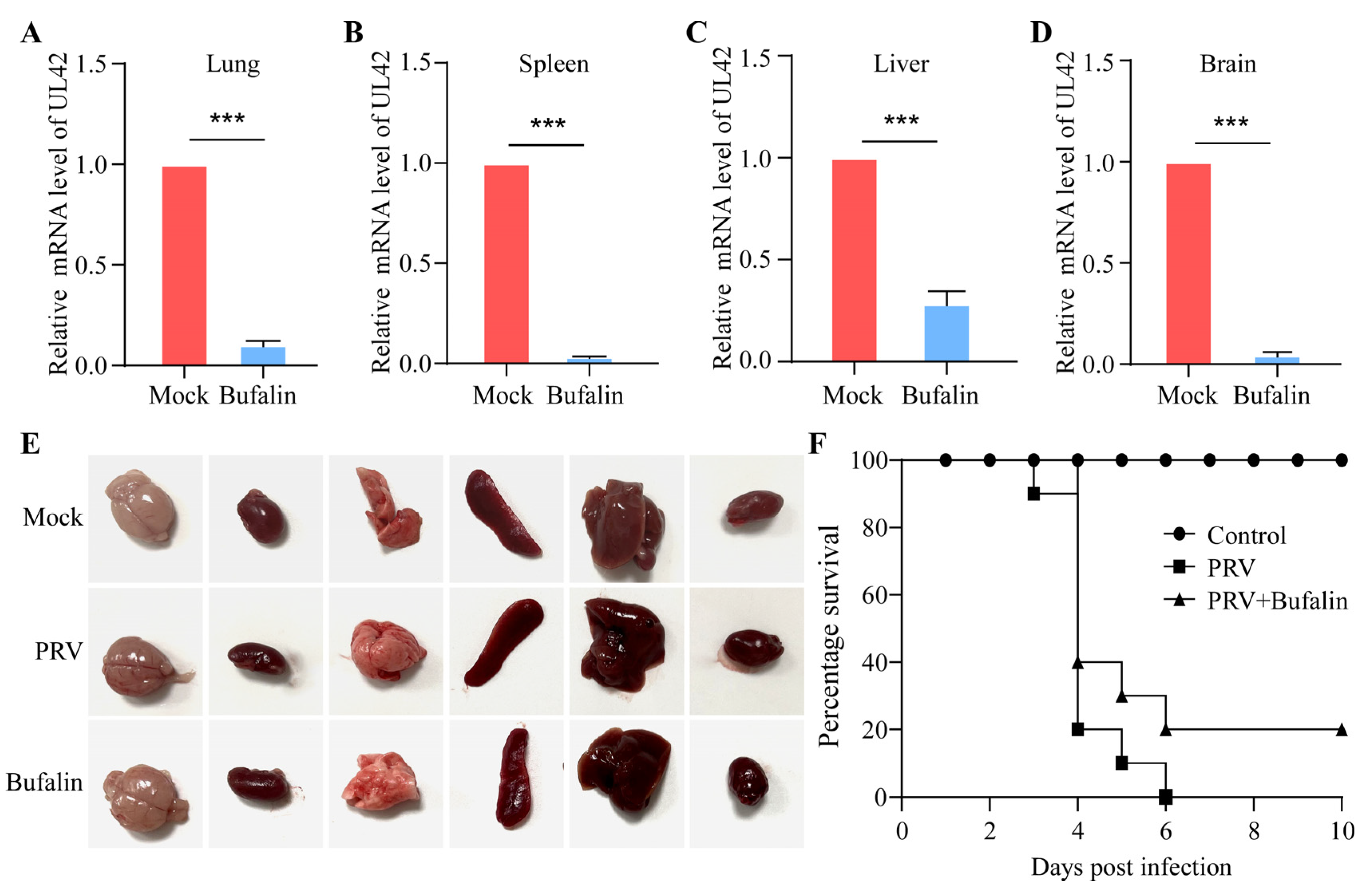
2.6. Bufalin Showed a Therapeutic Effect on PRV Infection in Mice
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Chemical Reagents and Antibodies
4.3. Immunoblotting Analysis
4.4. Cell Viability Assay
4.5. Cell-Based ELISA
4.6. qRT-PCR
4.7. Indirect Immunofluorescence Assay
4.8. Plaque Formation Assay
4.9. The Inhibitory Effect of Bufalin on Different Stages of PRV Replication
4.10. Sodium and Potassium Treatment Assay
4.11. The Effect of Bufalin on PRV Infection in BALB/C Mice
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.Y.; Wilson, M.R. A review of pseudorabies (Aujeszky’s disease) in pigs. Can. Vet. J. 1979, 20, 65–69. [Google Scholar] [PubMed]
- Nauwynck, H.; Glorieux, S.; Favoreel, H.; Pensaert, M. Cell biological and molecular characteristics of pseudorabies virus infections in cell cultures and in pigs with emphasis on the respiratory tract. Vet. Res. 2007, 38, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Aujeszky’s disease (pseudorabies) virus: The virus and molecular pathogenesis—State of the art, June 1999. Vet. Res. 2000, 31, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Li, X. A Review of Pseudorabies Virus Variants: Genomics, Vaccination, Transmission, and Zoonotic Potential. Viruses 2022, 14, 1003. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Xie, C.; Ding, S.; Yang, H.; Guo, S.; Li, J.; Qin, L.; Ban, F.; Wang, D.; et al. A Novel Human Acute Encephalitis Caused by Pseudorabies Virus Variant Strain. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e3690–e3700. [Google Scholar] [CrossRef]
- Wong, G.; Lu, J.; Zhang, W.; Gao, G.F. Pseudorabies virus: A neglected zoonotic pathogen in humans? Emerg. Microbes Infect. 2019, 8, 150–154. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, C.; Liu, Y.; Chen, S.; Zheng, Y.; Wang, Z.; Cao, R.; Wang, J. A noval strategy of deletion in PK gene for construction of a vaccine candidate with exellent safety and complete protection efficiency against high virulent Chinese pseudorabies virus variant. Virus Res. 2022, 313, 198740. [Google Scholar] [CrossRef]
- Delva, J.L.; Nauwynck, H.J.; Mettenleiter, T.C.; Favoreel, H.W. The Attenuated Pseudorabies Virus Vaccine Strain Bartha K61: A Brief Review on the Knowledge Gathered During 60 Years of Research. Pathogens 2020, 9, 897. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, Z.; Hu, D.; Zhang, Q.; Han, T.; Li, X.; Gu, X.; Yuan, L.; Zhang, S.; Wang, B.; et al. Pathogenic pseudorabies virus, China, 2012. Emerg. Infect. Dis. 2014, 20, 102–104. [Google Scholar] [CrossRef]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef]
- Fan, J.; Zeng, X.; Zhang, G.; Wu, Q.; Niu, J.; Sun, B.; Xie, Q.; Ma, J. Molecular characterization and phylogenetic analysis of pseudorabies virus variants isolated from Guangdong province of southern China during 2013–2014. J. Vet. Sci. 2016, 17, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Liu, F.; Zheng, H.; Liang, C.; Zhou, Y.J.; Jiang, Y.F.; Shan, T.L.; Gao, F.; Li, G.X.; Tong, G.Z. Emergence of a Pseudorabies virus variant with increased virulence to piglets. Vet. Microbiol. 2015, 181, 236–240. [Google Scholar] [CrossRef]
- Gu, Z.; Dong, J.; Wang, J.; Hou, C.; Sun, H.; Yang, W.; Bai, J.; Jiang, P. A novel inactivated gE/gI deleted pseudorabies virus (PRV) vaccine completely protects pigs from an emerged variant PRV challenge. Virus Res. 2015, 195, 57–63. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Liu, H.; Ye, G.; Huang, L.; Weng, C. Isolation and Characterization of Two Pseudorabies Virus and Evaluation of Their Effects on Host Natural Immune Responses and Pathogenicity. Viruses 2022, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L.; Tan, L.; Liang, X. Inhibition of Herpes Simplex Virus-1 Replication by Natural Compound Honokiol. Virol. Sin. 2019, 34, 315–323. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Conradie, A.M.; Hahn, F.; Wild, M.; Marschall, M.; Kaufer, B.B. Artesunate derivative TF27 inhibits replication and pathogenesis of an oncogenic avian alphaherpesvirus. Antivir. Res. 2019, 171, 104606. [Google Scholar] [CrossRef]
- Huan, C.; Xu, W.; Guo, T.; Pan, H.; Zou, H.; Jiang, L.; Li, C.; Gao, S. (-)-Epigallocatechin-3-Gallate Inhibits the Life Cycle of Pseudorabies Virus In Vitro and Protects Mice Against Fatal Infection. Front. Cell. Infect. Microbiol. 2020, 10, 616895. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Li, L.; Chen, Y.; Jia, R.; Zou, Y.; Wan, H.; Zhao, L.; Tang, H.; Lv, C.; et al. Resveratrol Inhibits Pseudorabies Virus Replication by Targeting IE180 Protein. Front. Microbiol. 2022, 13, 891978. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Li, Z.; Shangguan, A.; Jiang, J.; Zeng, W.; Zhang, S.; He, Q. Quercetin as an antiviral agent inhibits the Pseudorabies virus in vitro and in vivo. Virus Res. 2021, 305, 198556. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.X.; Wang, T.Y.; Tang, Y.D.; Wei, P. Isobavachalcone inhibits Pseudorabies virus by impairing virus-induced cell-to-cell fusion. Virol. J. 2020, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.J.; Ruan, L.J.; Tian, H.Y.; Liang, G.P.; Ye, W.C.; Hughes, E.; Esmann, M.; Fedosova, N.U.; Chung, T.Y.; Tzen, J.T.; et al. Novel stereoselective bufadienolides reveal new insights into the requirements for Na+, K+-ATPase inhibition by cardiotonic steroids. Sci. Rep. 2016, 6, 29155. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, Y.; Wang, C.H.; Yuan, J.; Li, N.; Song, K.; Qiu, H.J. Control of swine pseudorabies in China: Opportunities and limitations. Vet. Microbiol. 2016, 183, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, Y.; Zuo, Q.; Chen, T. Molecular mechanisms underlying the antimetastatic activity of bufalin. Mol. Clin. Oncol. 2018, 8, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Zhou, B.F.; Xie, Y.Y.; Lou, J.; Li, K.Q. Bufalin and 5-fluorouracil synergistically induce apoptosis in colorectal cancer cells. Oncol. Lett. 2018, 15, 8019–8026. [Google Scholar] [CrossRef]
- Miao, Q.; Bi, L.L.; Li, X.; Miao, S.; Zhang, J.; Zhang, S.; Yang, Q.; Xie, Y.H.; Zhang, J.; Wang, S.W. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 cells: Roles of apoptosis and autophagy. Int. J. Mol. Sci. 2013, 14, 1370–1382. [Google Scholar] [CrossRef]
- Xie, C.M.; Chan, W.Y.; Yu, S.; Zhao, J.; Cheng, C.H. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic. Biol. Med. 2011, 51, 1365–1375. [Google Scholar] [CrossRef]
- Lu, C.X.; Nan, K.J.; Lei, Y. Agents from amphibians with anticancer properties. Anti Cancer Drugs 2008, 19, 931–939. [Google Scholar] [CrossRef]
- Lan, Y.L.; Wang, X.; Lou, J.C.; Xing, J.S.; Yu, Z.L.; Wang, H.; Zou, S.; Ma, X.; Zhang, B. Bufalin inhibits glioblastoma growth by promoting proteasomal degradation of the Na+/K+-ATPase α1 subunit. Biomed. Pharmacother. 2018, 103, 204–215. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W. Bufalin induced apoptosis of bladder carcinoma cells through the inactivation of Na+K+-ATPase. Oncol. Lett. 2018, 16, 3826–3832. [Google Scholar] [CrossRef]
- Yu, Z.; Feng, H.; Zhuo, Y.; Li, M.; Zhu, X.; Huang, L.; Zhang, X.; Zhou, Z.; Zheng, C.; Jiang, Y.; et al. Bufalin inhibits hepatitis B virus-associated hepatocellular carcinoma development through androgen receptor dephosphorylation and cell cycle-related kinase degradation. Cell. Oncol. 2020, 43, 1129–1145. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, A.K.; Wu, K.X.; Qiang, J.; Chu, J.J.; Kwang, J. Natural compounds inhibiting the replication of Porcine reproductive and respiratory syndrome virus. Antivir. Res. 2012, 94, 188–194. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Zhang, Y.N.; Li, X.D.; Zhang, H.Q.; Xiao, S.Q.; Deng, F.; Yuan, Z.M.; Ye, H.Q.; Zhang, B. A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jia, X.; Liu, Y.; Wang, S.; Cao, J.; Zhang, B.; Xiao, G.; Wang, W. Screening of Natural Extracts for Inhibitors against Japanese Encephalitis Virus Infection. Antimicrob. Agents Chemother. 2020, 64, e02373-19. [Google Scholar] [CrossRef]
- Carvalho, D.C.M.; da Silva, P.G.; Dantas, W.M.; da Silva, S.J.R.; da Silva, C.T.A.; Chaves, E.J.F.; de Araújo, D.A.M.; de Oliveira, R.N.; Rodrigues-Mascarenhas, S.; Pena, L.J. Antiviral activity of ouabain against a Brazilian Zika virus strain. Sci. Rep. 2022, 12, 12598. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- Yasin, B.; Wang, W.; Pang, M.; Cheshenko, N.; Hong, T.; Waring, A.J.; Herold, B.C.; Wagar, E.A.; Lehrer, R.I. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 2004, 78, 5147–5156. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- Sun, L.; Chen, H.; Ming, X.; Bo, Z.; Shin, H.J.; Jung, Y.S.; Qian, Y. Porcine Epidemic Diarrhea Virus Infection Induces Caspase-8-Mediated G3BP1 Cleavage and Subverts Stress Granules To Promote Viral Replication. J. Virol. 2021, 95, e02344-20. [Google Scholar] [CrossRef]
- Lin, C.; Huang, C.; Shi, Z.; Ou, M.; Sun, S.; Yu, M.; Chen, T.; Yi, Y.; Ji, X.; Lv, F.; et al. Biodegradable calcium sulfide-based nanomodulators for H2S-boosted Ca2+-involved synergistic cascade cancer therapy. Acta Pharm. Sin. B 2022, 12, 4472–4485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Zhang, Q.; Sun, J.; Zhang, X.; Wu, Y. PQBP1 regulates the cellular inflammation induced by avian reovirus and interacts with the viral p17 protein. Virus Res. 2023, 332, 199119. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Miao, Y.; Xi, R.; Zhong, Q.; Bao, C.; Chen, H.; Sun, L.; Qian, Y.; Jung, Y.S.; Dai, J. PRV UL13 inhibits cGAS-STING-mediated IFN-β production by phosphorylating IRF3. Vet. Res. 2020, 51, 118. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, Z.; Zhang, Y.; Wan, H.; Yin, Z.; Li, L.; Liang, X.; Zhao, X.; Yin, L.; Ye, G.; et al. Myricetin inhibits pseudorabies virus infection through direct inactivation and activating host antiviral defense. Front. Microbiol. 2022, 13, 985108. [Google Scholar] [CrossRef]
- Tolo, F.M.; Rukunga, G.M.; Muli, F.W.; Njagi, E.N.; Njue, W.; Kumon, K.; Mungai, G.M.; Muthaura, C.N.; Muli, J.M.; Keter, L.K.; et al. Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. J. Ethnopharmacol. 2006, 104, 92–99. [Google Scholar] [CrossRef]




| Name | Sequence |
|---|---|
| PRV-UL42-F | 5′-GACCGTCTTCAACGTCACCT-3′ |
| PRV-UL42-R | 5′-GCATGATGCAGTAGTCGTTG-3′ |
| Pig-GAPDH-F | 5′-CCTTCATTGACCTCCACTACA-3′ |
| Pig-GAPDH-R | 5′-GATGGCCTTTCCATTGATGAC-3′ |
| HSV-UL42-F | 5′-GACACGGCCCTAAAGAAACC-3′ |
| HSV-UL42-R | 5′-GGAGGTCGCGAAAGTAACAC-3′ |
| Monkey-GAPDH-F | 5′-GCCTCAAGATCGTCAGCAAC-3′ |
| Monkey-GAPDH-R | 5′-GGTCATGAGTCCTTCCACGA-3′ |
| MDV-UL42-F | 5′- AGCGCATCCATCATTTGTCC-3′ |
| MDV-UL42-R | 5′- ACATCACAAATCGTTCCGGC-3′ |
| Chicken-GAPDH-F | 5′-CTGTTGTTGACCTGACCTGC-3′ |
| Chicken-GAPDH-R | 5′-TCAAAGGTGGAGGAATGGCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, Z.; Zhu, J.; Li, X.; Zhang, C.; Guo, M.; Cao, Y.; Zhang, X.; Wu, Y. Identification of Na+/K+-ATPase Inhibitor Bufalin as a Novel Pseudorabies Virus Infection Inhibitor In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 14479. https://doi.org/10.3390/ijms241914479
Bo Z, Zhu J, Li X, Zhang C, Guo M, Cao Y, Zhang X, Wu Y. Identification of Na+/K+-ATPase Inhibitor Bufalin as a Novel Pseudorabies Virus Infection Inhibitor In Vitro and In Vivo. International Journal of Molecular Sciences. 2023; 24(19):14479. https://doi.org/10.3390/ijms241914479
Chicago/Turabian StyleBo, Zongyi, Jinjin Zhu, Xiaojuan Li, Chengcheng Zhang, Mengjiao Guo, Yongzhong Cao, Xiaorong Zhang, and Yantao Wu. 2023. "Identification of Na+/K+-ATPase Inhibitor Bufalin as a Novel Pseudorabies Virus Infection Inhibitor In Vitro and In Vivo" International Journal of Molecular Sciences 24, no. 19: 14479. https://doi.org/10.3390/ijms241914479
APA StyleBo, Z., Zhu, J., Li, X., Zhang, C., Guo, M., Cao, Y., Zhang, X., & Wu, Y. (2023). Identification of Na+/K+-ATPase Inhibitor Bufalin as a Novel Pseudorabies Virus Infection Inhibitor In Vitro and In Vivo. International Journal of Molecular Sciences, 24(19), 14479. https://doi.org/10.3390/ijms241914479






