A Study on the Effect of Energy on the Development of Silkworm Embryos Using an Estrogen-Related Receptor
Abstract
:1. Introduction
2. Results
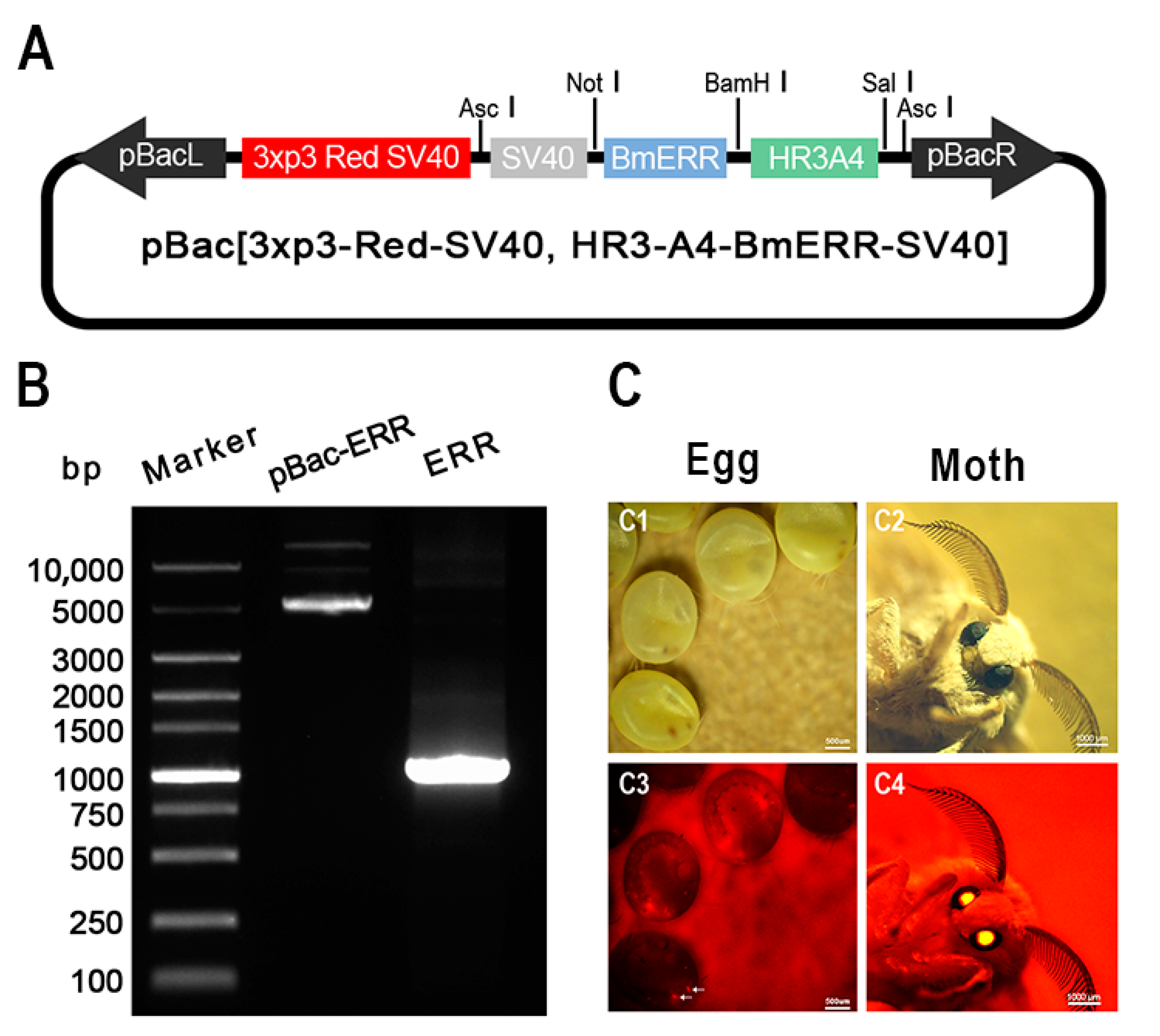
2.1. Overexpression of BmERRs in the Eggs of Transgenic Silkworms
2.2. Impact of Incremental BmERR Expression on Silkworm Eggs
2.3. The Impact of the Increased Expression of B. mori Estrogen-Related Receptors (BmERRs) on Embryonic Energy Metabolism
2.4. Impact on Energy Metabolism and Embryonic Development in Silkworms
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Construction of Transgenic Silkworms
4.3. RNA Extraction and cDNA Synthesis (Primer Sequences and Amplification Conditions)
4.4. Protein Extraction and Immunoblotting
4.5. Phenotypic Statistics
4.6. Newly Hatched Silkworm Activity Test
4.7. Detection of Glucose and ATP Levels
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scholtes, C.; Giguere, V. Transcriptional control of energy metabolism by nuclear receptors. Nat. Rev. Mol. Cell Biol. 2022, 23, 750–770. [Google Scholar] [CrossRef] [PubMed]
- Horard, B.; Vanacker, J.M. Estrogen receptor-related receptors: Orphan receptors desperately seeking a ligand. J. Mol. Endocrinol. 2003, 31, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Giguere, V. Transcriptional Control of Energy Homeostasis by the Estrogen-Related Receptors. Endocr. Rev. 2008, 29, 677–696. [Google Scholar] [CrossRef]
- Hubbard, W.J.; Bland, K.I.; Chaudry, I.H. The ERRor of Our Ways: Estrogen-Related Receptors are about Energy, Not Hormones, and are Potential New Targets for Trauma and Shock. Shock 2015, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Eichner, L.J.; Giguere, V. Estrogen related receptors (ERRs): A new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion 2011, 11, 544–552. [Google Scholar] [CrossRef]
- Tripathi, M.; Yen, P.M.; Singh, B.K. Estrogen-Related Receptor Alpha: An Under-Appreciated Potential Target for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2020, 21, 1645. [Google Scholar] [CrossRef]
- Kobayashi, A.; Azuma, K.; Ikeda, K.; Inoue, S. Mechanisms Underlying the Regulation of Mitochondrial Respiratory Chain Complexes by Nuclear Steroid Receptors. Int. J. Mol. Sci. 2020, 21, 6683. [Google Scholar] [CrossRef]
- Tremblay, A.M.; Giguere, V. The NR3B subgroup: An ovERRview. Nucl. Recept. Signal. 2007, 5, e009. [Google Scholar] [CrossRef]
- Luo, J.M.; Sladek, R.; Carrier, J.; Bader, J.A.; Richard, D.; Giguere, V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol. Cell. Biol. 2003, 23, 7947–7956. [Google Scholar] [CrossRef]
- Yoshihara, E.; Wei, Z.; Lin, C.S.; Fang, S.; Ahmadian, M.; Kida, Y.; Tseng, T.; Dai, Y.; Yu, R.T.; Liddle, C.; et al. ERR gamma Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive beta Cells. Cell Metab. 2016, 23, 622–634. [Google Scholar] [CrossRef]
- Lu, D.S.; Kiriyama, Y.; Lee, K.Y.; Giguere, V. Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Res. 2001, 61, 6755–6761. [Google Scholar] [PubMed]
- Maglich, J.M.; Sluder, A.; Guan, X.J.; Shi, Y.L.; Mckee, D.D.; Carrick, K.; Kamdar, K.; Willson, T.M.; Moore, J.T. Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol. 2001, 2, research0029.1. [Google Scholar] [CrossRef] [PubMed]
- Bardet, P.L.; Laudet, V.; Vanacker, J.M. Studying non-mammalian models? Not a fool’s ERRand! Trends Endocrinol. Metab. 2006, 17, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bozzolan, F.; Durand, N.; Demondion, E.; Bourgeois, T.; Gassias, E.; Debernard, S. Evidence for a role of oestrogen receptor-related receptor in the regulation of male sexual behaviour in the moth Agrotis ipsilon. Insect Mol. Biol. 2017, 26, 403–413. [Google Scholar] [CrossRef]
- Mazina, M.Y.; Kocheryzhkina, E.V.; Nikolenko, J.V.; Krasnov, A.N.; Georgieva, S.G.; Vorobyeva, N.E. Nuclear receptors EcR, Usp, E75, DHR3, and ERR regulate transcription of ecdysone cascade genes. Dokl. Biochem. Biophys. 2017, 473, 145–147. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I.S. Molecular effects of endocrine-disrupting chemicals on the Chironomus riparius estrogen-related receptor gene. Chemosphere 2010, 79, 934–941. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xi, G.S.; Zhao, J. Vitellogenin regulates estrogen-related receptor expression by crosstalk with the JH and IIS-TOR signaling pathway in Polyrhachis vicina Roger (Hymenoptera, Formicidae). Gen. Comp. Endocrinol. 2021, 310, 113836. [Google Scholar] [CrossRef]
- Jiang, X.J.; Zheng, S.W.; Bamu, A.; Dai, H.; Lin, X.D. Nilaparvata lugens ERR2 regulates moulting and ovary development is related to hormone signalling. Insect Mol. Biol. 2023, 32, 376–386. [Google Scholar] [CrossRef]
- Park, W.R.; Lim, D.J.; Sang, H.; Kim, E.; Moon, J.H.; Choi, H.S.; Kim, I.S.; Kim, D.K. Aphid estrogen-related receptor controls glycolytic gene expression and fecundity. Insect Biochem. Mol. 2021, 130, 103529. [Google Scholar] [CrossRef]
- Beebe, K.; Robins, M.M.; Hernandez, E.J.; Lam, G.; Horner, M.A.; Thummel, C.S. Drosophila estrogen-related receptor directs a transcriptional switch that supports adult glycolysis and lipogenesis. Gene Dev. 2020, 34, 701–714. [Google Scholar] [CrossRef]
- Kovalenko, E.V.; Mazina, M.Y.; Krasnov, A.N.; Vorobyeva, N.E. The Drosophila nuclear receptors EcR and ERR jointly regulate the expression of genes involved in carbohydrate metabolism. Insect Biochem. Mol. 2019, 112, 103184. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.M.; Baker, K.D.; Lam, G.; Evans, J.; Thummel, C.S. The Drosophila Estrogen-Related Receptor Directs a Metabolic Switch that Supports Developmental Growth. Cell Metab. 2011, 13, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.M.; Baker, K.D.; Lam, G.; Evans, J.; Thummel, C.S. The Drosophila Estrogen-Related Receptor Coordinates Carbohydrate Metabolism with Developmental Growth. Faseb J. 2011, 25, 917.7. [Google Scholar] [CrossRef]
- Li, Y.; Padmanabha, D.; Gentile, L.B.; Dumur, C.I.; Beckstead, R.B.; Baker, K.D. HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in Drosophila melanogaster. PLoS Genet. 2013, 9, e1003230. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, A.K.; Gupta, S.; Kumar, A.; Khanna, P.; Shankar, J.; Ram, K.R. Estrogen related receptor is required for the testicular development and for the normal sperm axoneme/mitochondrial derivatives in Drosophila males. Sci. Rep.-UK 2017, 7, 40372. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.W.; Wu, J.X.; Lin, Y.; Hua, X.T.; Xia, Q.Y.; Zhao, P. Estrogen-Related Receptor Influences the Hemolymph Glucose Content by Regulating Midgut Trehalase Gene Expression in the Last Instar Larvae of Bombyx mori. Int. J. Mol. Sci. 2021, 22, 4343. [Google Scholar] [CrossRef] [PubMed]
- Takesue, S.; Keino, H.; Endo, K. Studies on the yolk granules of the silkworm, Bombyx mori L.: The morphology of diapause and non-diapause eggs during early developmental stages. Wilehm Roux Arch. Dev. Biol. 1976, 180, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ponnuvel, K.M.; Murthy, G.N.; Awasthi, A.K.; Rao, G.; Vijayaprakash, N.B. Differential gene expression during early embryonic development in diapause and non-diapause eggs of multivoltine silkworm Bombyx mori. Indian J. Exp. Biol. 2010, 48, 1143–1151. [Google Scholar]
- Chino, H. Carbohydrate Metabolism in the Diapause Egg of the Silkworm, Bombyx-Mori—2. Conversion of Glycogen into Sorbitol and Glycerol during Diapause. J. Insect Physiol. 1958, 2, 1–4. [Google Scholar] [CrossRef]
- Mangé, A.; Couble, P.; Prudhomme, J. Two alternative promoters drive the expression of the cytoplasmic actin A4 gene of Bombyx mori. Gene 1996, 183, 191–199. [Google Scholar] [CrossRef]
- Lu, M.; Farrell, P.J.; Johnson, R.; Iatrou, K. A Baculovirus (Bombyx mori Nuclear Polyhedrosis Virus) Repeat Element Functions as a Powerful Constitutive Enhancer in Transfected Insect Cells. J. Biol. Chem. 1997, 272, 30724–30728. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yao, B.; Zhu, Z.; Yi, Y.; Lin, X.a.; Zhang, Z.; Shen, G. A constitutive super-enhancer: Homologous region 3 of Bombyx mori nucleopolyhedrovirus. Biochem. Biophys. Res. Commun. 2004, 318, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tingcai, C.; Ping, Z.; Qiong, Y.; Genhong, W.; Shengkai, J.; Ping, L.; Yang, X.; Qingyou, X.; Adam, Y.Y. Resistance to BmNPV via Overexpression of an Exogenous Gene Controlled by an Inducible Promoter and Enhancer in Transgenic Silkworm, Bombyx mori. PLoS ONE 2012, 7, e41838. [Google Scholar]
- Jiang, L.; Zhao, P.; Wang, G.; Cheng, T.; Yang, Q.; Jin, S.; Lin, P.; Xiao, Y.; Sun, Q.; Xia, Q. Comparison of factors that may affect the inhibitory efficacy of transgenic RNAi targeting of baculoviral genes in silkworm, Bombyx mori. Antivir. Res. 2013, 97, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. 1998, 28, 277–300. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef]
- Sasibhushan, S.; Rao, C.G.P.; Ponnuvel, K.M. Genome wide microarray based expression profiles during early embryogenesis in diapause induced and non-diapause eggs of polyvoltine silkworm Bombyx mori. Genomics 2013, 102, 379–387. [Google Scholar] [CrossRef]
- Chino, H. Insect Diapause and Its Unique Energy-Metabolism—A Citation Classic Commentary on Carbohydrate-Metabolism in the Diapause Egg of the Silkworm, Bombyx-mori—2. Conversion of Glycogen into Sorbitol and Glycerol during Diapause by Chino, H. Cc/Agric. Biol. Environ. 1989, 14. Available online: http://www.garfield.library.upenn.edu/classics1989/A1989U091800001.pdf (accessed on 18 September 2023).
- Shen, G.; Wu, J.; Han, C.; Liu, H.; Xu, Y.; Zhang, H.; Lin, Y.; Xia, Q. Oestrogen-related receptor reduces vitellogenin expression by crosstalk with the ecdysone receptor pathway in female silkworm, Bombyx mori. Insect Mol. Biol. 2018, 27, 454–463. [Google Scholar] [CrossRef]
- Nakagaki, M.; Takei, R.; Nagashima, E.; Yaginuma, T. Cell-Cycles in Embryos of the Silkworm, Bombyx-Mori–G2-Arrest at Diapause Stage. Roux Arch. Dev. Biol. 1991, 200, 223–229. [Google Scholar] [CrossRef]
- Azuma, M.; Yamashita, O. Immunohistochemical and Biochemical Localization of Trehalase in the Developing Ovaries of the Silkworm, Bombyx-Mori. Insect Biochem. 1985, 15, 589–596. [Google Scholar] [CrossRef]
- Lin, X.W.; Xu, W.H. Hexokinase is a key regulator of energy metabolism and ROS activity in insect lifespan extension. Aging 2016, 8, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Wu, J.; Shen, G.; Zhang, H.; Liu, H.; Xu, Y.; Gu, J.; Jia, L.; Lin, Y.; Xia, Q. Estrogen-related receptor participates in regulating glycolysis and influences embryonic development in silkworm Bombyx mori. Insect Mol. Biol. 2020, 29, 160–169. [Google Scholar] [CrossRef]
- Hasegawa, K. Diapause Hormone of the Silkworm, Bombyx-Mori. Nature 1957, 179, 1300–1301. [Google Scholar] [CrossRef]
- Imai, K.; Konno, T.; Nakazawa, Y.; Komiya, T.; Isobe, M.; Koga, K.; Goto, T.; Yaginuma, T.; Sakakibara, K.; Hasegawa, K.; et al. Isolation and Structure of Diapause Hormone of the Silkworm, Bombyx-Mori. Proc. Jpn. Acad. B-Phys. 1991, 67, 98–101. [Google Scholar] [CrossRef]
- Sun, J.S.; Chen, F.S.; Xu, W.H. Localization, expression, and secretion pathway of diapause hormone in embryo and larva of the silkworm, Bombyx mori. Chin. Sci. Bull. 2004, 49, 1386–1391. [Google Scholar] [CrossRef]
- Yamashita, O. Diapause hormone of the silkworm, Bombyx mori: Structure, gene expression and function. J. Insect Physiol. 1996, 42, 669–679. [Google Scholar] [CrossRef]
- Kurata, S.; Koga, K.; Sakaguchi, B. Rna-Content and Rna-Synthesis in Diapause and Non-Diapause Eggs of Bombyx-Mori. Insect Biochem. 1979, 9, 107–109. [Google Scholar] [CrossRef]
- Miura, K.; Shimizu, I. Changes in Lipid Components in Diapause, Non-Diapause and Hcl-Treated Eggs of the Silkworm, Bombyx-Mori. Zool. Sci. 1984, 1, 908. [Google Scholar]
- Fan, L.F.; Lin, J.R.; Zhong, Y.S.; Liu, J.Y. Shotgun Proteomic Analysis on the Diapause and Non-Diapause Eggs of Domesticated Silkworm Bombyx mori. PLoS ONE 2013, 8, e60386. [Google Scholar] [CrossRef]
- Takeda, S.; Kono, Y.; Kameda, Y. Induction of Non-Diapause Eggs in Bombyx-Mori by a Trehalase Inhibitor. Entomol. Exp. Appl. 1988, 46, 291–294. [Google Scholar] [CrossRef]
- Takahashi, M.; Niimi, T.; Ichimura, H.; Sasaki, T.; Yamashita, O.; Yaginuma, T. Cloning of a B-type cyclin homolog from Bombyx mori and the profiles of its mRNA level in non-diapause and diapause eggs. Dev. Genes Evol. 1996, 206, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, R.; Ponnuvel, K.M.; Qadri, S.M.H. Expression of metabolic enzyme genes and heat-shock protein genes during embryonic development in diapause and non-diapause egg of multivoltine silkworm Bombyx mori. Biologia 2008, 63, 737–744. [Google Scholar] [CrossRef]
- Sawada, H.; Yamahama, Y.; Yamamoto, T.; Togawa, T.; Mase, K. Developmental Changes in the Localization of Protein Kinase CK2 in Non-Diapause and Diapause Eggs of the Silkworm, Bombyx mori. Zool. Sci. 2012, 29, 6–10. [Google Scholar] [CrossRef]
- Morohoshi, S. Hormonal Studies on the Diapause and Non-Diapause Eggs of the Silkworm, Bombyx-Mori L. J. Insect Physiol. 1959, 3, 28–40. [Google Scholar] [CrossRef]
- Miyadai, T.; Yamashita, O. Diapause Hormone Action in the Silkworm, Bombyx-Mori L. (Lepidoptera, Bombycidae)–Enhancement of Trehalase Activity in Developing Ovaries Incubated Invitro. Appl. Entomol. Zool. 1980, 15, 439–446. [Google Scholar] [CrossRef]
- Su, Z.H.; Ikeda, M.; Sato, Y.; Saito, H.; Imai, K.; Isobe, M.; Yamashita, O. Molecular Characterization of Ovary Trehalase of the Silkworm, Bombyx-Mori and Its Transcriptional Activation by Diapause Hormone. BBA-Gene Struct. Exp. 1994, 1218, 366–374. [Google Scholar] [CrossRef]
- Kamei, Y.; Hasegawa, Y.; Niimi, T.; Yamashita, O.; Yaginuma, T. trehalase-2 protein contributes to trehalase activity enhanced by diapause hormone in developing ovaries of the silkworm, Bombyx mori. J. Insect Physiol. 2011, 57, 608–613. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, G.; Liu, D.; Xu, H.; Wu, J.; Hou, L.; Yang, C.; Xia, Q.; Lin, P. A Study on the Effect of Energy on the Development of Silkworm Embryos Using an Estrogen-Related Receptor. Int. J. Mol. Sci. 2023, 24, 14485. https://doi.org/10.3390/ijms241914485
Shen G, Liu D, Xu H, Wu J, Hou L, Yang C, Xia Q, Lin P. A Study on the Effect of Energy on the Development of Silkworm Embryos Using an Estrogen-Related Receptor. International Journal of Molecular Sciences. 2023; 24(19):14485. https://doi.org/10.3390/ijms241914485
Chicago/Turabian StyleShen, Guanwang, Die Liu, Haoran Xu, Jinxin Wu, Luyu Hou, Chunyan Yang, Qingyou Xia, and Ping Lin. 2023. "A Study on the Effect of Energy on the Development of Silkworm Embryos Using an Estrogen-Related Receptor" International Journal of Molecular Sciences 24, no. 19: 14485. https://doi.org/10.3390/ijms241914485





