OsBTBZ1 Confers Salt Stress Tolerance in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
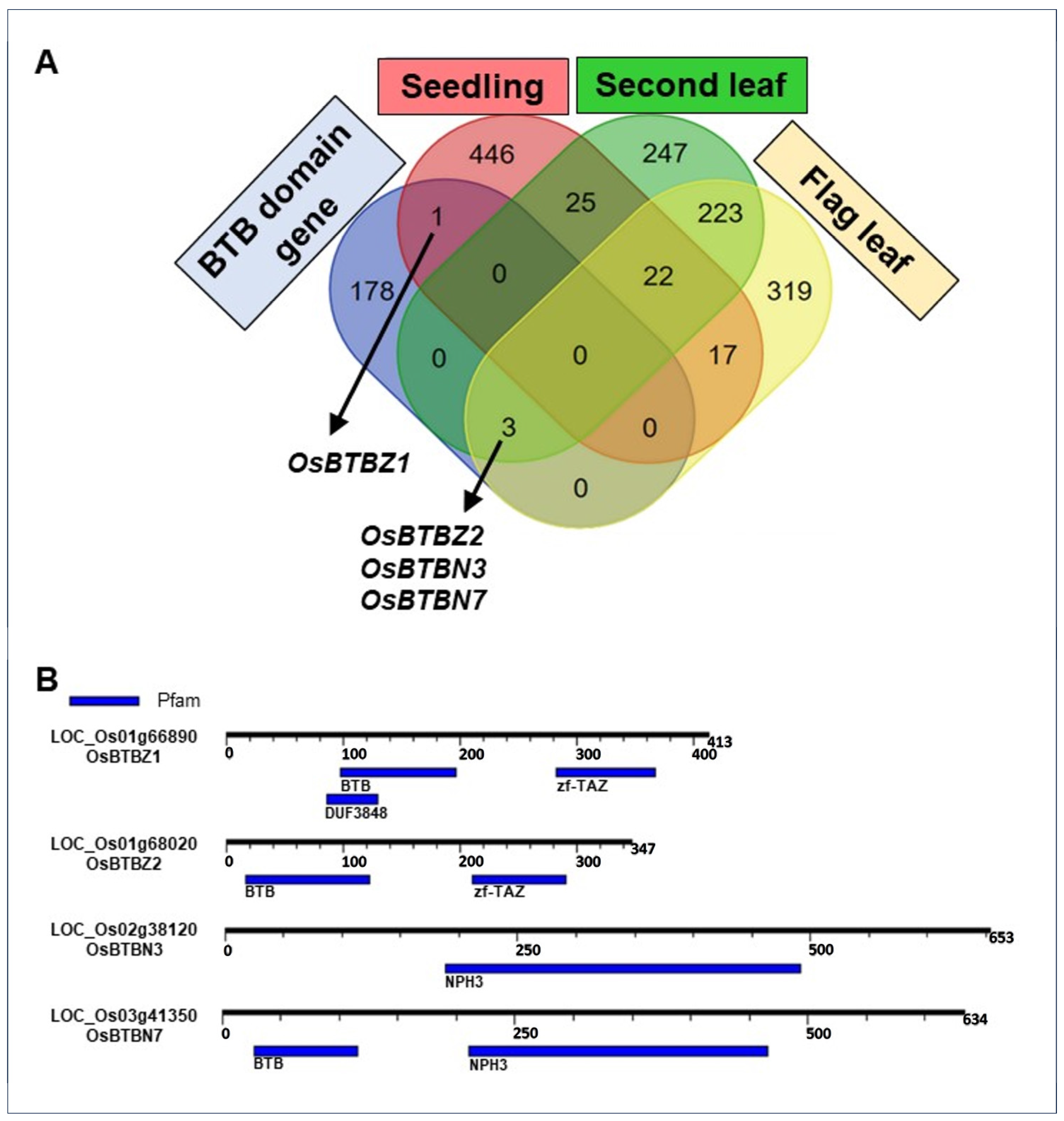
2.1. Only Four BTB Genes Were Expressed in CSSL16 under Salt Stress
2.2. OsBTBZ1, OsBTBZ2, OsBTBN3, and OsBTBN7 Promoters Contain Multiple Cis-Elements Related to the Water Stress Response
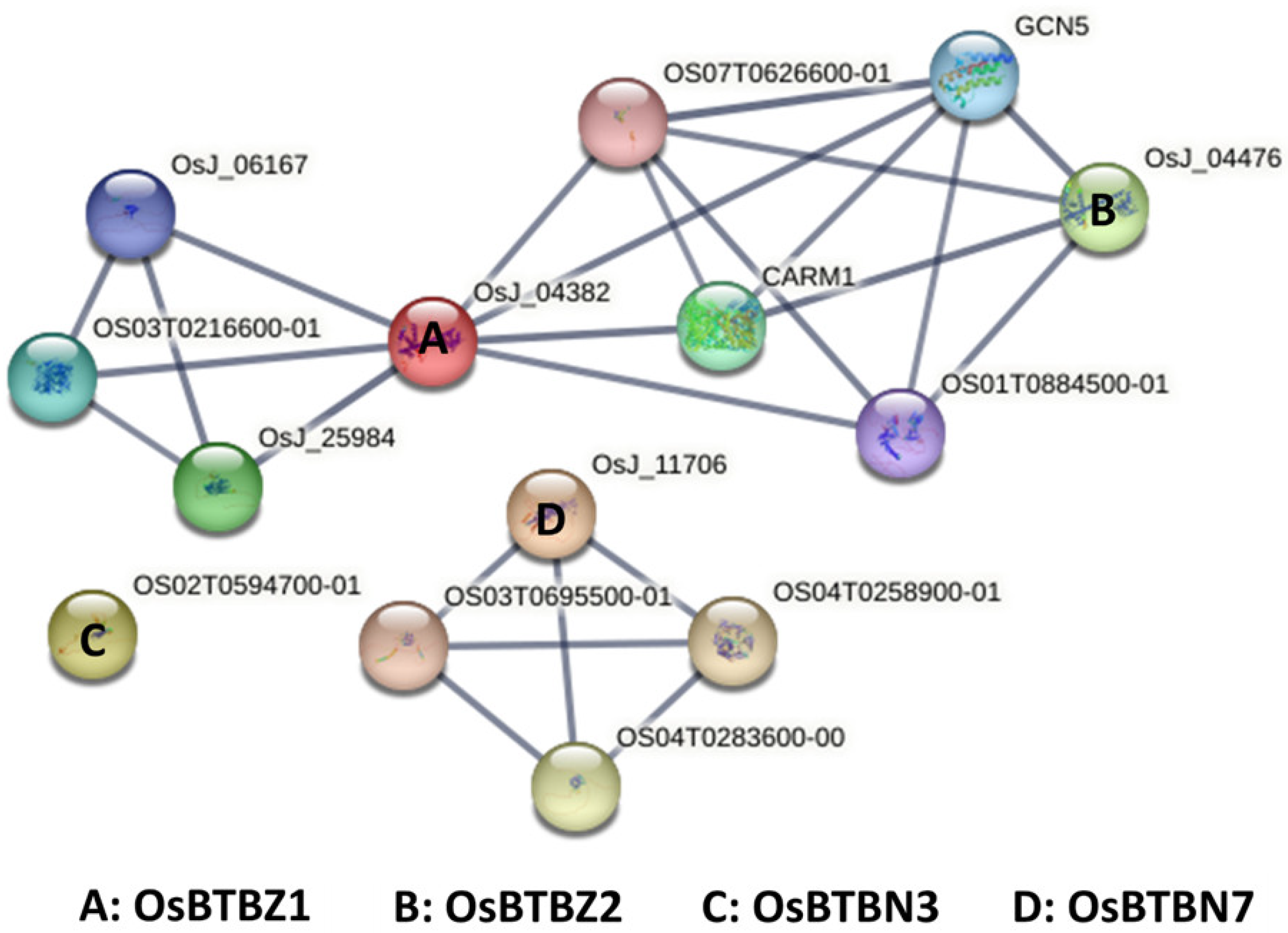
2.3. OsBTBZ1 and OsBTBZ2 Are in the Same Protein-Protein Interaction (PPI) Network
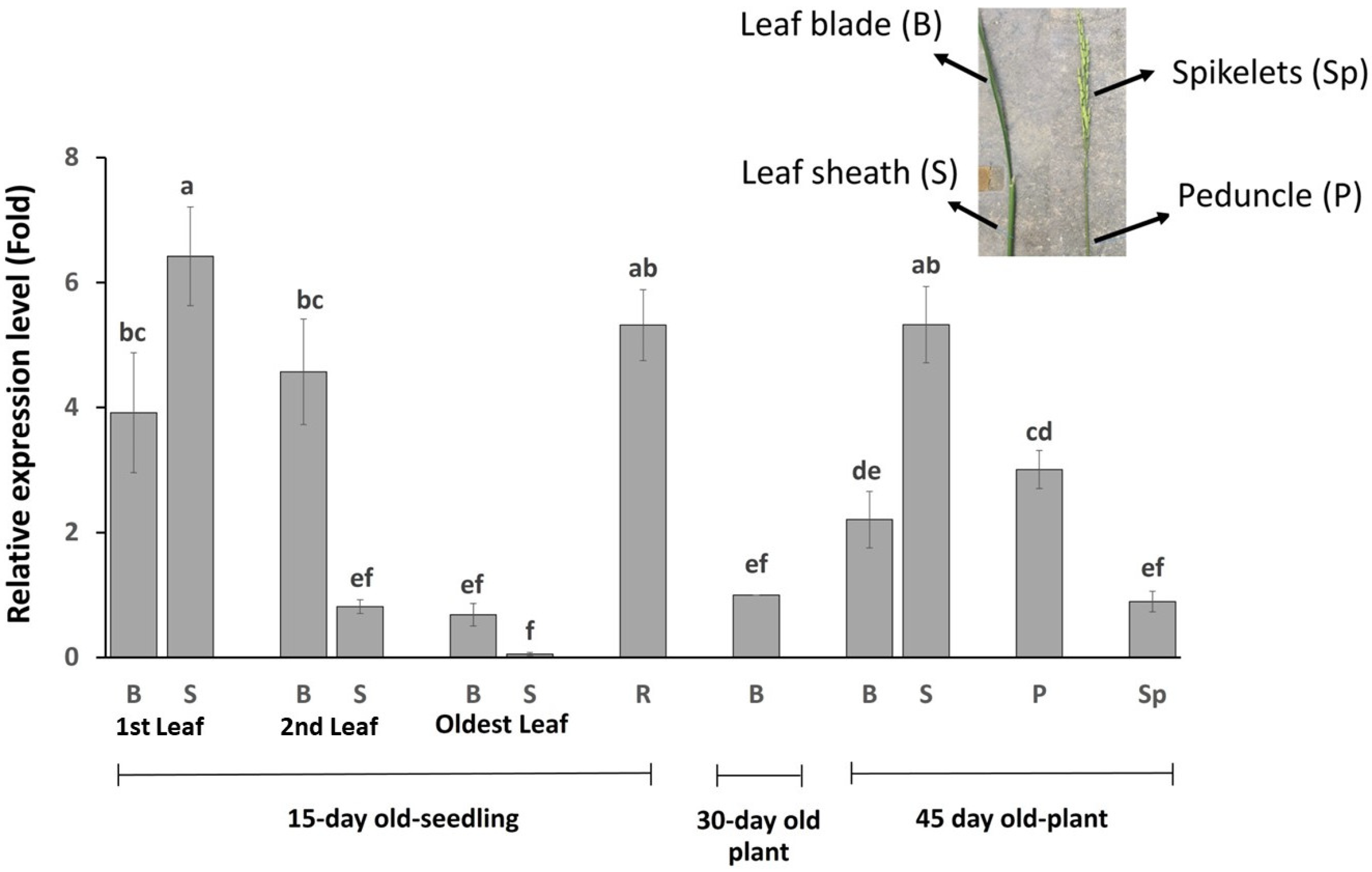
2.4. OsBTBZ1 Is Expressed in All Plant Tissues, Especially in Younger Leaf Sheaths
2.5. OsBTBZ1 Is Localized in the Nucleus, Suggesting the Role of the Transcription Factor
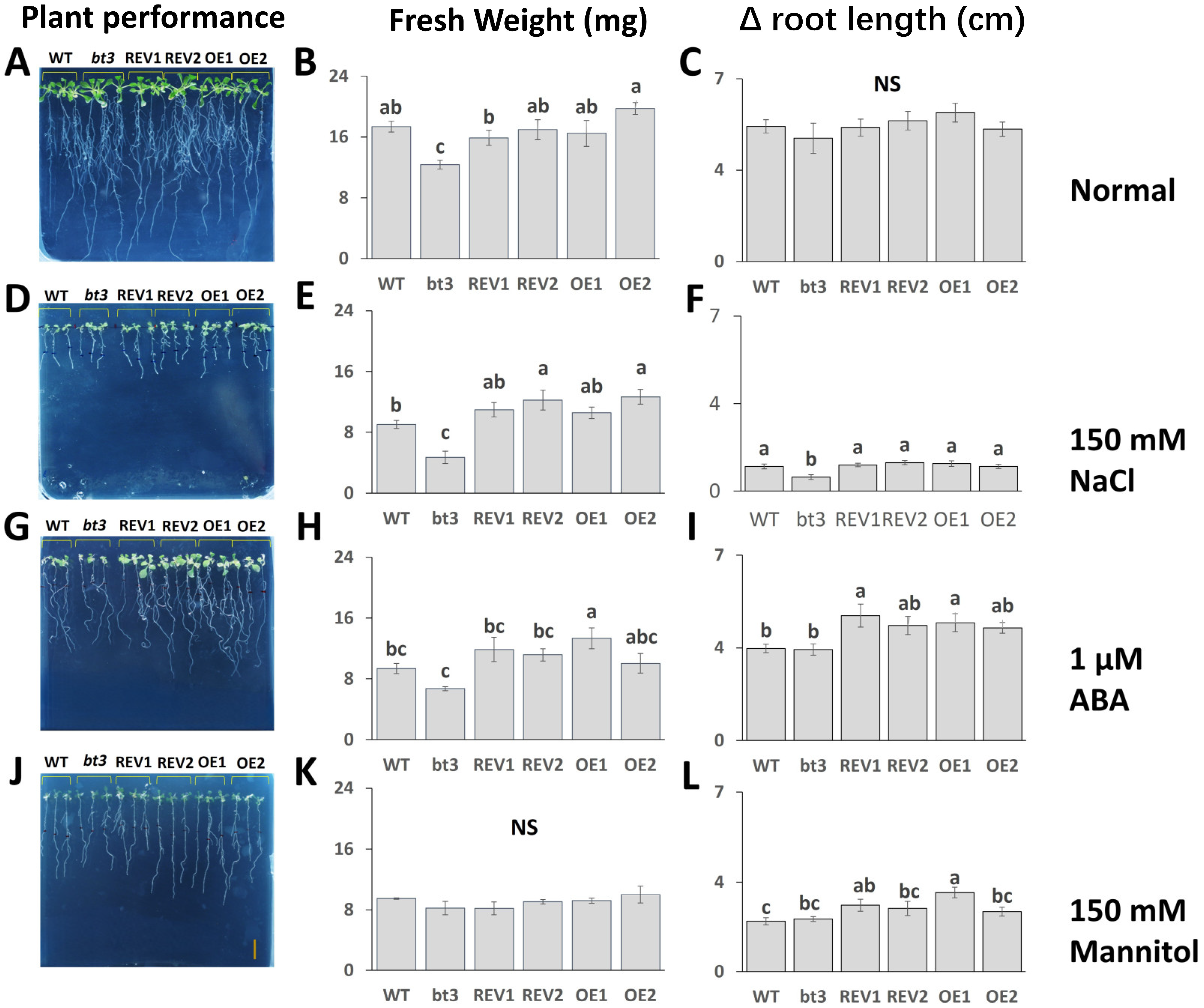
2.6. Ectopic Expression of OsBTBZ1 Could Revert the NaCl and ABA Susceptibility of the Atbt3 Arabidopsis Mutant at the Germination Stage
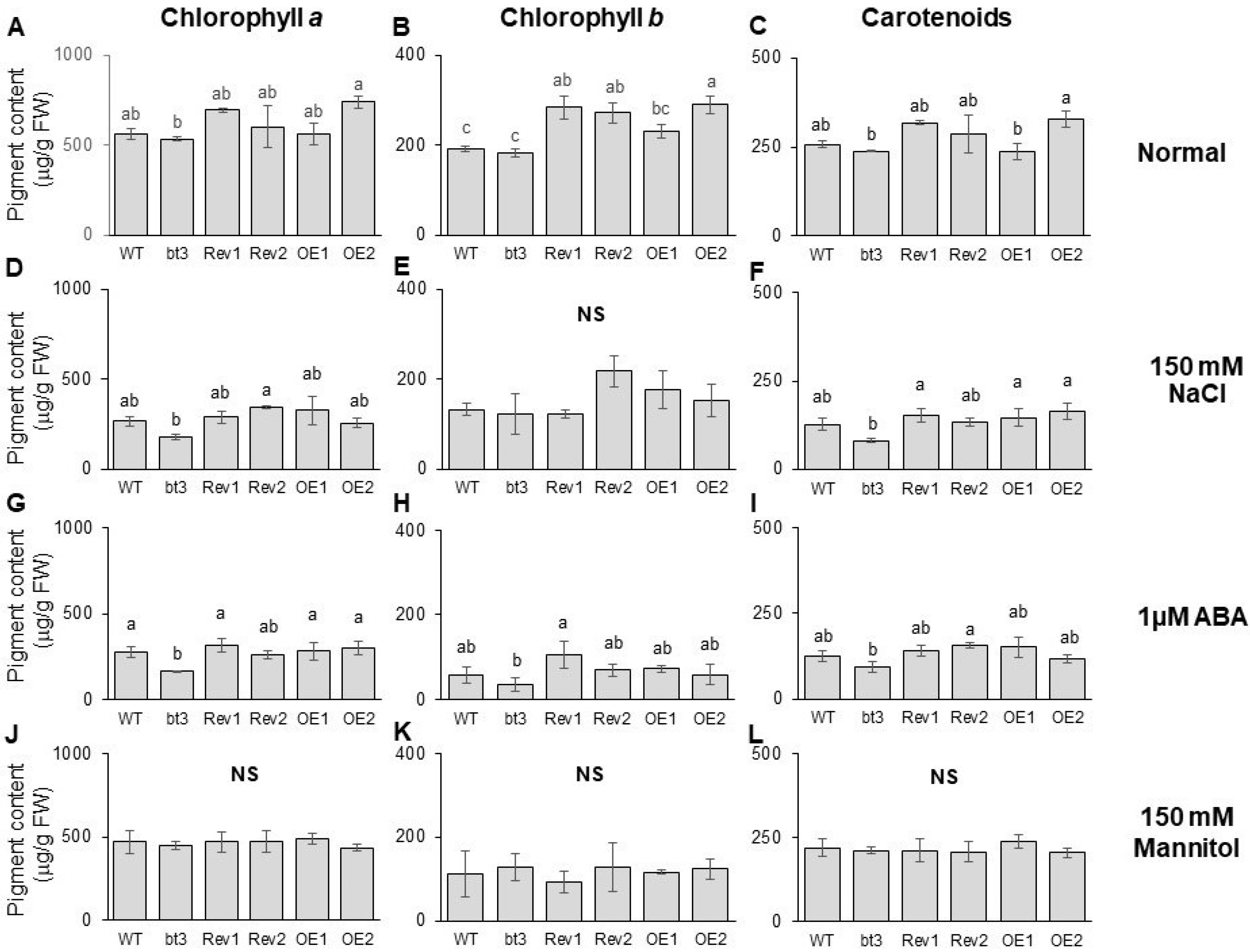
2.7. OsBTBZ1 Enhanced the Salt and ABA Tolerance in Transgenic Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Transcriptome Analysis
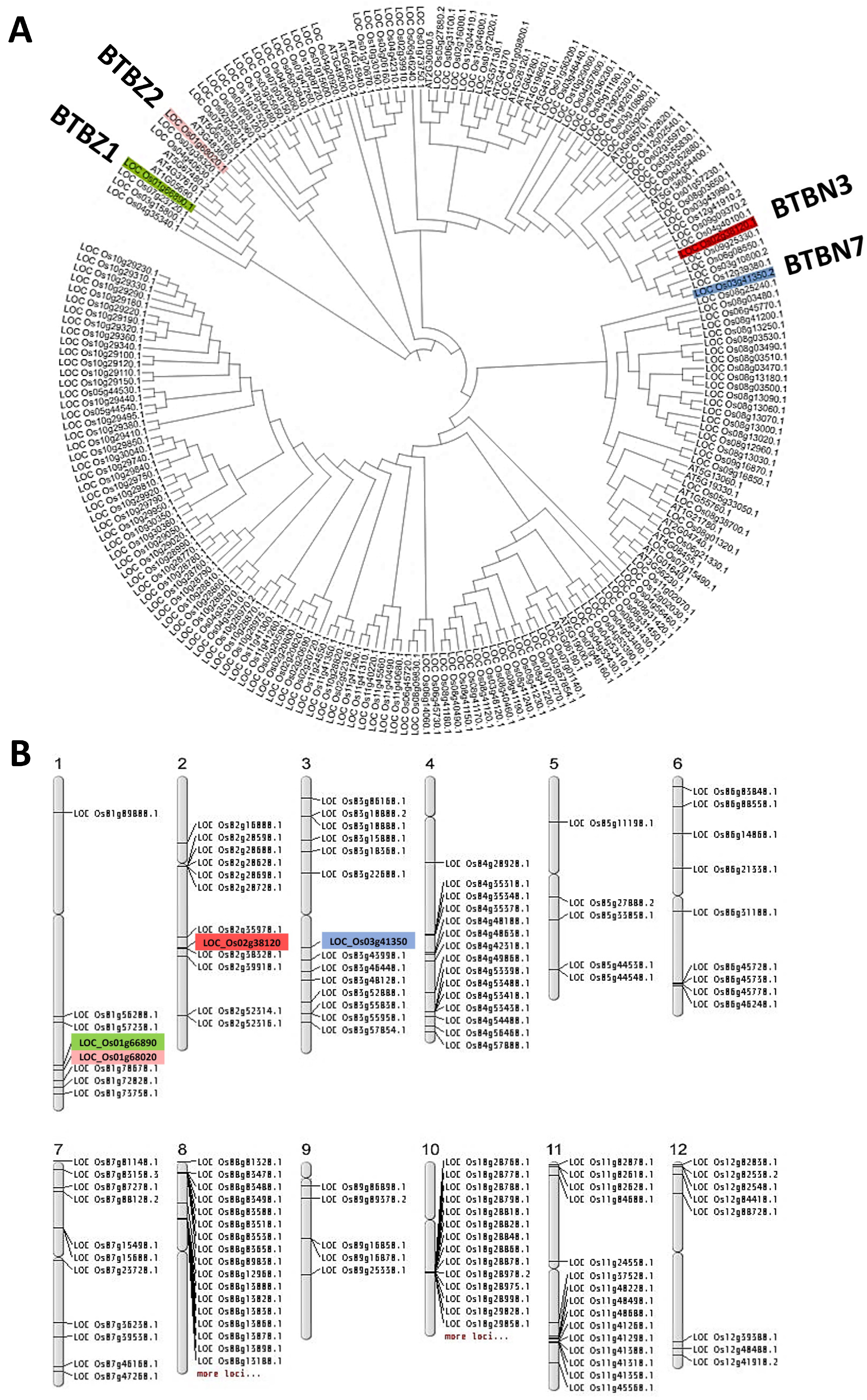
4.2. Phylogenetic Analysis and an In Silico Analysis of BTB Proteins in Oryza sativa
4.3. Putative Promoter Analysis
4.4. Protein-Protein Interaction (PPI) Based on the STRING Database
4.5. Detection of OsBTBZ1 Gene Expression
4.6. Generation of Complementation and Over-Expression of Arabidopsis Lines with the OsBTBZ1 Gene
4.7. Transformation of A. thaliana
4.8. Subcellular Localization in Onion Inner Epidermal Cells
4.9. Evaluation of the Effect of OsBTBZ1 Gene Expression in Transgenic Arabidopsis Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flower, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Flowers, T.J. Effects of salinity on seed set in rice. Plant Cell Environ. 1995, 18, 61–67. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C. Effects of salinity on grain yield and yield components of rice at different seeding densities. Agron. J. 2000, 92, 418–423. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment; Mitigation; and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef]
- Ammar, M.H.M.; Singh, R.K.; Singh, A.K.; Mohapatra, T.; Sharma, T.R.; Singh, N.K. Mapping QTLs for salinity tolerance at seedling stage in rice (Oryza sativa L.). In African Crop Science Conference Proceedings; African Crop Science Society: Kampala, Uganda, 2007; pp. 617–620. [Google Scholar]
- Bimpong, I.K.; Manneh, B.; Diop, B.; Ghislain, K.; Sow, A.; Amoah, N.K.A.; Gregorio, G.; Singh, R.K.; Ortiz, R.; Wopereis, M. New quantitative trait loci for enhancing adaptation to salinity in rice from Hasawi, a Saudi landrace into three african cultivars at the reproductive stage. Euphytica 2014, 200, 45–60. [Google Scholar] [CrossRef]
- Ghomi, K.; Rabiei, B.; Sabouri, H.; Sabouri, A. Mapping QTLs for traits related to salinity tolerance at seedling stage of rice (Oryza sativa L.): An agrigenomics study of an Iranian rice population. OMICS 2013, 17, 242–251. [Google Scholar] [CrossRef]
- Hossain, H.; Rahman, M.A.; Alam, M.S.; Singh, R.K. Mapping of quantitative trait loci associated with reproductive-stage salt tolerance in rice. J. Agron. Crop. Sci. 2015, 201, 17–31. [Google Scholar] [CrossRef]
- Koyama, M.L.; Levesley, A.; Koebner, R.M.; Flowers, T.J.; Yeo, A.R. Quantitative trait loci for component physiological traits determining salt tolerance in rice. Plant Physiol. 2001, 125, 406–422. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ahn, J.H.; Cha, Y.S.; Yun, D.W.; Lee, M.C.; Ko, J.C.; Lee, K.S.; Eun, M.Y. Mapping QTLs related to salinity tolerance of rice at the young seedling stage. Plant Breed. 2007, 126, 43–46. [Google Scholar] [CrossRef]
- Lin, H.X.; Zhu, M.Z.; Yano, M.; Gao, J.P.; Liang, Z.W.; Su, W.A.; Hu, X.H.; Ren, Z.H.; Chao, D.Y. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor. Appl. Genet. 2004, 108, 253–260. [Google Scholar] [CrossRef]
- Yao, M.; Wang, J.; Chen, H.; Zhang, H. Inheritance and QTL mapping of salt tolerance in rice. Rice Sci. 2005, 12, 25–32. [Google Scholar]
- Mohammadi, R.; Mendioro, M.S.; Diaz, G.Q.; Gregorio, G.B.; Singh, R.K. Mapping quantitative trait loci associated with yield and yield components under reproductive stage salinity stress in rice (Oryza sativa L.). J. Genet. 2013, 92, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.R.; Bagali, P.G.; Hittalmani, S.; Shashidhar, H.E. Molecular mapping of quantitative trait loci associated with seedling tolerance to salt stress in rice (Oryza sativa L.). Curr. Sci. 2000, 78, 162–164. [Google Scholar]
- Qiu, X.; Yuan, Z.; Liu, H.; Xiang, X.; Yang, L.; He, W.; Du, B.; Ye, G.; Xu, J.; Xing, D. Identification of salt tolerance-improving quantitative trait loci alleles from a salt-susceptible rice breeding line by introgression breeding. Plant Breed. 2015, 134, 653–660. [Google Scholar] [CrossRef]
- Ullah, M.A.; Abdullah-zawawi, M.-R.; Zainal-abidin, R.-A.; Sukiran, N.L.; Uddin, M.I.; Zainal, Z. A Review of Integrative Omic Approaches for Understanding Rice Salt Response Mechanisms. Plants 2022, 11, 1430. [Google Scholar] [CrossRef]
- Kojonna, T.; Suttiyut, T.; Khunpolwattana, N.; Pongpanich, M.; Suriya-arunroj, D.; Comai, L.; Buaboocha, T.; Chadchawan, S. Identification of a Negative Regulator for Salt Tolerance at Seedling Stage via a Genome-Wide Association Study of Thai Rice Populations. Int. J. Mol. Sci. 2022, 23, 1842. [Google Scholar] [CrossRef]
- Punchkhon, C.; Chutimanukul, P.; Chokwiwatkul, R.; Saputro, T.B.; Grennan, A.K.; Diego, N.D.; Spíchal, L.; Chadchawan, S. Role of LOC_Os01g68450, Containing DUF2358, in Salt Tolerance Is Mediated via Adaptation of Absorbed Light Energy Dissipation. Plants 2022, 11, 1233. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, W.; Tong, W.; He, Q.; Yoon, M.Y.; Li, F.P.; Choi, B.; Heo, E.B.; Kim, K.W.; Park, Y.J. A genome-wide association study reveals candidate genes related to salt tolerance in rice (Oryza sativa) at the germination stage. Int. J. Mol. Sci. 2018, 19, 3145. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, H.; Wen, H.; Qu, D.; Cui, J.; Li, C.; Wang, J.; Liu, H.; Yang, L.; Jia, Y.; et al. Identification of Candidate Genes for Salt Tolerance at the Germination Stage in Japonica Rice by Genome-Wide Association Analysis. Agriculture 2022, 12, 1588. [Google Scholar] [CrossRef]
- Batayeva, D.; Labaco, B.; Ye, C.; Li, X.; Usenbekov, B.; Rysbekova, A.; Dyuskalieva, G.; Vergara, G.; Reinke, R.; Leung, H. Genome-wide association study of seedling stage salinity tolerance in temperate japonica rice germplasm. BMC Genet. 2018, 19, 2. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, S.-M. Identification of Candidate Genes for Salt Tolerance at the Seedling Stage Using Integrated Genome-Wide Association Study and Transcriptome Analysis in Rice. Plants 2023, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cui, J.; Cao, H.; Liang, S.; Ma, T.; Liu, H.; Wang, J.; Yang, L.; Xin, W.; Jia, Y.; et al. Identification of candidate genes for salinity tolerance in Japonica rice at the seedling stage based on genome-wide association study and linkage mapping. Front. Plant Sci. 2023, 14, 1184416. [Google Scholar] [CrossRef] [PubMed]
- Nayyeripasand, L.; Garoosi, G.A.; Ahmadikhah, A. Genome-Wide Association Study (GWAS) to Identify Salt-Tolerance QTLs Carrying Novel Candidate Genes in Rice During Early Vegetative Stage. Rice (N. Y.) 2021, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Kumar, A.; Grover, N.; Ellur, R.K.; Bollinedi, H.; Krishnan, S.G.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Singh, A.K. Genome-wide association study reveals marker–trait associations for early vegetative stage salinity tolerance in rice. Plants 2021, 10, 559. [Google Scholar] [CrossRef]
- Warraich, A.S.; Krishnamurthy, S.L.; Sooch, B.S.; Vinaykumar, N.M.; Dushyanthkumar, B.M.; Bose, J.; Sharma, P.C. Rice GWAS reveals key genomic regions essential for salinity tolerance at reproductive stage. Acta Physiol. Plant 2020, 42, 134. [Google Scholar] [CrossRef]
- Lekklar, C.; Pongpanich, M.; Suriya-arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, N.; Li, W.; Gao, X.; Cha, M.; Qin, L.; Liu, L. Advances in transcriptomics in the response to stress in plants. Glob. Med. Genet. 2020, 7, 30–34. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, J.; Wei, H.; Xiao, F.; Wang, Y.; Jahan, N.; Hazman, M.; Qian, Q.; Shang, L.; Guo, L. Combining GWAS, Genome-Wide Domestication and a Transcriptomic Analysis Reveals the Loci and Natural Alleles of Salt Tolerance in Rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 912637. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Saputro, T.B.; Mahaprom, P.; Plaimas, K.; Comai, L.; Buaboocha, T.; Siangliw, M.; Toojinda, T.; Chadchawan, S. Combining genome and gene co-expression network analyses for the identification of genes potentially regulating salt tolerance in rice. Front. Plant Sci. 2021, 12, 704549. [Google Scholar] [CrossRef]
- Kanjoo, V.; Jearakongman, S.; Punyawaew, K.; Siangliw, J.L.; Siangliw, M.; Vanavichit, A.; Toojinda, T. Co-location of quantitative trait loci for drought and salinity tolerance in rice. Thai J. Genet. 2011, 4, 126–138. [Google Scholar] [CrossRef]
- Kanjoo, V.; Punyawaew, K.; Siangliw, J.L.; Jearakongman, S.; Vanavichit, A.; Toojinda, T. Evaluation of agronomic traits in chromosome segment substitution lines of KDML105 containing drought tolerance QTL under drought stress. Rice Sci. 2012, 19, 117–124. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, G.; Zhang, L.; Xu, J.; Hu, L.; Jiang, L.; Liu, S. Comprehensive genomic analysis and expression profiling of the BTB and TAZ (BT) genes in cucumber (Cucumis sativus L.). Czech J. Genet. Plant Breed 2020, 56, 15–23. [Google Scholar] [CrossRef]
- He, Y.-M.; Liu, K.-K.; Zhang, H.-X.; Cheng, G.-X.; Ali, M.; Ul Haq, S.; Wei, A.-M.; Gong, Z.-H. Contribution of CaBPM4; a BTB domain-containing gene; to the response of pepper to Phytophthora capsici infection and abiotic stresses. Agronomy 2019, 9, 417. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, H.; He, S.; Zhu, H.; Gao, S.; Xing, S.; Wei, Z.; Zhao, N.; Liu, Q. The sweet potato BTB-TAZ protein gene; IbBT4; enhances drought tolerance in transgenic Arabidopsis. Front. Plant Sci. 2020, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhu, W.; Hamilton, J.; Lin, H.; Campbell, M.; Childs, K.; Thibaud-Nissen, F.; Malek, R.L.; Lee, Y.; Zheng, L.; et al. The TIGR Rice Genome Annotation Resource: Improvements and new features. Nucleic Acids Res. 2007, 35, D883–D887. [Google Scholar] [CrossRef]
- Kang, H.; Fan, T.; Wu, J.; Zhu, Y.; Shen, W.H. Histone modification and chromatin remodeling in plant response to pathogens. Front. Plant Sci. 2022, 13, 986940. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.T.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P. The STRING database in 2021: Customizable protein-protein networks; and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Weber, H.; Hellmann, H. Arabidopsis thaliana BTB/POZ-MATH proteins interact with members of the ERF/AP2 transcription factor family. FEBS J. 2009, 276, 6624–6635. [Google Scholar] [CrossRef]
- Wan, X.; Peng, L.; Xiong, J.; Li, X.; Wang, J.; Li, X.; Yang, Y. AtSIBP1, a novel BTB domain-containing protein; positively regulates salt signaling in Arabidopsis thaliana. Plants 2019, 8, 573. [Google Scholar] [CrossRef]
- Li, J.; Su, X.; Wang, Y.; Yang, W.; Pan, Y.; Su, C.; Zhang, X. Genome-wide identification and expression analysis of the BTB domain-containing protein gene family in tomato. Genes Genom. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Robert, H.S.; Quint, A.; Brand, D.; Vivian-Smith, A.; Offringa, R. BTB and TAZ domain scaffold proteins perform a crucial function in Arabidopsis development. Plant J. 2009, 58, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torrado, R.; Yamada, D.; Defossez, P.A. Born to bind: The BTB protein-protein interaction domain. BioEssays 2006, 28, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, D.J.; Hanada, K.; Shiu, S.H.; Vierstra, R.D. Large-scale; lineage-specific expansion of a bric-a-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice. Plant Cell 2007, 19, 2329–2348. [Google Scholar] [CrossRef] [PubMed]
- Ban, Z.; Estelle, M. CUL3 E3 ligases in plant development and environmental response. Nat. Plants 2021, 7, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Pedmale, U.V.; Morrow, J.; Sachdev, S.; Lechner, E.; Tang, X.; Zheng, N.; Hannink, M.; Genschik, P.; Liscum, E. Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-ring E3 ubiquitin ligase CRL3(NPH3). Plant Cell 2011, 23, 3627–3640. [Google Scholar] [CrossRef]
- Inada, S.; Ohgishi, M.; Mayama, T.; Okada, K.; Sakai, T. RPT2 is a signal transducer involved in a phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 2004, 16, 887–896. [Google Scholar] [CrossRef]
- Hashiguchi, A.; Komatsu, S. Impact of post-translational modifications of crop proteins under abiotic stress. Proteomes 2016, 4, 42. [Google Scholar] [CrossRef]
- Hernando, C.E.; Sanchez, S.E.; Mancini, E.; Yanovsky, M.J. Genome wide comparative analysis of the effects of PRMT5 and PRMT4/CARM1 arginine methyltransferases on the Arabidopsis thaliana transcriptome. BMC Genom. 2015, 16, 192. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, X.; Lin, J.; Liu, X.; Wang, Z.; Xin, M.; Yao, Y.; Peng, H.; Zhou, D.X.; Ni, Z.; et al. Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J. 2019, 97, 587–602. [Google Scholar] [CrossRef]
- Ullah, F.; Xu, Q.; Zhao, Y.; Zhou, D.-X. Histone deacetylase HDA710 controls salt tolerance by regulating ABA signaling in rice. J. Integr. Plant Biol. 2021, 63, 451–467. [Google Scholar] [CrossRef]
- Li, H.; Yan, S.; Zhao, L.; Tan, J.; Zhang, Q.; Gao, F.; Wang, P.; Hou, H.; Li, L. Histone acetylation associated up-regulation of the cell wall related genes is involved in salt stress induced maize root swelling. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Qiao, Z.; Li, Y.; Wang, C.; Wang, B. The roles of CCCH zinc-finger proteins in plant abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 8327. [Google Scholar] [CrossRef] [PubMed]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Luan, Y.S.; Liu, Z. Overexpression of SpWRKY1 promotes resistance to Phytophthora nicotianae and tolerance to salt and drought stress in transgenic tobacco. Physiol. Plant 2015, 155, 248–266. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Ansari, M.W.; Tuteja, N. Multiple abiotic stress responsive rice cyclophilin: (OsCYP-25) mediates a wide range of cellular responses. Commun. Integr. Biol. 2013, 6, e25260. [Google Scholar] [CrossRef]
- Xiao, J.; Cheng, H.; Li, X.; Xiao, J.; Xu, C.; Wang, S. Rice WRKY13 regulates cross talk between abiotic and biotic stress signaling pathways by selective binding to different cis-elements. Plant Physiol. 2013, 163, 1868–1882. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought; salt; and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Kaplan, B.; Davydov, O.; Knight, H.; Galon, Y.; Knight, M.R.; Fluhr, R.; Fromm, H. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell 2006, 18, 2733–2748. [Google Scholar] [CrossRef]
- Noman, M.; Jameel, A.; Qiang, W.D.; Ahmad, N.; Liu, W.C.; Wang, F.W.; Li, H.Y. Overexpression of GmCAMTA12 enhanced drought tolerance in Arabidopsis and soybean. Int. J. Mol. Sci. 2019, 20, 4849. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ethylene RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Constabel, C.P. MYB repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 2019, 24, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, F.; Duan, D. Function analysis and stress-mediated cis-element identification in the promoter region of VqMYB15. Plant Signal. Behav. 2020, 15, 1773664. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Guo, H.; Wang, L.; Yang, C.; Zhang, Y.; Zhang, C.; Wang, C. Identification of novel cis-elements bound by BplMYB46 involved in abiotic stress responses and secondary wall deposition. J. Integr. Plant Biol. 2018, 60, 1000–1014. [Google Scholar] [CrossRef]
- Berendzen, K.W.; Weiste, C.; Wanke, D.; Kilian, J.; Harter, K.; Dröge-Laser, W. Bioinformatic cis-element analyses performed in Arabidopsis and rice disclose bZIP- and MYB-related binding sites as potential AuxRE-coupling elements in auxin-mediated transcription. BMC Plant Biol. 2012, 12, 125. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Baral, A. Bananas tackling drought and heat—With DREBs and more. Physiol. Plant 2019, 165, 128–130. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Vu, L.T.K.; Nguyen, L.T.N.; Pham, N.T.T.; Nguyen, Y.T.H.; Le, S.V.; Chu, M.H. Overexpression of the GmDREB6 gene enhances proline accumulation and salt tolerance in genetically modified soybean plants. Sci. Rep. 2019, 9, 19663. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Nakaya, A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Kurata, N.; Yamazaki, Y. Oryzabase. an integrated biological and genome information database for rice. Plant Physiol. 2006, 140, 12–17. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Udomchalothorn, T.; Plaimas, K.; Sripinyowanich, S.; Boonchai, C.; Kojonna, T.; Chutimanukul, P.; Comai, L.; Buaboocha, T.; Chadchawan, S. OsNucleolin1-L expression in Arabidopsis enhances photosynthesis via transcriptome modification under salt stress conditions. Plant Cell Physiol. 2017, 58, 717–734. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Endo, A.; Egawa, C.; Oohashi, M.; Maoka, A.M.; Shimosaka, E.; Sato, Y. Ectopic expression of mutated type 2C protein phosphatase OsABI-LIKE2 decreases abscisic acid sensitivity in Arabidopsis and rice. Sci. Rep. 2018, 8, 12320. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Wang, J.L.; Huang, X.; Xu, W.H.; Liu, Z.M.; Fang, R.X. The promoter of a rice glycine-rich protein gene; Osgrp-2; confers vascular-specific expression in transgenic plants. Planta 2003, 216, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Huang, X.; Wu, M.; Wang, Y.; Chang, Y.; Liu, K.; Zhang, J.; Zhang, Y.; Zhang, F.; Yi, L.; et al. A rapid, highly efficient and economical method of Agrobacterium-mediated in planta transient transformation in living onion epidermis. PLoS ONE 2014, 9, e83556. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral determination of chlorophyll a and b; as well as total carotenoids; using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Kositsup, B.; Plaimas, K.; Buaboocha, T.; Siangliw, M.; Toojinda, T.; Comai, L.; Chadchawan, S. Photosynthetic responses and identification of salt tolerance genes in a chromosome segment substitution line of ‘Khao Dawk Mali 105′ rice. Environ. Exp. Bot. 2018, 155, 497–508. [Google Scholar] [CrossRef]
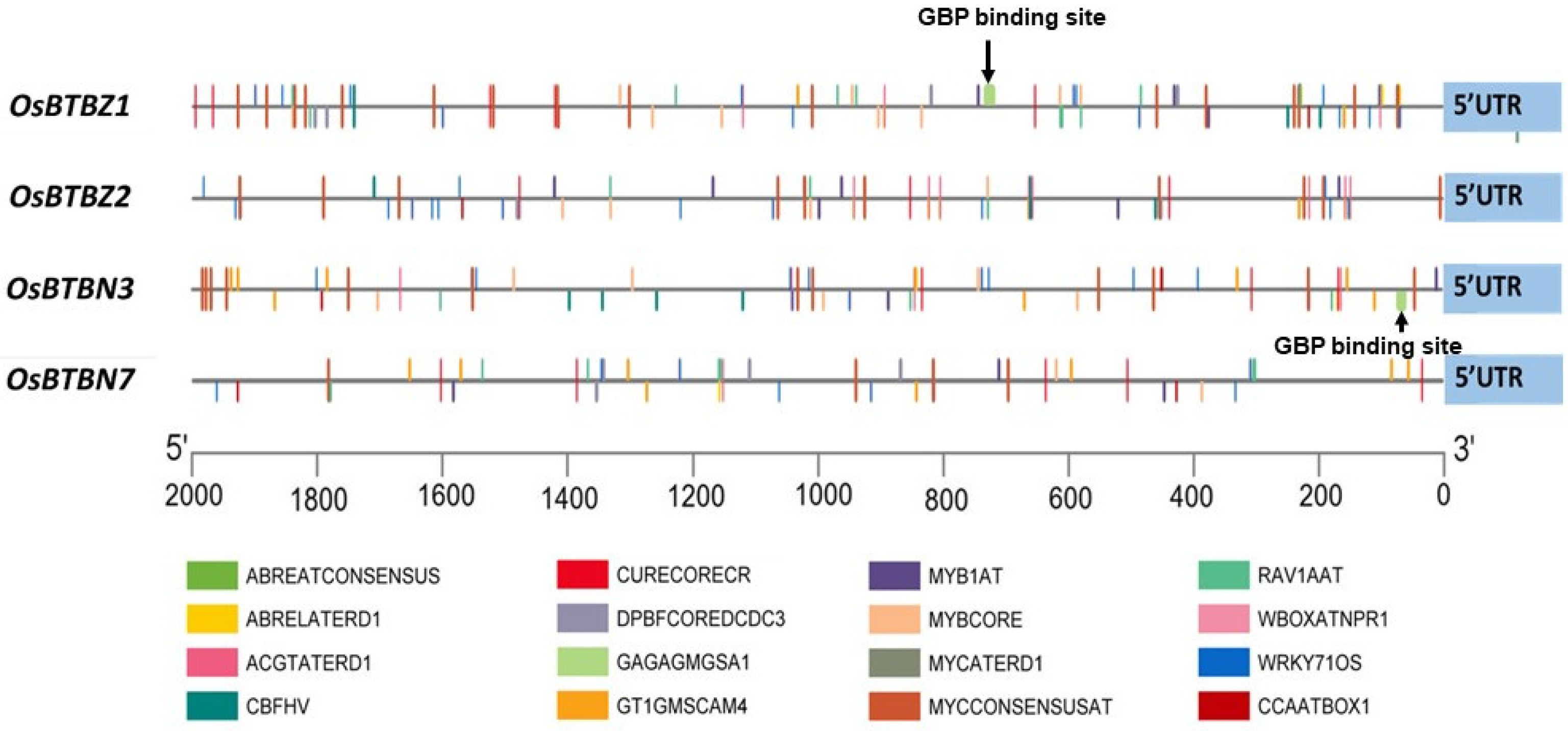


| Factor or Site Name | Signal Sequence | OsBTBZ1 | OsBTBZ2 | OsBTBN3 | OsBTBN7 | Functions | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strand (+) | Strand (−) | Strand (+) | Strand (−) | Strand (+) | Strand (−) | Strand (+) | Strand (−) | |||
| ABREATCONSENSUS | YACGTGGC | 1 | - | - | - | - | - | - | - | ABA-responsive element |
| ABRELATERD1 | ACGTG | 3 | 3 | - | 6 | 1 | 1 | - | 1 | ERD-related gene (early responsive to dehydration) |
| ACGTATERD1 | ACGT | 5 | 5 | 8 | 8 | 4 | 4 | 1 | 1 | Abiotic stresses (drought, salt); response to light |
| CBFHV | RYCGAC | 1 | 3 | 2 | 2 | - | 4 | - | - | Dehydration-responsive element (DRE) binding proteins (DREBs) |
| CCAATBOX1 | CCAAT | - | 2 | - | 1 | 1 | 1 | - | 2 | Heat-shock element |
| CURECORECR | GTAC | 5 | 5 | 3 | 3 | 3 | 3 | 5 | 5 | Oxygen-responsive element |
| DPBFCOREDCDC3 | ACACNNG | 4 | 3 | 1 | 3 | 1 | 1 | 3 | 2 | Dehydration and ABA response |
| GAGAGMGSA1 | GAGAGAGAGAGAGAGAGA | 2 | - | - | - | - | 1 | - | - | GAGA binding protein (GBP) binding site |
| GT1GMSCAM4 | GAAAAA | 1 | 1 | - | 1 | 5 | 3 | 6 | 2 | Plays a role in pathogen- and salt-induced SCaM-4 gene expression |
| MYB1AT | WAACCA | 2 | 1 | 4 | 2 | 1 | 1 | 1 | 2 | Element for the dehydration-responsive gene in Arabidopsis |
| MYBCORE | CNGTTR | 5 | 6 | 2 | 4 | 3 | 3 | 1 | 1 | Responsive to water stress, induced by dehydration stress |
| MYCATERD1 | CATGTG | 1 | - | 2 | - | 1 | 2 | 2 | - | Necessary for ERD1 expression, the binding site of the NAC protein |
| MYCCONSENSUSAT | CANNTG | 16 | 16 | 10 | 10 | 12 | 12 | 4 | 4 | Element for the dehydration-responsive gene in Arabidopsis |
| RAV1AAT | CAACA | 5 | 4 | 2 | 1 | - | 3 | 6 | 1 | Rosette leaves and the root-specific element, growth, and development, hormonal regulation brassinosteroid (BR); metabolism |
| WBOXATNPR1 | TTGAC | 2 | 2 | 1 | 3 | 1 | 1 | 2 | 1 | Salicylic acid-induced WRKY DNA binding proteins |
| WRKY71OS | TGAC | 8 | 6 | 3 | 11 | 8 | 2 | 3 | 4 | Involved in gibberellic acid (GA), ABA-mediated pathways, and pathogen-related protein (PR) |
| Name | Sequence 5′→3′ | Tm (°C) |
|---|---|---|
| qPCR_OsBTBZ1_FW | TTCCTGCCTGCAAGGGCATC | 63 |
| qPCR_OsBTBZ1_REV | TCCTTGAAATGCCTACAGAGGGG | 60 |
| qPCR_OsEF1α_FW | ATGGTTGTGGAGACCTTC | 53 |
| qPCR_OsEF1α_REV | TCACCTTGGCACCGGTTG | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputro, T.B.; Jakada, B.H.; Chutimanukul, P.; Comai, L.; Buaboocha, T.; Chadchawan, S. OsBTBZ1 Confers Salt Stress Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 14483. https://doi.org/10.3390/ijms241914483
Saputro TB, Jakada BH, Chutimanukul P, Comai L, Buaboocha T, Chadchawan S. OsBTBZ1 Confers Salt Stress Tolerance in Arabidopsis thaliana. International Journal of Molecular Sciences. 2023; 24(19):14483. https://doi.org/10.3390/ijms241914483
Chicago/Turabian StyleSaputro, Triono B., Bello H. Jakada, Panita Chutimanukul, Luca Comai, Teerapong Buaboocha, and Supachitra Chadchawan. 2023. "OsBTBZ1 Confers Salt Stress Tolerance in Arabidopsis thaliana" International Journal of Molecular Sciences 24, no. 19: 14483. https://doi.org/10.3390/ijms241914483
APA StyleSaputro, T. B., Jakada, B. H., Chutimanukul, P., Comai, L., Buaboocha, T., & Chadchawan, S. (2023). OsBTBZ1 Confers Salt Stress Tolerance in Arabidopsis thaliana. International Journal of Molecular Sciences, 24(19), 14483. https://doi.org/10.3390/ijms241914483





