Interaction between Rumen Epithelial miRNAs-Microbiota-Metabolites in Response to Cold-Season Nutritional Stress in Tibetan Sheep
Abstract
:1. Introduction
2. Results
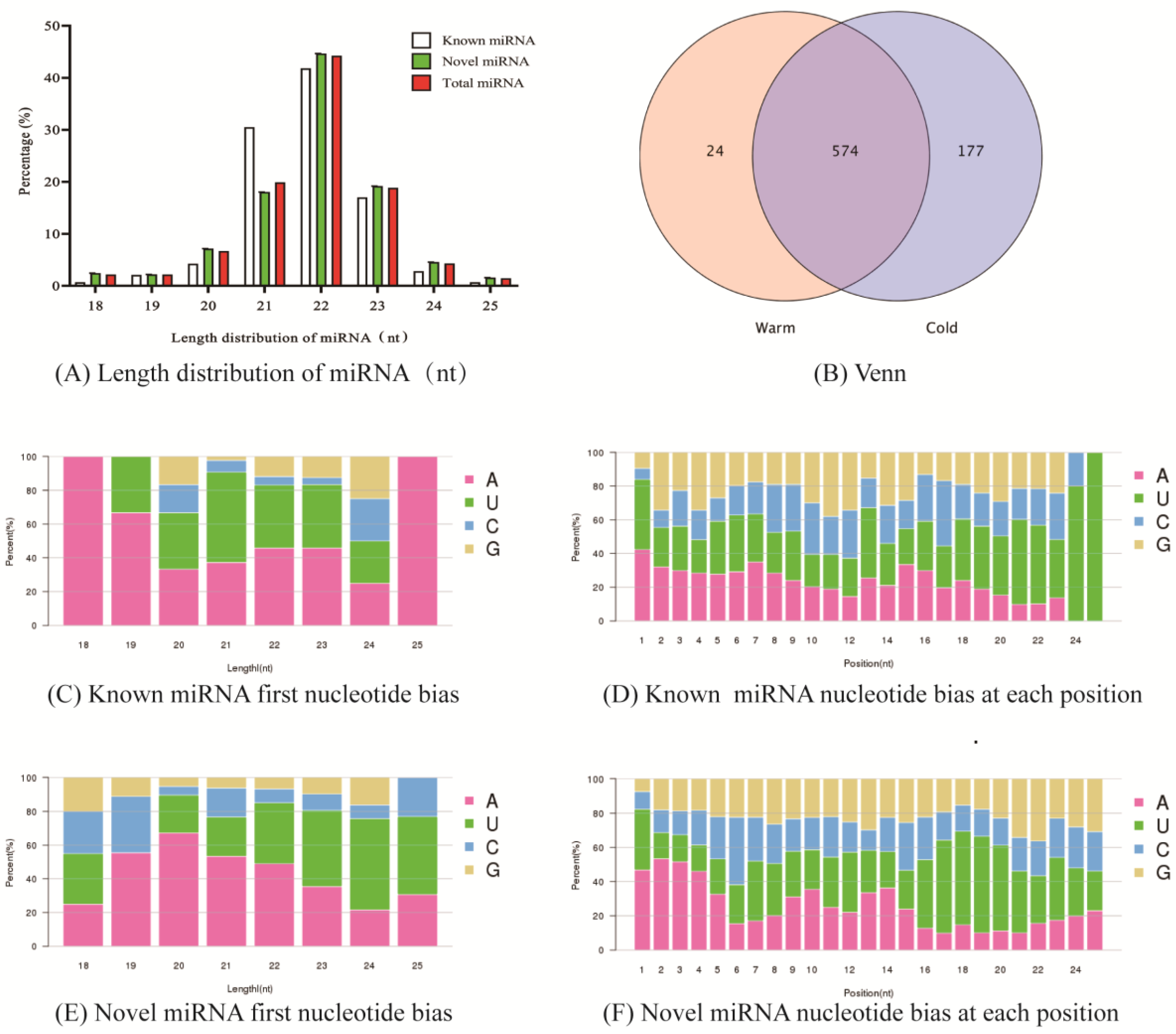
2.1. Data Quality Control and miRNA Classification Annotation
2.2. Identification of Tibetan Sheep Rumen Epithelial miRNAs
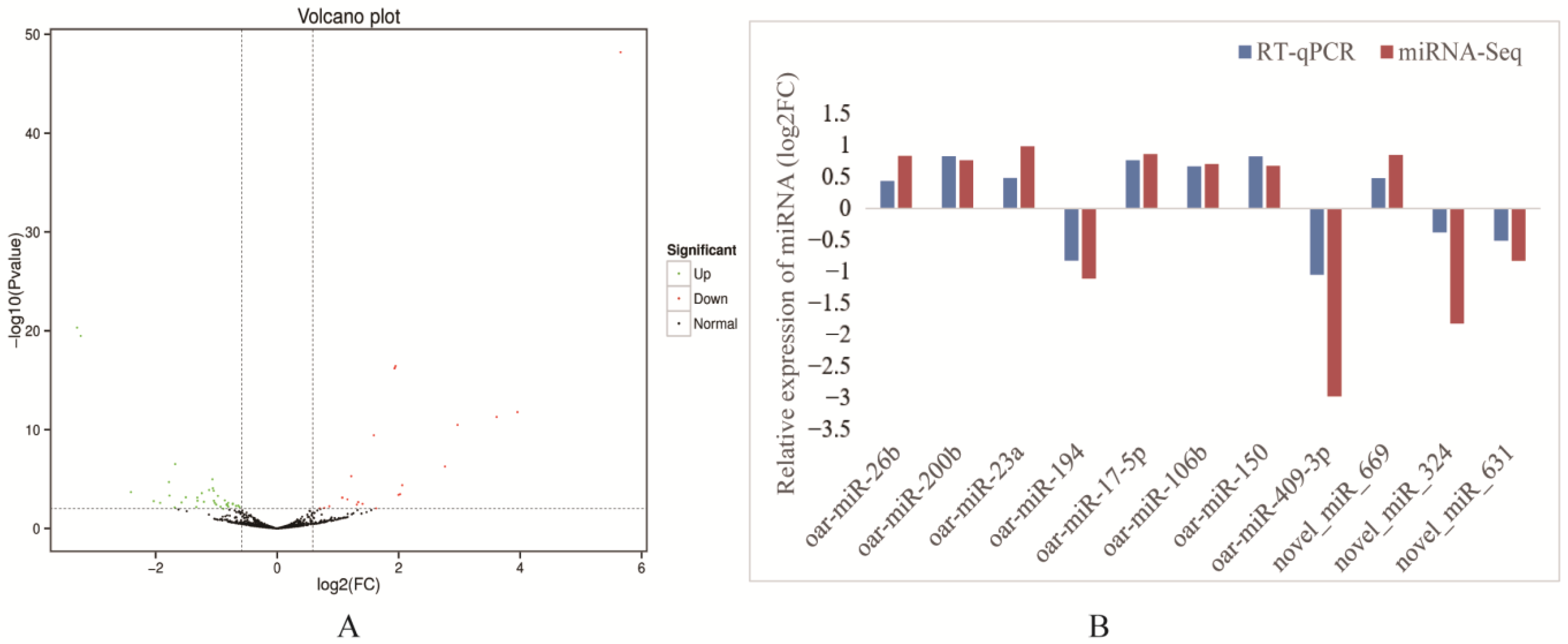
2.3. Screening of Cold and Warm Season Rumen Epithelial DE miRNAs and Validation by RT-qPCR
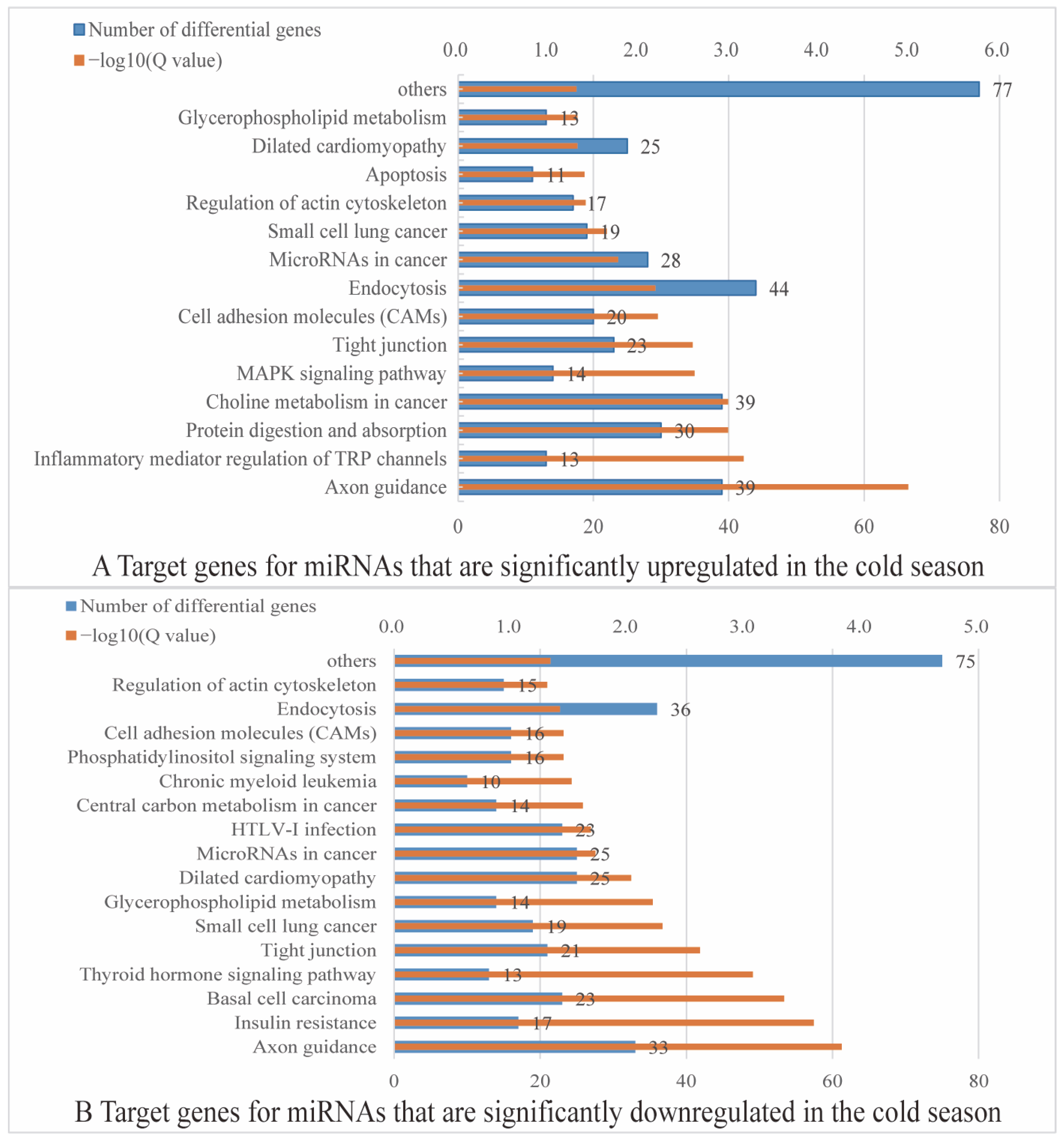
2.4. Functional Annotation of Rumen Epithelial DE miRNA Targeted Genes
2.4.1. GO Functional Annotation
2.4.2. KEGG Functional Annotation
2.5. Correlation Analysis of Rumen Epithelial miRNA-Microbiota-Metabolite
2.5.1. Correlation Analysis of Cold and Warm Season Rumen DE miRNAs with Rumen Microbiota
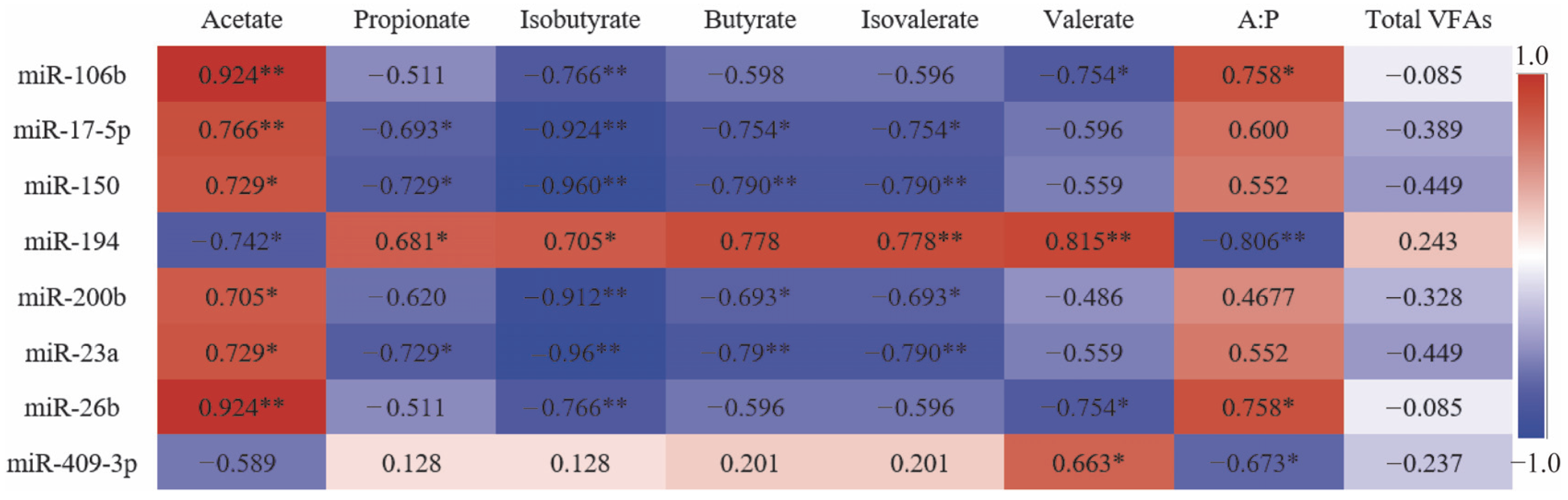
2.5.2. Correlation Analysis of Cold and Warm Season Ruminal DE miRNAs with Ruminal VFAs
2.5.3. Correlation Analysis of the Cold and Warm Season Rumen Epithelial DE miRNA Targeted Genes with Microbiota Metabolites
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Sample Collection
4.2. Microbiota 16S rRNA Sequencing of Rumen Contents in Tibetan Sheep
4.3. Determination of VFAs in Rumen Contents of Tibetan Sheep
4.4. MiRNA Sequencing of Tibetan Sheep Rumen Epithelium
4.4.1. Extraction of Total RNA from Rumen Epithelial Tissue of Tibetan Sheep
4.4.2. Preparation and Quantification of miRNA Libraries
4.4.3. Identification of miRNAs and Targeted Gene Prediction, Functional Annotation and Pathway Enrichment Analysis in the Rumen Epithelium of Tibetan Sheep
4.5. Real-Time Quantitative PCR (RT-qPCR) Validation
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, B.; Zhao, X.Q.; Zhang, Y.S. Seasonal changes in weight and body composition of yak grazing on alpine-meadow grassland in the Qinghai-Tibetan plateau of China. J. Anim. Sci. 2005, 83, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, P.; Hou, F.; Yan, T.; Chang, S.; Chen, X.; Wang, Z. Relationship between chemical composition of native forage and nutrient digestibility by Tibetan sheep on the Qinghai-Tibetan Plateau. J. Anim. Sci. 2018, 96, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, W.; Degen, A.; Guo, Y.; Kang, J.; Liu, P.; Ding, L.; Shang, Z.; Fievez, V.; Zhou, J.; et al. Tibetan sheep have a high capacity to absorb and to regulate metabolism of SCFA in the rumen epithelium to adapt to low energy intake. Br. J. Nutr. 2020, 123, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Baldwin, R.L., 6th; Li, C.J.; Li, R.W.; Chung, H. Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Funct. Integr. Genom. 2013, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qiu, Q.; Jiang, Y.; Wang, K.; Lin, Z.; Li, Z.; Bibi, F.; Yang, Y.; Wang, J.; Nie, W.; et al. Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science 2019, 364, eaav6202. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Oddy, V.H.; Archibald, A.L.; Vercoe, P.E.; Dalrymple, B.P. Epithelial, metabolic and innate immunity transcriptomic signatures differentiating the rumen from other sheep and mammalian gastrointestinal tract tissues. PeerJ 2016, 4, e1762. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.A.; Crowley, J.R.; Sambandam, N.; Muegge, B.D.; Costello, E.K.; Hamady, M.; Knight, R.; Gordon, J.I. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl. Acad. Sci. USA 2009, 106, 11276–11281. [Google Scholar] [CrossRef]
- Janis, C. The evolutionary strategy of the equidae and the origins of rumen and cecal digestion. Evolution 1976, 30, 757–774. [Google Scholar] [CrossRef]
- Beharka, A.A.; Nagaraja, T.G.; Morrill, J.L.; Kennedy, G.A.; Klemm, R.D. Effects of form of the diet on anatomical, microbial, and fermentative development of the rumen of neonatal calves. J. Dairy Sci. 1998, 81, 1946–1955. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, L.; Chen, X.; Zhong, Y.; Yang, Y.; Xia, W.; Liu, C.; Zhu, W.; Wang, H.; Yan, B.; et al. Biological adaptations in the Arctic cervid, the reindeer (Rangifer tarandus). Science 2019, 64, eaav6312. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.B.; Zhang, X.Y.; Wen, J.; Deng, K.; Qin, X.W.; Wang, D.H. The microbiota-gut-brain interaction in regulating host metabolic adaptation to cold in male Brandt’s voles (Lasiopodomys brandtii). ISME J. 2019, 13, 3037–3053. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Shukla, G.C.; Singh, J.; Barik, S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol. Cell. Pharmacol. 2011, 3, 83–92. [Google Scholar]
- Grosshans, H.; Filipowicz, W. Molecular biology: The expanding world of small RNAs. Nature 2008, 451, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Santos, R.M.; Moreno, C.; Zhang, W.C. Non-Coding RNAs in Lung Tumor Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2774. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lv, W.; Tong, Q.; Jin, J.; Xu, Z.; Zuo, B. Functional Non-coding RNA During Embryonic Myogenesis and Postnatal Muscle Development and Disease. Front. Cell Dev. Biol. 2021, 9, 628339. [Google Scholar] [CrossRef]
- Gu, Y.R.; Liang, Y.; Gong, J.J.; Zeng, K.; Li, Z.Q.; Lei, Y.F.; He, Z.P.; Lv, X.B. Suitable internal control microRNA genes for measuring miRNA abundance in pig milk during different lactation periods. Genet. Mol. Res. 2012, 11, 2506–2512. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Ambros, V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell 2003, 113, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Chen, Y.; Bert, A.; Hu, Y.; Sheridan, J.M.; Beck, T.; Shi, W.; Satterley, K.; Jamieson, P.; Goodall, G.J.; et al. Integration of microRNA signatures of distinct mammary epithelial cell types with their gene expression and epigenetic portraits. Breast Cancer Res. 2015, 17, 85. [Google Scholar] [CrossRef]
- Yoo, A.S.; Staahl, B.T.; Chen, L.; Crabtree, G.R. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 2009, 460, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, Z.; Wang, F. Genetically Encoded Reporter Genes for MicroRNA Imaging in Living Cells and Animals. Mol. Ther. Nucleic Acids 2020, 21, 555–567. [Google Scholar] [CrossRef]
- Peck, B.C.; Sincavage, J.; Feinstein, S.; Mah, A.T.; Simmons, J.G.; Lund, P.K.; Sethupathy, P. miR-30 Family Controls Proliferation and Differentiation of Intestinal Epithelial Cell Models by Directing a Broad Gene Expression Program That Includes SOX9 and the Ubiquitin Ligase Pathway. J. Biol. Chem. 2016, 291, 15975–15984. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Dalmasso, G.; Yan, Y.; Laroui, H.; Dahan, S.; Mayer, L.; Sitaraman, S.V.; Merlin, D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J. Biol. Chem. 2010, 285, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, G.; Wang, J.Y.; Zou, T.; Liu, L.; Xiao, L.; Chung, H.K.; Rao, J.N.; Wang, J.Y. Post-transcriptional regulation of Wnt co-receptor LRP6 and RNA-binding protein HuR by miR-29b in intestinal epithelial cells. Biochem. J. 2016, 473, 1641–1649. [Google Scholar] [CrossRef]
- Xue, X.; Feng, T.; Yao, S.; Wolf, K.J.; Liu, C.G.; Liu, X.; Elson, C.O.; Cong, Y. Microbiota downregulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. J. Immunol. 2011, 187, 5879–5886. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Guan, L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019, 20, 172. [Google Scholar] [CrossRef]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Gene Ontol. Consort. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Liu, X.; Sha, Y.; Dingkao, R.; Zhang, W.; Lv, W.; Wei, H.; Shi, H.; Hu, J.; Wang, J.; Li, S.; et al. Interactions Between Rumen Microbes, VFAs, and Host Genes Regulate Nutrient Absorption and Epithelial Barrier Function During Cold Season Nutritional Stress in Tibetan Sheep. Front. Microbiol. 2020, 11, 593062. [Google Scholar] [CrossRef]
- Liu, X.; Sha, Y.; Lv, W.; Cao, G.; Guo, X.; Pu, X.; Wang, J.; Li, S.; Hu, J.; Luo, Y. Multi-Omics Reveals That the Rumen Transcriptome, Microbiome, and Its Metabolome Co-regulate Cold Season Adaptability of Tibetan Sheep. Front. Microbiol. 2022, 13, 859601. [Google Scholar] [CrossRef]
- Zhu, Z.; Di, J.; Lu, Z.; Gao, K.; Zheng, J. Rap2B GTPase: Structure, functions, and regulation. Tumor Biol. 2016, 37, 7085–7093. [Google Scholar] [CrossRef]
- Liu, M.; Bi, F.; Zhou, X.; Zheng, Y. Rho GTPase regulation by miRNAs and covalent modifications. Trends Cell Biol. 2012, 22, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Karve, S.S.; Pradhan, S.; Ward, D.V.; Weiss, A.A. Intestinal organoids model human responses to infection by commensal and Shiga toxin producing Escherichia coli. PLoS ONE 2017, 12, e0178966. [Google Scholar] [CrossRef]
- Sepulveda, J.; Moeller, A.H. The Effects of Temperature on Animal Gut Microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Zepeda Mendoza, M.L.; Xiong, Z.; Escalera-Zamudio, M.; Runge, A.K.; Thézé, J.; Streicker, D.; Frank, H.K.; Loza-Rubio, E.; Liu, S.; Ryder, O.A.; et al. Hologenomic adaptations underlying the evolution of sanguivory in the common vampire bat. Nat. Ecol. Evol. 2018, 2, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, J.; Hou, Q.; Wang, Y.; Hu, Z.; Shi, K.; Yan, Z.; Wang, Z. Effect of hay supplementation timing on rumen microbiota in suckling calves. MicrobiologyOpen 2018, 7, e00430. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liang, R.; Zhang, W.; Tian, K.; Li, J.; Chen, X.; Yu, T.; Chen, Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes 2020, 12, 224–236. [Google Scholar] [CrossRef]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Ye, J.A.; Liu, J.X. Effects of tea saponins on rumen microbiota, rumen fermentation, methane production and growth performance--a review. Trop. Anim. Health Prod. 2012, 44, 697–706. [Google Scholar] [CrossRef]
- Strobel, H.J. Vitamin B12-dependent propionate production by the ruminal bacterium Prevotella ruminicola 23. Appl. Environ. Microbiol. 1992, 58, 2331–2333. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Ajami, N.J.; Michalek, R.D.; Tian, X.; Wong, M.; Losee-Olson, S.H.; Petrosino, J.F.; Yoo, S.H.; Shimomura, K.; et al. Transmissible microbial and metabolomic remodeling by soluble dietary fiber improves metabolic homeostasis. Sci. Rep. 2015, 5, 10604. [Google Scholar] [CrossRef]
- Peng, B.; Huang, S.; Liu, T.; Geng, A. Bacterial xylose isomerases from the mammal gut Bacteroidetes cluster function in Saccharomyces cerevisiae for effective xylose fermentation. Microb. Cell Factories 2015, 14, 70. [Google Scholar] [CrossRef]
- Tajima, K.; Aminov, R.I.; Nagamine, T.; Matsui, H.; Nakamura, M.; Benno, Y. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 2001, 67, 2766–2774. [Google Scholar] [CrossRef]
- Abdul Rahman, N.; Parks, D.H.; Vanwonterghem, I.; Morrison, M.; Tyson, G.W.; Hugenholtz, P. A Phylogenomic Analysis of the Bacterial Phylum Fibrobacteres. Front. Microbiol. 2016, 6, 1469. [Google Scholar] [CrossRef]
- Fisel, P.; Schaeffeler, E.; Schwab, M. Clinical and Functional Relevance of the Monocarboxylate Transporter Family in Disease Pathophysiology and Drug Therapy. Clin. Transl. Sci. 2018, 11, 352–364. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Gan, T.Q.; Chen, W.J.; Qin, H.; Huang, S.N.; Yang, L.H.; Fang, Y.Y.; Pan, L.J.; Li, Z.Y.; Chen, G. Clinical Value and Prospective Pathway Signaling of MicroRNA-375 in Lung Adenocarcinoma: A Study Based on the Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO) and Bioinformatics Analysis. Med. Sci. Monit. 2017, 23, 2453–2464. [Google Scholar] [CrossRef]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef]
- Voelker, D.R. Phosphatidylserine decarboxylase. Biochim. Biophys. Acta 1997, 1348, 236–244. [Google Scholar] [CrossRef]
- Vance, D.E. Phospholipid methylation in mammals: From biochemistry to physiological function. Biochim. Biophys. Acta 2014, 1838, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Wellbrock, C.; Karasarides, M.; Marais, R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 2004, 5, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.F.; Dar, A.C.; Hertz, N.T.; Chao, W.C.; Burlingame, A.L.; Shokat, K.M.; Barford, D. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 2011, 472, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, R.C.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging 2011, 3, 192–222. [Google Scholar] [CrossRef] [PubMed]
- Alekhina, O.; Burstein, E.; Billadeau, D.D. Cellular functions of WASP family proteins at a glance. J. Cell Sci. 2017, 130, 2235–2241. [Google Scholar] [CrossRef]
- Fernando, H.S.; Sanders, A.J.; Kynaston, H.G.; Jiang, W.G. WAVE1 is associated with invasiveness and growth of prostate cancer cells. J. Urol. 2008, 180, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, L.; Shen, L.; Zhou, S.; Duan, Z.; Xiao, L.; Cao, Y.; Mu, X.; Zha, L.; Wang, H. High level of WAVE1 expression is associated with tumor aggressiveness and unfavorable prognosis of epithelial ovarian cancer. Gynecol. Oncol. 2012, 127, 223–230. [Google Scholar] [CrossRef]
- Kang, R.; Tang, D.; Yu, Y.; Wang, Z.; Hu, T.; Hu, T.; Cao, L. WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells. Leukemia 2010, 24, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, S.; Suetsugu, S.; Yamazaki, D.; Yamaguchi, H.; Takenawa, T. Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene 2005, 24, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Surace, E.I.; Lusis, E.; Haipek, C.A.; Gutmann, D.H. Functional significance of S6K overexpression in meningioma progression. Ann. Neurol. 2004, 56, 295–298. [Google Scholar] [CrossRef]
- Lai, K.P.; Leong, W.F.; Chau, J.F.; Jia, D.; Zeng, L.; Liu, H.; He, L.; Hao, A.; Zhang, H.; Meek, D.; et al. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. EMBO J. 2010, 29, 2994–3006. [Google Scholar] [CrossRef]
- Halicka, H.D.; Zhao, H.; Li, J.; Lee, Y.S.; Hsieh, T.C.; Wu, J.M.; Darzynkiewicz, Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage-signaling. Aging 2012, 4, 952–965. [Google Scholar] [CrossRef]
- Brown, H.A.; Gutowski, S.; Moomaw, C.R.; Slaughter, C.; Sternweis, P.C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 1993, 75, 1137–1144. [Google Scholar] [CrossRef]
- Kanaho, Y.; Funakoshi, Y.; Hasegawa, H. Phospholipase D signalling and its involvement in neurite outgrowth. Biochim. Biophys. Acta 2009, 1791, 898–904. [Google Scholar] [CrossRef]
- Huang, P.; Altshuller, Y.M.; Hou, J.C.; Pessin, J.E.; Frohman, M.A. Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol. Biol. Cell 2005, 16, 2614–2623. [Google Scholar] [CrossRef]
- Foster, D.A.; Salloum, D.; Menon, D.; Frias, M.A. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J. Biol. Chem. 2014, 289, 22583–22588. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, B.L.; Neufeld, E.B.; Freeman, L.A.; Gordon, S.M.; Sampson, M.L.; Pryor, M.; Hillman, E.; Axley, M.J.; Karathanasis, S.K.; Remaley, A.T. LCAT Enzyme Replacement Therapy Reduces LpX and Improves Kidney Function in a Mouse Model of Familial LCAT Deficiency. J. Pharmacol. Exp. Ther. 2019, 368, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Unoki, T.; Matsuda, S.; Kakegawa, W.; Van, N.T.; Kohda, K.; Suzuki, A.; Funakoshi, Y.; Hasegawa, H.; Yuzaki, M.; Kanaho, Y. NMDA receptor-mediated PIP5K activation to produce PI(4,5)P2 is essential for AMPA receptor endocytosis during LTD. Neuron 2012, 73, 135–148. [Google Scholar] [CrossRef]
- Turk, V.; Turk, B.; Turk, D. Lysosomal cysteine proteases: Facts and opportunities. EMBO J. 2001, 20, 4629–4633. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fu, Q.; Su, B.; Song, H.; Zhou, S.; Tan, F.; Li, C. The involvement of cathepsin F gene (CTSF) in turbot (Scophthalmus maximus L.) mucosal immunity. Fish Shellfish Immunol. 2017, 66, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.C.; Martínez-Romero, C.; Sempere Borau, M.; Pham, C.T.N.; García-Sastre, A.; Stertz, S. Proteomic Identification of Potential Target Proteins of Cathepsin W for Its Development as a Drug Target for Influenza. Microbiol. Spectr. 2022, 10, e0092122. [Google Scholar] [CrossRef]




| miRNAs/Genes | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| miR-26b | TTCAAGTAATTCAGGATAGGT | Universal reverse prime r * |
| miR-200b | TAATACTGCCTGGTAATGATG | Universal reverse primer * |
| miR-23a | ATCACATTGCCAGGGATTTCCA | Universal reverse primer * |
| miR-194 | TGTAACAGCAACTCCATGTGGA | Universal reverse primer * |
| miR-17-5p | CAAAGTGCTTACAGTGCAGGTA | Universal reverse primer * |
| miR-106b | TAAAGTGCTGACAGTGCAGAT | Universal reverse primer * |
| miR-150 | TCTCCCAACCCTTGTACCAGTG | Universal reverse primer * |
| miR-409-3p | CGAATGTTGCTCGGTGAACCCCT | Universal reverse primer * |
| miR-669 | UCCUUCAUUCCACCGGAGUCUGU | Universal reverse primer * |
| miR-324 | GUCCAGUUUUCCCAGGAAUCCCU | Universal reverse primer * |
| miR-631 | CUGACCUAUGAAUUGACAGCCAG | Universal reverse primer * |
| U6 | ACGGACAGGATTGACAGATT | TCGCTCCACCAACTAAGAA |
| 18S RNA | GTGGTGTTGAGGAAAGCAGACA | TGATCACACGTTCCACCTCATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.; Sha, Y.; Liu, X.; He, Y.; Hu, J.; Wang, J.; Li, S.; Guo, X.; Shao, P.; Zhao, F.; et al. Interaction between Rumen Epithelial miRNAs-Microbiota-Metabolites in Response to Cold-Season Nutritional Stress in Tibetan Sheep. Int. J. Mol. Sci. 2023, 24, 14489. https://doi.org/10.3390/ijms241914489
Lv W, Sha Y, Liu X, He Y, Hu J, Wang J, Li S, Guo X, Shao P, Zhao F, et al. Interaction between Rumen Epithelial miRNAs-Microbiota-Metabolites in Response to Cold-Season Nutritional Stress in Tibetan Sheep. International Journal of Molecular Sciences. 2023; 24(19):14489. https://doi.org/10.3390/ijms241914489
Chicago/Turabian StyleLv, Weibing, Yuzhu Sha, Xiu Liu, Yanyu He, Jiang Hu, Jiqing Wang, Shaobin Li, Xinyu Guo, Pengyang Shao, Fangfang Zhao, and et al. 2023. "Interaction between Rumen Epithelial miRNAs-Microbiota-Metabolites in Response to Cold-Season Nutritional Stress in Tibetan Sheep" International Journal of Molecular Sciences 24, no. 19: 14489. https://doi.org/10.3390/ijms241914489
APA StyleLv, W., Sha, Y., Liu, X., He, Y., Hu, J., Wang, J., Li, S., Guo, X., Shao, P., Zhao, F., & Li, M. (2023). Interaction between Rumen Epithelial miRNAs-Microbiota-Metabolites in Response to Cold-Season Nutritional Stress in Tibetan Sheep. International Journal of Molecular Sciences, 24(19), 14489. https://doi.org/10.3390/ijms241914489






