The Plant Virus Tomato Spotted Wilt Orthotospovirus Benefits Its Vector Frankliniella occidentalis by Decreasing Plant Toxic Alkaloids in Host Plant Datura stramonium
Abstract
1. Introduction
2. Results
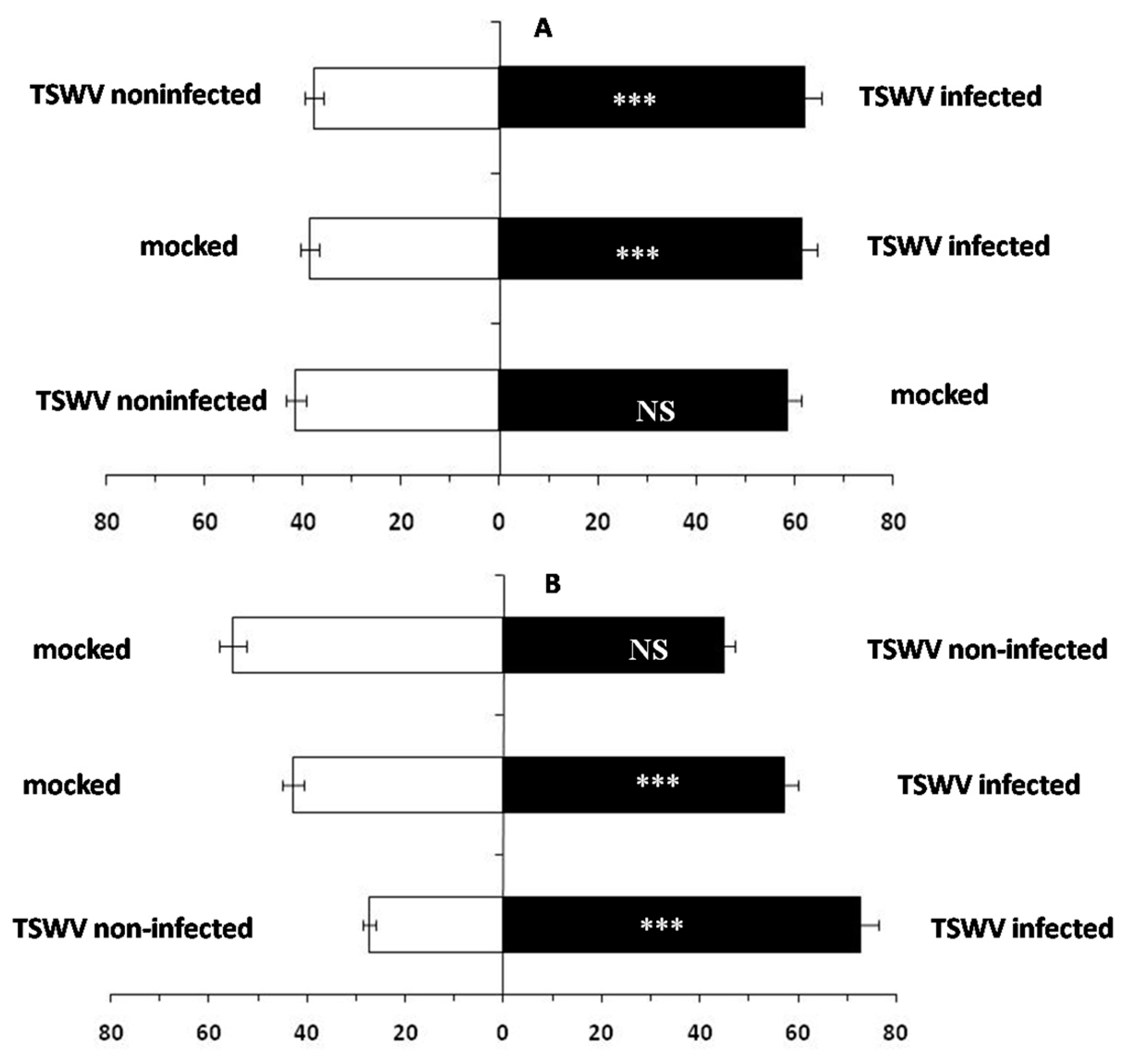
2.1. F. occidentalis Shows a Feeding Preference for TSWV-Infected D. stramonium
2.2. TSWV Infection Reduces the Accumulation of Alkaloids in D. stramonium
2.3. Alkaloid Interactions with F. occidentalis: Repellence and Toxicity
2.3.1. Repellent Effect of Scopolamine and Atropine on F. occidentalis
2.3.2. Toxicity of Scopolamine and Atropine to F. occidentalis
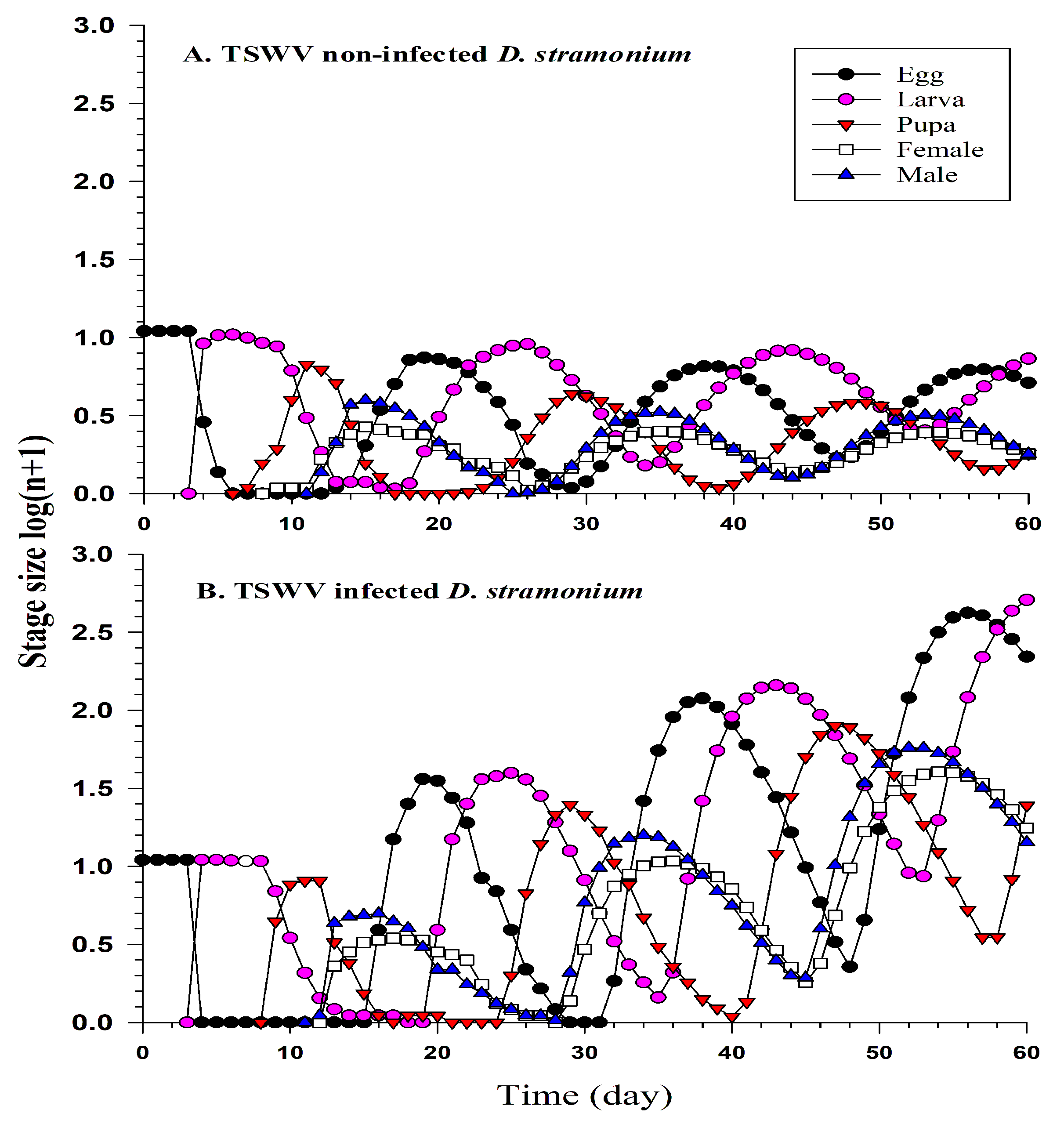
2.4. Performance of F. occidentalis on TSWV-Infected or TSWV Non-Infected D. stramonium
3. Discussion
4. Materials and Methods
4.1. Plants, Virus Isolates, and Insect Populations
4.2. Thrips’ Preference for TSWV-Infected and TSWV Non-Infected Plants
4.2.1. Preference of Larvae Thrips
4.2.2. Preferences of Adult Thrips
4.3. Analysis of Alkaloids Scopolamine and Atropine
4.4. Effects of the Alkaloids Scopolamine and Atropine on Thrips’ Preference and Survival
4.4.1. F. occidentalis Choice between Feeding Solutions
4.4.2. F. occidentalis Survival on Feeding Solutions
4.5. Thrips Performance Experiments
4.5.1. Development and Juvenile Survival
4.5.2. Adult Survival and Oviposition
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belliure, B.; Janssen, A.; Maris, P.C.; Peters, D.; Sabelis, M.W. Herbivore Arthropods Benefit from Vectoring Plant Viruses. Ecol. Lett. 2005, 8, 70–79. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from Potato Plants Infected with Potato Leafroll Virus Attract and Arrest the Virus Vector, Myzus Persicae (Homoptera: Aphididae). Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 455–460. [Google Scholar] [CrossRef]
- Luan, J.B.; Yao, D.M.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.J.; Liu, S.S. Suppression of Terpenoid Synthesis in Plants by a Virus Promotes Its Mutualism with Vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Maluta, N.; Fereres, A.; Lopes, J.R.S. Plant-Mediated Indirect Effects of Two Viruses with Different Transmission Modes on Bemisia Tabaci Feeding Behavior and Fitness. J. Pest. Sci. 2019, 92, 405–416. [Google Scholar] [CrossRef]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission Mechanisms Shape Pathogen Effects on Host–Vector Interactions: Evidence from Plant Viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive Chemical Signals Induced by a Plant Virus Attract Insect Vectors to Inferior Hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and Physiological Mechanisms Underlying Effects of Cucumber Mosaic Virus on Host-Plant Traits That Mediate Transmission by Aphid Vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Wei, H.; Chen, Y.D.; Zheng, K.Y.; Mu, Y.; Zhao, X.Y.; Zhao, L.H.; Gao, Y.L.; Zheng, L.M.; et al. A Plant Virus Mediates Interspecific Competition between Its Insect Vectors in Capsicuum Annuum. J. Pest. Sci. 2021, 94, 17–28. [Google Scholar] [CrossRef]
- Chesnais, Q.; Sun, P.; Mauck, K.E. Advanced Infections by Cucurbit Yellow Stunting Disorder Virus Encourage Whitefly Vector Colonization While Discouraging Non-Vector Aphid Competitors. J. Pest. Sci. 2022, 95, 231–247. [Google Scholar] [CrossRef]
- Belliure, B.; Janssen, A.; Sabelis, M.W. Herbivore Benefits from Vectoring Plant Virus through Reduction of Period of Vulnerability to Predation. Oecologia 2008, 156, 797–806. [Google Scholar] [CrossRef]
- Blanc, S.; Michalakis, Y. Manipulation of Hosts and Vectors by Plant Viruses and Impact of the Environment. Curr. Opin. Insect Sci. 2016, 16, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Smyers, E.; De Moraes, C.M.; Mescher, M.C. Virus Infection Influences Host Plant Interactions with Non-Vector Herbivores and Predators. Funct. Ecol. 2015, 29, 662–673. [Google Scholar] [CrossRef]
- Liu, G.; Ji, Y.; Bhuiyan, N.H.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino Acid Homeostasis Modulates Salicylic Acid–Associated Redox Status and Defense Responses in Arabidopsis. Plant Cell 2010, 22, 3845–3863. [Google Scholar] [CrossRef] [PubMed]
- Llave, C. Dynamic Cross-Talk between Host Primary Metabolism and Viruses during Infections in Plants. Curr. Opin. Virol. 2016, 19, 50–55. [Google Scholar] [CrossRef]
- Chesnais, Q.; Mauck, K.E.; Bogaert, F.; Bamière, A.; Catterou, M.; Spicher, F.; Brault, V.; Tepfer, M.; Ameline, A. Virus Effects on Plant Quality and Vector Behavior Are Species Specific and Do Not Depend on Host Physiological Phenotype. J. Pest. Sci. 2019, 92, 791–804. [Google Scholar] [CrossRef]
- Blua, M.J.; Perring, T.M.; Madore, M.A. Plant Virus-Induced Changes in Aphid Population Development and Temporal Fluctuations in Plant Nutrients. J. Chem. Ecol. 1994, 20, 691–707. [Google Scholar] [CrossRef]
- Kern, M.; Meiners, T.; Schliephake, E.; Habekuss, A.; Ordon, F.; Will, T. Infection of Susceptible/Tolerant Barley Genotypes with Barley Yellow Dwarf Virus Alters the Host Plant Preference of Rhopalosiphum Padi Clones Depending upon Their Ability to Transmit BYDV. J. Pest. Sci. 2022, 95, 215–229. [Google Scholar] [CrossRef]
- Wu, X.; Xu, S.; Zhao, P.; Zhang, X.; Yao, X.; Sun, Y.; Fang, R.; Ye, J. The Orthotospovirus Nonstructural Protein NSs Suppresses Plant MYC-Regulated Jasmonate Signaling Leading to Enhanced Vector Attraction and Performance. PLoS Pathog. 2019, 15, e1007897. [Google Scholar] [CrossRef]
- Su, Q.; Mescher, M.C.; Wang, S.; Chen, G.; Xie, W.; Wu, Q.; Wang, W.; Zhang, Y. Tomato Yellow Leaf Curl Virus Differentially Influences Plant Defence Responses to a Vector and a Non-Vector Herbivore. Plant Cell Environ. 2016, 39, 597–607. [Google Scholar] [CrossRef]
- Madden, L.V.; Jeger, M.J.; van den Bosch, F. A Theoretical Assessment of the Effects of Vector-Virus Transmission Mechanism on Plant Virus Disease Epidemics. Phytopathology 2000, 90, 576–594. [Google Scholar] [CrossRef]
- Rashed, A.; Nash, T.D.; Paetzold, L.; Workneh, F.; Rush, C.M. Transmission Efficiency of ‘Candidatus Liberibacter Solanacearum’ and Potato Zebra Chip Disease Progress in Relation to Pathogen Titer, Vector Numbers, and Feeding Sites. Phytopathology 2012, 102, 1079–1085. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shalileh, S.; Ogada, P.A.; Moualeu, D.P.; Poehling, H.M. Manipulation of Frankliniella Occidentalis (Thysanoptera: Thripidae) by Tomato Spotted Wilt Virus (Tospovirus) Via the Host Plant Nutrients to Enhance Its Transmission and Spread. Environ. Entomol. 2016, 45, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Sisterson, M.S. Effects of Insect-Vector Preference for Healthy or Infected Plants on Pathogen Spread: Insights from a Model. J. Econ. Entomol. 2008, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ulate, B.; Hopfer, H.; Figueroa-Balderas, R.; Ye, Z.; Rivero, R.M.; Albacete, A.; Pérez-Alfocea, F.; Koyama, R.; Anderson, M.M.; Smith, R.J.; et al. Red Blotch Disease Alters Grape Berry Development and Metabolism by Interfering with the Transcriptional and Hormonal Regulation of Ripening. J. Exp. Bot. 2017, 68, 1225–1238. [Google Scholar] [CrossRef]
- Mishra, J.; Srivastava, R.; Trivedi, P.K.; Verma, P.C. Effect of Virus Infection on the Secondary Metabolite Production and Phytohormone Biosynthesis in Plants. 3 Biotech 2020, 10, 547. [Google Scholar] [CrossRef]
- Srivastava, A.; Agrawal, L.; Raj, R.; Jaidi, M.; Raj, S.K.; Gupta, S.; Dixit, R.; Singh, P.C.; Tripathi, T.; Sidhu, O.P.; et al. Ageratum Enation Virus Infection Induces Programmed Cell Death and Alters Metabolite Biosynthesis in Papaver Somniferum. Front. Plant Sci. 2017, 8, 1172. [Google Scholar] [CrossRef]
- Pan, L.L.; Miao, H.; Wang, Q.; Walling, L.L.; Liu, S.S. Virus-Induced Phytohormone Dynamics and Their Effects on Plant–Insect Interactions. New Phytologist 2021, 230, 1305–1320. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. Tospovirus-Thrips Interactions. Annu. Rev. Phytopathol. 2005, 43, 459–489. [Google Scholar] [CrossRef]
- Kormelink, R.; Verchot, J.; Tao, X.; Desbiez, C. The Bunyavirales: The Plant-Infecting Counterparts. Viruses 2021, 13, 842. [Google Scholar] [CrossRef] [PubMed]
- Wijkamp, I.; Almarza, N.; Goldbach, R.; Peters, D. Distinct Levels of Specifity in Thrips Transmission of Tospovirus. Phytopathology 1995, 85, 1069–1074. [Google Scholar] [CrossRef]
- Roselló, S.; Díez, M.J.; Nuez, F. Viral Diseases Causing the Greatest Economic Losses to the Tomato Crop. I. The Tomato Spotted Wilt Virus—A Review. Sci. Hortic. 1996, 67, 117–150. [Google Scholar] [CrossRef]
- Oliver, J.E.; Whitfield, A.E. The Genus Tospovirus: Emerging Bunyaviruses That Threaten Food Security. Annu. Rev. Virol. 2016, 3, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the Insect Supervectors Bemisia Tabaci and Frankliniella Occidentalis in the Emergence and Global Spread of Plant Viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Bautista, R.C.; Mau, R.F.L.; Cho, J.J.; Custer, D.M. Potential of Tomato Spotted Wilt Tospovirus Plant Hosts in Hawaii as Virus Reservoirs for Transmission by Frankliniella Occidentalis (Thysanoptera: Thripidae). Phytopathology 1995, 85, 953–958. [Google Scholar] [CrossRef]
- Maris, P.C.; Joosten, N.N.; Goldbach, R.W.; Peters, D. Tomato Spotted Wilt Virus Infection Improves Host Suitability for Its Vector Frankliniella Occidentalis. Phytopathology 2004, 94, 706–711. [Google Scholar] [CrossRef]
- Ogada, P.A.; Maiss, E.; Poehling, H.M. Influence of Tomato Spotted Wilt Virus on Performance and Behaviour of Western Flower Thrips (Frankliniella Occidentalis). J. Appl. Entomol. 2013, 137, 488–498. [Google Scholar] [CrossRef]
- Abe, H.; Tomitaka, Y.; Shimoda, T.; Seo, S.; Sakurai, T.; Kugimiya, S.; Tsuda, S.; Kobayashi, M. Antagonistic Plant Defense System Regulated by Phytohormones Assists Interactions Among Vector Insect, Thrips and a Tospovirus. Plant Cell Physiol. 2012, 53, 204–212. [Google Scholar] [CrossRef]
- Abe, H.; Ohnishi, J.; Narusaka, M.; Seo, S.; Narusaka, Y.; Tsuda, S.; Kobayashi, M. Function of Jasmonate in Response and Tolerance of Arabidopsis to Thrip Feeding. Plant Cell Physiol. 2008, 49, 68–80. [Google Scholar] [CrossRef]
- Abe, H.; Shimoda, T.; Ohnishi, J.; Kugimiya, S.; Narusaka, M.; Seo, S.; Narusaka, Y.; Tsuda, S.; Kobayashi, M. Jasmonate-Dependent Plant Defense Restricts Thrips Performance and Preference. BMC Plant Biol. 2009, 9, 97. [Google Scholar] [CrossRef]
- Egger, B.; Koschier, E.H. Behavioural Responses of Frankliniella Occidentalis Pergande Larvae to Methyl Jasmonate and Cis-Jasmone. J. Pest. Sci. 2014, 87, 53–59. [Google Scholar] [CrossRef][Green Version]
- Escobar-Bravo, R.; Chen, G.; Kim, H.K.; Grosser, K.; van Dam, N.M.; Leiss, K.A.; Klinkhamer, P.G.L. Ultraviolet Radiation Exposure Time and Intensity Modulate Tomato Resistance to Herbivory through Activation of Jasmonic Acid Signaling. J. Exp. Bot. 2019, 70, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; McRoberts, J.; Shi, F.; Moreno, J.E.; Jones, A.D.; Howe, G.A. The Flavonoid Biosynthetic Enzyme Chalcone Isomerase Modulates Terpenoid Production in Glandular Trichomes of Tomato. Plant Physiol. 2014, 164, 1161–1174. [Google Scholar] [CrossRef]
- Kariñho-Betancourt, E. Plant-Herbivore Interactions and Secondary Metabolites of Plants: Ecological and Evolutionary Perspectives. Bot. Sci. 2018, 96, 35–51. [Google Scholar] [CrossRef]
- Leiss, K.A.; Cristofori, G.; van Steenis, R.; Verpoorte, R.; Klinkhamer, P.G.L. An Eco-Metabolomic Study of Host Plant Resistance to Western Flower Thrips in Cultivated, Biofortified and Wild Carrots. Phytochemistry 2013, 93, 63–70. [Google Scholar] [CrossRef]
- Liu, X.; Klinkhamer, P.G.L.; Vrieling, K. The Effect of Structurally Related Metabolites on Insect Herbivores: A Case Study on Pyrrolizidine Alkaloids and Western Flower Thrips. Phytochemistry 2017, 138, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vrieling, K.; Klinkhamer, P.G.L. Interactions between Plant Metabolites Affect Herbivores: A Study with Pyrrolizidine Alkaloids and Chlorogenic Acid. Front. Plant Sci. 2017, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Philippi, J.; Schliephake, E.; Jürgens, H.-U.; Jansen, G.; Ordon, F. Correlation of the Alkaloid Content and Composition of Narrow-Leafed Lupins (Lupinus angustifolius L.) to Aphid Susceptibility. J. Pest. Sci. 2016, 89, 359–373. [Google Scholar] [CrossRef]
- Castillo, G.; Valverde, P.L.; Cruz, L.L.; Hernández-Cumplido, J.; Andraca-Gómez, G.; Fornoni, J.; Sandoval-Castellanos, E.; Olmedo-Vicente, E.; Flores-Ortiz, C.M.; Núñez-Farfán, J. Adaptive Divergence in Resistance to Herbivores in Datura Stramonium. PeerJ 2015, 3, e1411. [Google Scholar] [CrossRef]
- Kariñho-Betancourt, E.; Agrawal, A.A.; Halitschke, R.; Núñez-Farfán, J. Phylogenetic Correlations among Chemical and Physical Plant Defenses Change with Ontogeny. N. Phytol. 2015, 206, 796–806. [Google Scholar] [CrossRef]
- Arab, A.; Alves, M.; Sartoratto, A.; Ogasawara, D.; Trigo, J. Methyl Jasmonate Increases the Tropane Alkaloid Scopolamine and Reduces Natural Herbivory in Brugmansia Suaveolens: Is Scopolamine Responsible for Plant Resistance? Neotrop. Entomol. 2012, 41, 2–8. [Google Scholar] [CrossRef]
- Koschier, E.H. Chapter 10 Plant Allelochemicals in Thrips Control Strategies. In Advances in Phytomedicine; Rai, M., Carpinella, M.C., Eds.; Naturally Occurring Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2006; Volume 3, pp. 221–249. [Google Scholar]
- Miranda-Pérez, A.; Castillo, G.; Hernández-Cumplido, J.; Valverde, P.L.; Borbolla, M.; Cruz, L.L.; Tapia-López, R.; Fornoni, J.; Flores-Ortiz, C.M.; Núñez-Farfán, J. Natural Selection Drives Chemical Resistance of Datura Stramonium. PeerJ 2016, 4, e1898. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Bravo, R.; Klinkhamer, P.G.L.; Leiss, K.A. Interactive Effects of UV-B Light with Abiotic Factors on Plant Growth and Chemistry, and Their Consequences for Defense against Arthropod Herbivores. Front. Plant Sci. 2017, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of Chlorogenic Acid as a Resistance Factor for Thrips in Chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Schütz, I.; Moritz, G.B.; Roos, W. Alkaloid Metabolism in Thrips-Papaveraceae Interaction: Recognition and Mutual Response. J. Plant Physiol. 2014, 171, 119–126. [Google Scholar] [CrossRef]
- Macel, M. Attract and Deter: A Dual Role for Pyrrolizidine Alkaloids in Plant–Insect Interactions. Phytochem. Rev. 2011, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, M.; Abd-el-Haliem, A.; Bleeker, P.; Dicke, M.; Escobar-Bravo, R.; Cheng, G.; Haring, M.A.; Kant, M.R.; Kappers, I.; Klinkhamer, P.G.L.; et al. Thrips Advisor: Exploiting Thrips-Induced Defences to Combat Pests on Crops. J. Exp. Bot. 2018, 69, 1837–1848. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, R.; Chai, Y.; Bonfill, M.; Moyano, E.; Oksman-Caldentey, K.-M.; Xu, T.; Pi, Y.; Wang, Z.; Zhang, H.; et al. Engineering Tropane Biosynthetic Pathway in Hyoscyamus Niger Hairy Root Cultures. Proc. Natl. Acad. Sci. USA 2004, 101, 6786–6791. [Google Scholar] [CrossRef]
- Jirschitzka, J.; Dolke, F.; D’Auria, J.C. Chapter Two—Increasing the Pace of New Discoveries in Tropane Alkaloid Biosynthesis. In Advances in Botanical Research; Giglioli-Guivarc’h, N., Ed.; New Light on Alkaloid Biosynthesis and Future Prospects; Academic Press: Cambridge, MA, USA, 2013; Volume 68, pp. 39–72. [Google Scholar]
- Qiu, F.; Zeng, J.; Wang, J.; Huang, J.P.; Zhou, W.; Yang, C.; Lan, X.; Chen, M.; Huang, S.X.; Kai, G.; et al. Functional Genomics Analysis Reveals Two Novel Genes Required for Littorine Biosynthesis. New Phytologist 2020, 225, 1906–1914. [Google Scholar] [CrossRef]
- Zhao, K.; Zeng, J.; Zhao, T.; Zhang, H.; Qiu, F.; Yang, C.; Zeng, L.; Liu, X.; Chen, M.; Lan, X.; et al. Enhancing Tropane Alkaloid Production Based on the Functional Identification of Tropine-Forming Reductase in Scopolia Lurida, a Tibetan Medicinal Plant. Front. Plant Sci. 2017, 8, 1745. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Li, W.; Zhang, J.; Huang, F.; Yang, J.; Bei, Y.; Lu, Y. De Novo Transcriptome Sequencing in Frankliniella Occidentalis to Identify Genes Involved in Plant Virus Transmission and Insecticide Resistance. Genomics 2013, 101, 296–305. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Feng, Z.K.; Zhang, Z.J.; Liu, Y.-B.; Tao, X.R. Complete Genome Sequence of a Tomato Spotted Wilt Virus Isolate from China and Comparison to Other TSWV Isolates of Different Geographic Origin. Arch. Virol. 2011, 156, 1905–1908. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wu, Q.J.; Li, X.F.; Zhang, Y.J.; Xu, B.Y.; Zhu, G.R. Life History of Western Flower Thrips, Frankliniella Occidentalis (Thysan., Thripae), on Five Different Vegetable Leaves. J. Appl. Entomol. 2007, 131, 347–354. [Google Scholar] [CrossRef]
- Jakabová, S.; Vincze, L.; Farkas, Á.; Kilár, F.; Boros, B.; Felinger, A. Determination of Tropane Alkaloids Atropine and Scopolamine by Liquid Chromatography–Mass Spectrometry in Plant Organs of Datura Species. J. Chromatogr. A 2012, 1232, 295–301. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, P.C.J.; Mollema, C.; Steenhuis-Broers, G.M. Comparative Life History Studies of Frankliniella Occidentalis and Thrips Tabaci (Thysanoptera: Thripidae) on Cucumber. Bull. Entomol. Res. 1995, 85, 285–297. [Google Scholar] [CrossRef]
- Soria, C.; Mollema, C. Life-History Parameters of Western Flower Thrips on Susceptible and Resistant Cucumber Genotypes. Entomol. Exp. Appl. 1995, 74, 177–184. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Chi, H. Demographic Comparison of Sweetpotato Weevil Reared on a Major Host, Ipomoea Batatas, and an Alternative Host, I. triloba. Sci. Rep. 2015, 5, 11871. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, Two-Sex Life Table: An Introduction to Theory, Data Analysis, and Application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H.; Guncan, A.; Kavousi, A.; Gholamhossein, G.; Atlıhan, R.; Özgökçe, M.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Taghizadeh, R. TWOSEX-MSChart: The Key Tool for Life Table Research and Education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MS Chart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis 2023. Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart.rar (accessed on 15 July 2023).
- Akköprü, E.P.; Atlıhan, R.; Okut, H.; Chi, H. Demographic Assessment of Plant Cultivar Resistance to Insect Pests: A Case Study of the Dusky-Veined Walnut Aphid (Hemiptera: Callaphididae) on Five Walnut Cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla Chinensis (Hemiptera: Psyllidae) Reared on Four Cultivars of Pyrus Bretschneideri (Rosales: Rosaceae) and P. Communis Pears With Estimations of Confidence Intervals of Specific Life Table Statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and Population Projection of Aphis Fabae (Hemiptera: Aphididae): With Additional Comments on Life Table Research Criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table 2023. Available online: http://140.120.197.173/Ecology/Download/Timing-MSChart.rar (accessed on 15 July 2023).
- Chi, H. Timing of Control Based on the Stage Structure of Pest Populations: A Simulation Approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]


| Treatment (mg/g) | Atropine (%) | Scopolamine (%) | Atropine + Scopolamine (%) |
|---|---|---|---|
| CK | 16.04 ± 1.09 a | ||
| 0.07 | 35.18 ± 2.73 b | 36.79 ± 2.43 b | 42.05 ± 2.54 b |
| 0.7 | 40.63 ± 4.65 b | 44.79 ± 3.91 b | 43.75 ± 2.88 b |
| 7 | 64.06 ± 5.99 c | 66.07 ± 3.54 c | 62.50 ± 5.55 c |
| Negative | 77.08 ± 4.31 c | ||
| Positive | 94.79 ± 2.41 d | ||
| Treatment | N | Intrinsic Rate of Increase rm (/d−1) | Finite Rate of Increase λ (/d−1) | Net Reproduction Rate R0 (Offspring/Individual) | Mean Generation Time T (d) |
|---|---|---|---|---|---|
| TSWV non-infected | 107 | 0.013 ± 0.017 b | 1.09 ± 0.018 b | 11.11 ± 0.33 b | 18.24 ± 2.06 a |
| TSWV-infected | 93 | 0.178 ± 0.013 a | 1.58 ± 0.014 a | 40.32 ± 0.96 a | 18.39 ± 0.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, J.; Li, X.; Zhang, J.; Wang, Y.; Lu, Y. The Plant Virus Tomato Spotted Wilt Orthotospovirus Benefits Its Vector Frankliniella occidentalis by Decreasing Plant Toxic Alkaloids in Host Plant Datura stramonium. Int. J. Mol. Sci. 2023, 24, 14493. https://doi.org/10.3390/ijms241914493
Zhang Z, Zhang J, Li X, Zhang J, Wang Y, Lu Y. The Plant Virus Tomato Spotted Wilt Orthotospovirus Benefits Its Vector Frankliniella occidentalis by Decreasing Plant Toxic Alkaloids in Host Plant Datura stramonium. International Journal of Molecular Sciences. 2023; 24(19):14493. https://doi.org/10.3390/ijms241914493
Chicago/Turabian StyleZhang, Zhijun, Jiahui Zhang, Xiaowei Li, Jinming Zhang, Yunsheng Wang, and Yaobin Lu. 2023. "The Plant Virus Tomato Spotted Wilt Orthotospovirus Benefits Its Vector Frankliniella occidentalis by Decreasing Plant Toxic Alkaloids in Host Plant Datura stramonium" International Journal of Molecular Sciences 24, no. 19: 14493. https://doi.org/10.3390/ijms241914493
APA StyleZhang, Z., Zhang, J., Li, X., Zhang, J., Wang, Y., & Lu, Y. (2023). The Plant Virus Tomato Spotted Wilt Orthotospovirus Benefits Its Vector Frankliniella occidentalis by Decreasing Plant Toxic Alkaloids in Host Plant Datura stramonium. International Journal of Molecular Sciences, 24(19), 14493. https://doi.org/10.3390/ijms241914493







