The Effect of Maternal Exposure to a Diet High in Fats and Cholesterol on the Placental Function and Phenotype of the Offspring in a Rabbit Model: A Summary Review of About 15 Years of Research
Abstract
:1. Introduction
2. The Rabbit Model
3. The High-Cholesterol and High-Fat Diet (H Diet)
4. Effects of the H Diet on the Phenotype of Female Does (F0)
5. Effects of the H Diet on the Embryo
6. Effects of the H Diet on Feto-Placental Development
7. Effects of a Maternal Diet Enriched with Cholesterol and Fat on the Post-Natal Phenotype of F1 Offspring
7.1. Cardiometabolic Phenotype
7.2. Gonadal Phenotype
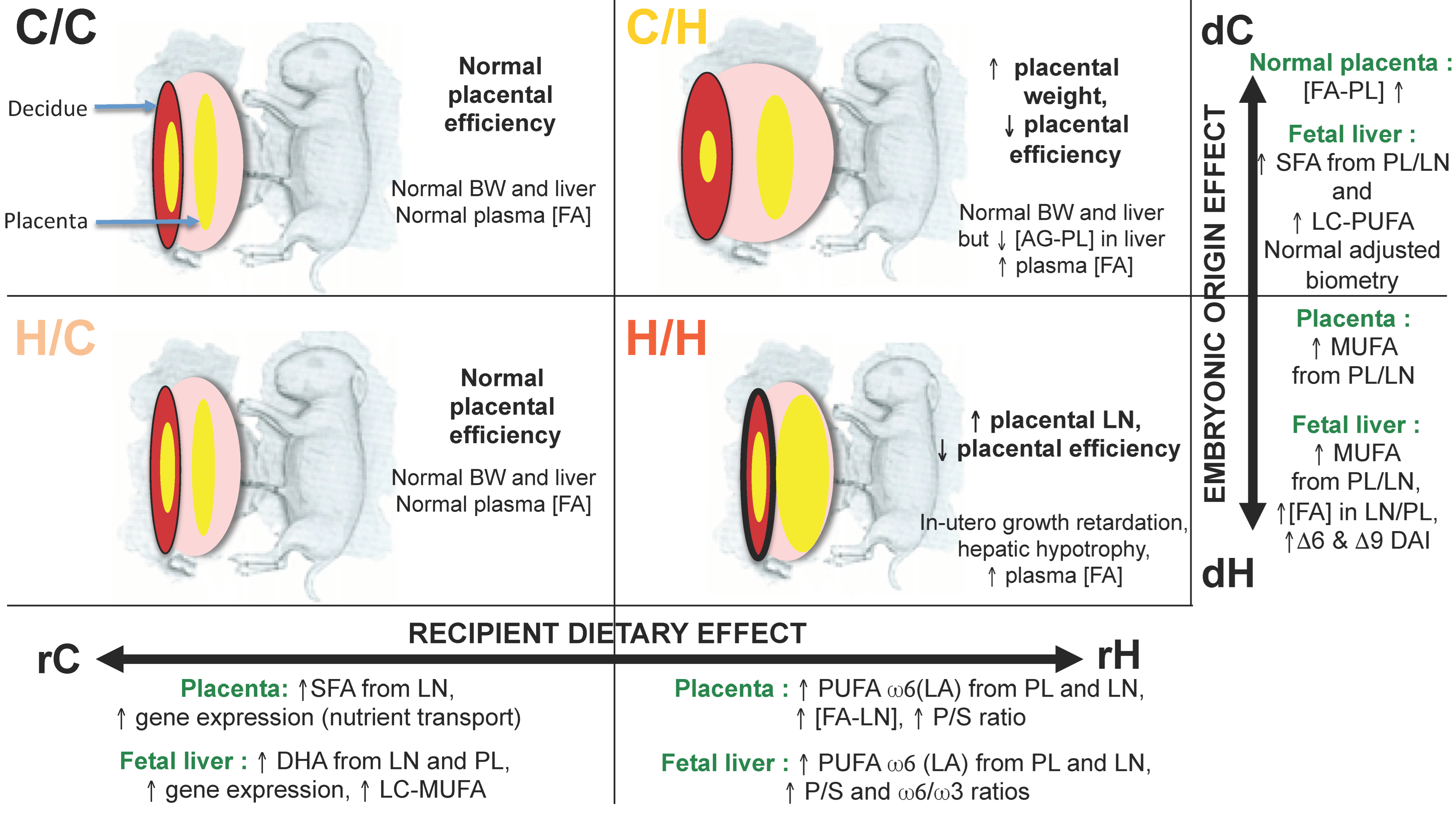
8. Identification of the Exposure Windows Responsible for the Fetal-Placental Phenotype: What Is the Importance of the Preconception and Gestation Windows?
8.1. Feto-Placental Phenotype
8.2. Quantification and Profiles of Placental FA
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Armitage, J.A.; Taylor, P.D.; Poston, L. Experimental models of developmental programming: Consequences of exposure to an energy rich diet during development. J. Physiol. 2005, 565, 3–8. [Google Scholar] [CrossRef]
- Dubuisson, C.; Lioret, S.; Touvier, M.; Dufour, A.; Calamassi-Tran, G.; Volatier, J.L.; Lafay, L. Trends in food and nutritional intakes of French adults from 1999 to 2007: Results from the INCA surveys. Br. J. Nutr. 2010, 103, 1035–1048. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic acid: Between doubts and certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. [Google Scholar] [CrossRef]
- Kritchevsky, D. History of recommendations to the public about dietary fat. J. Nutr. 1998, 128, 449S–452S. [Google Scholar] [CrossRef] [PubMed]
- Massiera, F.; Barbry, P.; Guesnet, P.; Joly, A.; Luquet, S.; Moreilhon-Brest, C.; Mohsen-Kanson, T.; Amri, E.Z.; Ailhaud, G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J. Lipid Res. 2010, 51, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, P.; Kose, J.; Fezeu, L.K.; Baudry, J.; Kesse-Guyot, E.; Julia, C.; Galan, P.; Péneau, S.; Oppert, J.M.; Hercberg, S.; et al. Determinants and consequences of obesity—Contribution of the French NutriNet-Santé cohort. Cah. Nutr. Diet. 2023, 58, 96–110. [Google Scholar] [CrossRef]
- Inserm; Kantar Health; Roche. Enquête Épidémiologique Nationale sur le Surpoids et L’obésité. ObEpi 2012. Available online: https://presse.inserm.fr/wp-content/uploads/2012/10/obepi_2012.pdf (accessed on 28 August 2023).
- Langley-Evans, S.C. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009, 215, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D.; Chavatte-Palmer, P. Review shows that maternal obesity induces serious adverse neonatal effects and is associated with childhood obesity in their offspring. Acta Paediatr. 2018, 107, 1156–1165. [Google Scholar] [CrossRef]
- Harmancıoğlu, B.; Kabaran, S. Maternal high fat diets: Impacts on offspring obesity and epigenetic hypothalamic programming. Front. Genet. 2023, 14, 1158089. [Google Scholar] [CrossRef]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am. J. Hum. Biol. 2007, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Kwong, W.Y.; Porter, R.; Ursell, E.; Fesenko, I.; Wilkins, A.; Miller, D.J.; Watkins, A.J.; Eckert, J.J. The embryo and its future. Biol. Reprod. 2004, 71, 1046–1054. [Google Scholar] [CrossRef]
- Shankar, K.; Harrell, A.; Liu, X.; Gilchrist, J.M.; Ronis, M.J.; Badger, T.M. Maternal obesity at conception programs obesity in the offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R528–R538. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Ursell, E.; Panton, R.; Papenbrock, T.; Hollis, L.; Cunningham, C.; Wilkins, A.; Perry, V.H.; Sheth, B.; Kwong, W.Y.; et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol. Reprod. 2008, 78, 299–306. [Google Scholar] [CrossRef]
- Lane, M.; Zander-Fox, D.L.; Robker, R.L.; McPherson, N.O. Peri-conception parental obesity, reproductive health, and transgenerational impacts. Trends Endocrinol. Metab. 2015, 26, 84–90. [Google Scholar] [CrossRef]
- Stang, J.; Huffman, L.G. Position of the Academy of Nutrition and Dietetics: Obesity, Reproduction, and Pregnancy Outcomes. J. Acad. Nutr. Diet. 2016, 116, 677–691. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Wu, Y.; Zhang, D.; Huang, H. Association between abnormal body mass index and pregnancy outcomes in patients following frozen embryo transfer: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, H.K.; Fleming, A.; Frey, D.; Barsoom, M.; Satpathy, C.; Khandalavala, J. Maternal obesity and pregnancy. Postgrad. Med. 2008, 120, E01–E09. [Google Scholar] [CrossRef] [PubMed]
- Smedts, H.P.; Rakhshandehroo, M.; Verkleij-Hagoort, A.C.; de Vries, J.H.; Ottenkamp, J.; Steegers, E.A.; Steegers-Theunissen, R.P. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects. Eur. J. Nutr. 2008, 47, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wax, J.R. Risks and management of obesity in pregnancy: Current controversies. Curr. Opin. Obstet. Gynecol. 2009, 21, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Panchenko, P.E.; Lacroix, M.C.; Jouin, M.; Voisin, S.; Badonnel, K.; Lemaire, M.; Meunier, N.; Safi-Stibler, S.; Persuy, M.A.; Jouneau, L.; et al. Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice. Nutrients 2019, 11, 948. [Google Scholar] [CrossRef]
- Ortiz, M.; Álvarez, D.; Muñoz, Y.; Crisosto, N.; Valenzuela, R.; Maliqueo, M. Linoleic and arachidonic fatty acids and their potential relationship with inflammation, pregnancy, and fetal development. Curr. Med. Chem. 2023. [Google Scholar] [CrossRef]
- Tarrade, A.; Panchenko, P.; Junien, C.; Gabory, A. Placental contribution to nutritional programming of health and diseases: Epigenetics and sexual dimorphism. J. Exp. Biol. 2015, 218, 50–58. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Staud, F.; Karahoda, R. Trophoblast: The central unit of fetal growth, protection and programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. Review: The placenta and developmental programming: Balancing fetal nutrient demands with maternal resource allocation. Placenta 2012, 33, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Rousseau-Ralliard, D.; Aubriere, M.C.; Peynot, N.; Dahirel, M.; Bertrand-Michel, J.; Aguirre-Lavin, T.; Morel, O.; Beaujean, N.; Duranthon, V.; et al. Sexual dimorphism of the feto-placental phenotype in response to a high fat and control maternal diets in a rabbit model. PLoS ONE 2013, 8, e83458. [Google Scholar] [CrossRef]
- Picone, O.; Laigre, P.; Fortun-Lamothe, L.; Archilla, C.; Peynot, N.; Ponter, A.; Berthelot, V.; Cordier, A.-G.; Duranthon, V.; Chavatte-Palmer, P. Hyperlipidic hypercholesterolemic diet in prepubertal rabbits affects gene expression in the embryo, restricts fetal growth and increases offspring susceptibility to obesity. Theriogenology 2011, 75, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Cordier, A.G.; Léveillé, P.; Dupont, C.; Tarrade, A.; Picone, O.; Larcher, T.; Dahirel, M.; Poumerol, E.; Mandon-Pepin, B.; Lévy, R.; et al. Dietary lipid and cholesterol induce ovarian dysfunction and abnormal LH response to stimulation in rabbits. PLoS ONE 2013, 8, e63101. [Google Scholar] [CrossRef]
- Dupont, C.; Cordier, A.G.; Junien, C.; Mandon-Pepin, B.; Levy, R.; Chavatte-Palmer, P. Maternal environment and the reproductive function of the offspring. Theriogenology 2012, 78, 1405–1414. [Google Scholar] [CrossRef]
- Léveillé, P.; Tarrade, A.; Dupont, C.; Larcher, T.; Dahirel, M.; Poumerol, E.; Cordier, A.G.; Picone, O.; Mandon-Pepin, B.; Jolivet, G.; et al. Maternal high-fat diet induces follicular atresia but does not affect fertility in adult rabbit offspring. J. Dev. Orig. Health Dis. 2014, 5, 88–97. [Google Scholar] [CrossRef]
- Rousseau-Ralliard, D.; Aubrière, M.C.; Daniel, N.; Dahirel, M.; Morin, G.; Prézelin, A.; Bertrand, J.; Rey, C.; Chavatte-Palmer, P.; Couturier-Tarrade, A. Importance of Windows of Exposure to Maternal High-Fat Diet and Feto-Placental Effects: Discrimination Between Pre-conception and Gestational Periods in a Rabbit Model. Front. Physiol. 2021, 12, 784268. [Google Scholar] [CrossRef]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef]
- Yoshinaga, K. A sequence of events in the uterus prior to implantation in the mouse. J. Assist. Reprod. Genet. 2013, 30, 1017–1022. [Google Scholar] [CrossRef]
- Calderari, S.; Daniel, N.; Mourier, E.; Richard, C.; Dahirel, M.; Lager, F.; Marchiol, C.; Renault, G.; Gatien, J.; Nadal-Desbarats, L.; et al. Metabolomic differences in blastocoel and uterine fluids collected in vivo by ultrasound biomicroscopy on rabbit embryos. Biol. Reprod. 2021, 104, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Animal models of human pregnancy and placentation: Alternatives to the mouse. Reproduction 2020, 160, R129–R143. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.F. Electron microscopy of the uterine epithelium in the rabbit. J. Cell Biol. 1962, 14, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Laigre, P.; Simonoff, E.; Chesne, P.; Challah-Jacques, M.; Renard, J.P. In utero characterisation of fetal growth by ultrasound scanning in the rabbit. Theriogenology 2008, 69, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, E.; Morel, O.; Tarrade, A.; Dahirel, M.; Bonneau, M.; Gayat, E.; Evain-Brion, D.; Chavatte-Palmer, P.; Tsatsaris, V. Quantification of utero-placental vascularization in a rabbit model of IUGR with three-dimensional power Doppler angiography. Placenta 2012, 33, 769–775. [Google Scholar] [CrossRef]
- Polisca, A.; Scotti, L.; Orlandi, R.; Brecchia, G.; Boiti, C. Doppler evaluation of maternal and fetal vessels during normal gestation in rabbits. Theriogenology 2010, 73, 358–366. [Google Scholar] [CrossRef]
- Napoli, C.; Witztum, J.L.; Calara, F.; de Nigris, F.; Palinski, W. Maternal hypercholesterolemia enhances atherogenesis in normocholesterolemic rabbits, which is inhibited by antioxidant or lipid-lowering intervention during pregnancy: An experimental model of atherogenic mechanisms in human fetuses. Circ. Res. 2000, 87, 946–952. [Google Scholar] [CrossRef]
- Li, X.F.; Lin, Y.S.; Kinsey-Jones, J.S.; O’Byrne, K.T. High-fat diet increases LH pulse frequency and kisspeptin-neurokinin B expression in puberty-advanced female rats. Endocrinology 2012, 153, 4422–4431. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Jagannathan, L.; Mahaley, R.E.; Subramanian, M.; Gilbreath, E.T.; Mohankumar, P.S.; Mohankumar, S.M. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J. Neuroendocrinol. 2012, 24, 748–755. [Google Scholar] [CrossRef]
- Lund, S.A.; Murdoch, J.; Van Kirk, E.A.; Murdoch, W.J. Mitogenic and antioxidant mechanisms of estradiol action in preovulatory ovine follicles: Relevance to luteal function. Biol. Reprod. 1999, 61, 388–392. [Google Scholar] [CrossRef]
- El-Sayyad, H.I.H.; El-Shershaby, E.M.F.; El-Mansi, A.A.; El-Ashry, N.E. Anti-hypercholesterolemic impacts of barley and date palm fruits on the ovary of Wistar albino rats and their offspring. Reprod. Biol. 2018, 18, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Larigauderie, G.; Furman, C.; Jaye, M.; Lasselin, C.; Copin, C.; Fruchart, J.C.; Castro, G.; Rouis, M. Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: Potential role in atherogenesis. Arter. Thromb. Vasc. Biol. 2004, 24, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Gan, J.W.; Brzostowski, J.; Parisi, M.J.; Schultz, C.J.; Londos, C.; Oliver, B.; Kimmel, A.R. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 2002, 277, 32253–32257. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.L.; Van Hoeck, V.; Clemente, M.; Rizos, D.; Gutierrez-Adan, A.; Van Soom, A.; Uytterhoeven, M.; Bols, P.E. The effect of nutritionally induced hyperlipidaemia on in vitro bovine embryo quality. Hum. Reprod. 2010, 25, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Larigauderie, G.; Cuaz-Perolin, C.; Younes, A.B.; Furman, C.; Lasselin, C.; Copin, C.; Jaye, M.; Fruchart, J.C.; Rouis, M. Adipophilin increases triglyceride storage in human macrophages by stimulation of biosynthesis and inhibition of beta-oxidation. FEBS J. 2006, 273, 3498–3510. [Google Scholar] [CrossRef]
- Calderari, S.; Archilla, C.; Jouneau, L.; Daniel, N.; Peynot, N.; Dahirel, M.; Richard, C.; Mourier, E.; Schmaltz-Panneau, B.; Vitorino Carvalho, A.; et al. Alteration of the embryonic microenvironment and sex-specific responses of the preimplantation embryo related to a maternal high-fat diet in the rabbit model. J. DOHaD 2023, in press. [Google Scholar]
- Bausenwein, J.; Serke, H.; Eberle, K.; Hirrlinger, J.; Jogschies, P.; Hmeidan, F.A.; Blumenauer, V.; Spanel-Borowski, K. Elevated levels of oxidized low-density lipoprotein and of catalase activity in follicular fluid of obese women. Mol. Hum. Reprod. 2010, 16, 117–124. [Google Scholar] [CrossRef]
- Wehrman, M.E.; Welsh, T.H., Jr.; Williams, G.L. Diet-induced hyperlipidemia in cattle modifies the intrafollicular cholesterol environment, modulates ovarian follicular dynamics, and hastens the onset of postpartum luteal activity. Biol. Reprod. 1991, 45, 514–522. [Google Scholar] [CrossRef]
- Burke, K.T.; Colvin, P.L.; Myatt, L.; Graf, G.A.; Schroeder, F.; Woollett, L.A. Transport of maternal cholesterol to the fetus is affected by maternal plasma cholesterol concentrations in the golden Syrian hamster. J. Lipid Res. 2009, 50, 1146–1155. [Google Scholar] [CrossRef]
- Aoun, M.; Feillet-Coudray, C.; Fouret, G.; Chabi, B.; Crouzier, D.; Ferreri, C.; Chatgilialoglu, C.; Wrutniak-Cabello, C.; Cristol, J.P.; Carbonneau, M.A.; et al. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: Effect of different nutritional lipid patterns. Br. J. Nutr. 2012, 107, 647–659. [Google Scholar] [CrossRef]
- Navarrete Santos, A.; Tonack, S.; Kirstein, M.; Kietz, S.; Fischer, B. Two insulin-responsive glucose transporter isoforms and the insulin receptor are developmentally expressed in rabbit preimplantation embryos. Reproduction 2004, 128, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Ramin, N.; Thieme, R.; Fischer, S.; Schindler, M.; Schmidt, T.; Fischer, B.; Navarrete Santos, A. Maternal diabetes impairs gastrulation and insulin and IGF-I receptor expression in rabbit blastocysts. Endocrinology 2010, 151, 4158–4167. [Google Scholar] [CrossRef] [PubMed]
- Glazier, J.D.; Cetin, I.; Perugino, G.; Ronzoni, S.; Grey, A.M.; Mahendran, D.; Marconi, A.M.; Pardi, G.; Sibley, C.P. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr. Res. 1997, 42, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Ylven, K.; Wennergren, M.; Powell, T.L. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta 2002, 23, 392–399. [Google Scholar] [CrossRef]
- Tajaddini, A.; Kendig, M.D.; Prates, K.V.; Westbrook, R.F.; Morris, M.J. Male Rat Offspring Are More Impacted by Maternal Obesity Induced by Cafeteria Diet than Females-Additive Effect of Postweaning Diet. Int. J. Mol. Sci. 2022, 23, 1442. [Google Scholar] [CrossRef]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.F.; Burcelin, R.; Gourdy, P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilsson, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef]
- Shrestha, N.; Ezechukwu, H.C.; Holland, O.J.; Hryciw, D.H. Developmental programming of peripheral diseases in offspring exposed to maternal obesity during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R507–R516. [Google Scholar] [CrossRef]
- Shrestha, N.; Melvin, S.D.; McKeating, D.R.; Holland, O.J.; Cuffe, J.S.M.; Perkins, A.V.; McAinch, A.J.; Hryciw, D.H. Sex-Specific Differences in Lysine, 3-Hydroxybutyric Acid and Acetic Acid in Offspring Exposed to Maternal and Postnatal High Linoleic Acid Diet, Independent of Diet. Int. J. Mol. Sci. 2021, 22, 10223. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, J.; Chen, M.; Huang, J.; Liu, J.; Wei, X.; Jia, R.; Song, L.; Sun, B.; Luo, X.; et al. Sex-Specific Effects of Maternal and Post-Weaning High-Fat Diet on Adipose Tissue Remodeling and Asprosin Expression in Mice Offspring. Mol. Nutr. Food Res. 2022, 66, e2100470. [Google Scholar] [CrossRef]
- Scheidl, T.B.; Wager, J.L.; Baker, L.G.; Brightwell, A.L.; Melan, K.M.; Larion, S.; Sarr, O.; Regnault, T.R.; Urbanski, S.J.; Thompson, J. High Maternal Adiposity during Pregnancy Programs an Imbalance in the Lipidome and Predisposes to Diet-induced Hepatosteatosis in the Offspring. Biosci. Rep. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.K.; Buyukdere, Y.; Gulec, A.; Akyol, A. Taurine supplementation reduces adiposity and hepatic lipid metabolic activity in adult offspring following maternal cafeteria diet. Nutr. Res. 2023, 117, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Rideout, T.C.; Andreani, G.A.; Pembroke, J.; Choudhary, D.; Browne, R.W.; Mahmood, S.; Patel, M.S. Maternal Pea Protein Intake Provides Sex-Specific Protection against Dyslipidemia in Offspring from Obese Pregnancies. Nutrients 2023, 15, 867. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Guiducci, L.; Guzzardi, M.A.; Cacciato Insilla, A.; Burchielli, S.; Brunetto, M.R.; Bonino, F.; Campani, D.; Iozzo, P. Maternal High-Fat Feeding Affects the Liver and Thymus Metabolic Axis in the Offspring and Some Effects Are Attenuated by Maternal Diet Normalization in a Minipig Model. Metabolites 2021, 11, 800. [Google Scholar] [CrossRef]
- Lin, X.H.; Gao, L.; Tian, S.; Klausen, C.; Guo, M.X.; Gao, Q.; Liu, M.E.; Wang, H.; Wu, D.D.; Zhou, C.L.; et al. Maternal high-fat-diet exposure is associated with elevated blood pressure and sustained increased leptin levels through epigenetic memory in offspring. Sci. Rep. 2021, 11, 316. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef]
- Kelsey, T.W.; Wallace, W.H. Ovarian volume correlates strongly with the number of nongrowing follicles in the human ovary. Obstet. Gynecol. Int. 2012, 2012, 305025. [Google Scholar] [CrossRef]
- Wu, L.L.; Dunning, K.R.; Yang, X.; Russell, D.L.; Lane, M.; Norman, R.J.; Robker, R.L. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 2010, 151, 5438–5445. [Google Scholar] [CrossRef]
- Wei, W.; Qin, F.; Gao, J.; Chang, J.; Pan, X.; Jiang, X.; Che, L.; Zhuo, Y.; Wu, D.; Xu, S. The effect of maternal consumption of high-fat diet on ovarian development in offspring. Anim. Reprod. Sci. 2023, 255, 107294. [Google Scholar] [CrossRef]
- Lee, W.; Xu, M.; Li, Y.; Gu, Y.; Chen, J.; Wong, D.; Fung, P.C.; Shen, J. Free cholesterol accumulation impairs antioxidant activities and aggravates apoptotic cell death in menadione-induced oxidative injury. Arch. Biochem. Biophys. 2011, 514, 57–67. [Google Scholar] [CrossRef]
- Hue-Beauvais, C.; Chavatte-Palmer, P.; Aujean, E.; Dahirel, M.; Laigre, P.; Pechoux, C.; Bouet, S.; Devinoy, E.; Charlier, M. An obesogenic diet started before puberty leads to abnormal mammary gland development during pregnancy in the rabbit. Dev. Dyn. 2011, 240, 347–356. [Google Scholar] [CrossRef]
- Da Silva, P.; Aitken, R.P.; Rhind, S.M.; Racey, P.A.; Wallace, J.M. Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs. Reproduction 2001, 122, 375–383. [Google Scholar] [CrossRef]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Toth, T.L.; Wright, D.L.; Meeker, J.D.; Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 2010, 93, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Faure, C.; Sermondade, N.; Boubaya, M.; Eustache, F.; Clément, P.; Briot, P.; Berthaut, I.; Levy, V.; Cedrin-Durnerin, I.; et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J. Androl. 2013, 15, 622–625. [Google Scholar] [CrossRef]
- Bakos, H.W.; Mitchell, M.; Setchell, B.P.; Lane, M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int. J. Androl. 2011, 34, 402–410. [Google Scholar] [CrossRef]
- Daniel-Carlier, N.; Harscoët, E.; Thépot, D.; Auguste, A.; Pailhoux, E.; Jolivet, G. Gonad differentiation in the rabbit: Evidence of species-specific features. PLoS ONE 2013, 8, e60451. [Google Scholar] [CrossRef]
- Chavatte-Palmer, P.; Dupont, C.; Debus, N.; Camous, S. Nutritional programming and the reproductive function of the offspring. Anim. Prod. Sci. 2014, 54, 1166–1176. [Google Scholar] [CrossRef]
- Kasper, P.; Selle, J.; Vohlen, C.; Wilke, R.; Kuiper-Makris, C.; Klymenko, O.; Bae-Gartz, I.; Schömig, C.; Quaas, A.; Schumacher, B.; et al. Perinatal Obesity Induces Hepatic Growth Restriction with Increased DNA Damage Response, Senescence, and Dysregulated Igf-1-Akt-Foxo1 Signaling in Male Offspring of Obese Mice. Int. J. Mol. Sci. 2022, 23, 5609. [Google Scholar] [CrossRef]
- Zambrano, E.; Rodríguez-González, G.L.; Reyes-Castro, L.A.; Bautista, C.J.; Castro-Rodríguez, D.C.; Juárez-Pilares, G.; Ibáñez, C.A.; Hernández-Rojas, A.; Nathanielsz, P.W.; Montaño, S.; et al. DHA Supplementation of Obese Rats throughout Pregnancy and Lactation Modifies Milk Composition and Anxiety Behavior of Offspring. Nutrients 2021, 13, 4243. [Google Scholar] [CrossRef] [PubMed]
- Andreu, A.; Casals, G.; Vinagre, I.; Flores, L. Obesity management in women of reproductive age. Endocrinol. Diabetes Nutr. 2023, 70 (Suppl. 1), 85–94. [Google Scholar] [CrossRef]
- Kusuyama, J.; Makarewicz, N.S.; Albertson, B.G.; Alves-Wagner, A.B.; Conlin, R.H.; Prince, N.B.; Alves, C.R.R.; Ramachandran, K.; Kozuka, C.; Xiudong, Y.; et al. Maternal Exercise-Induced SOD3 Reverses the Deleterious Effects of Maternal High-Fat Diet on Offspring Metabolism through Stabilization of H3K4me3 and Protection against WDR82 Carbonylation. Diabetes 2022, 71, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Liu, S.; Kiyoi, T.; Mogi, M.; Sugiyama, T. Long-term impact of maternal dietary intervention on metabolic homeostasis in male offspring in mice. J. Nutr. Biochem. 2022, 104, 108971. [Google Scholar] [CrossRef] [PubMed]
- Hieronimus, B.; Ensenauer, R. Influence of maternal and paternal pre-conception overweight/obesity on offspring outcomes and strategies for prevention. Eur. J. Clin. Nutr. 2021, 75, 1735–1744. [Google Scholar] [CrossRef]
- Legro, R.S. Preconception weight loss in the obese patient: Overhyped or underutilized? Fertil. Steril. 2022, 118, 431–433. [Google Scholar] [CrossRef]
- Satokar, V.V.; Derraik, J.G.B.; Harwood, M.; Okesene-Gafa, K.; Beck, K.; Cameron-Smith, D.; Garg, M.L.; O’Sullivan, J.M.; Sundborn, G.; Pundir, S.; et al. Fish oil supplementation during pregnancy and postpartum in mothers with overweight and obesity to improve body composition and metabolic health during infancy: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2023, 117, 883–895. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kwon, Y.H. Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet. Lipids Health Dis. 2023, 22, 50. [Google Scholar] [CrossRef]

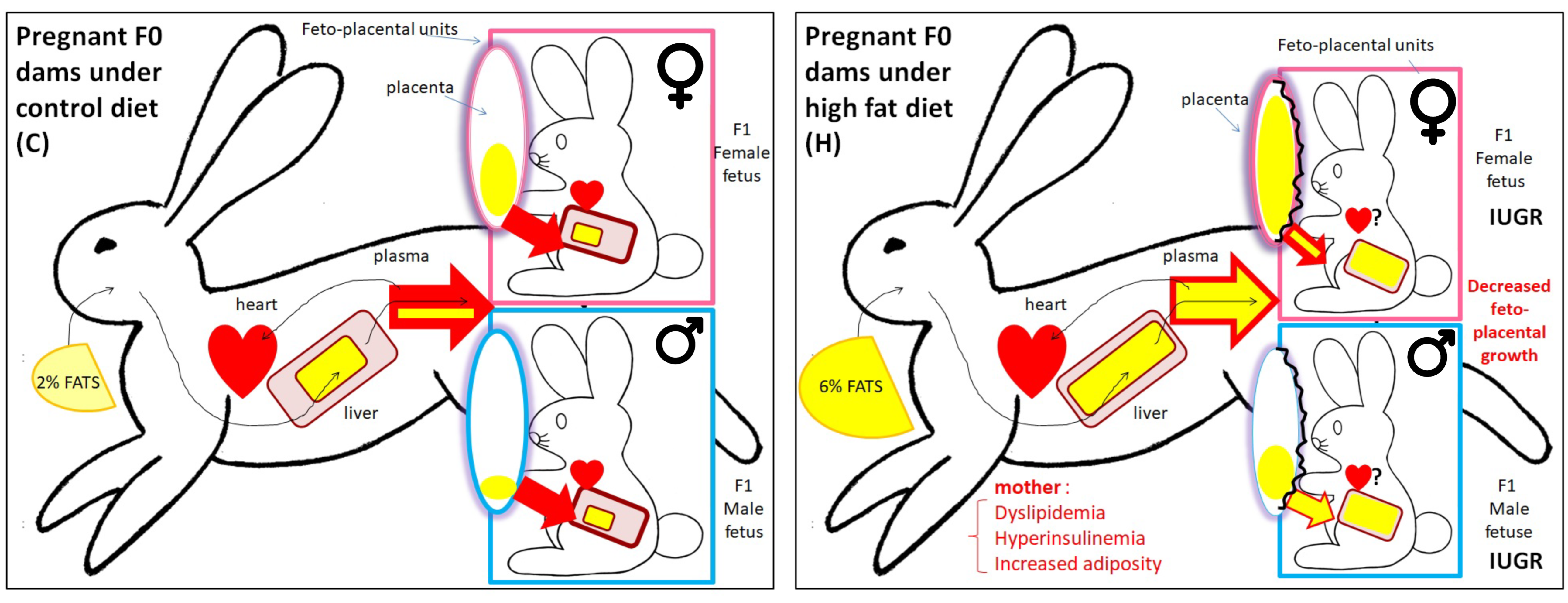

| F1 Male Offspring | F1 Female Offspring | |
|---|---|---|
| Prenatal period F0 does were exposed to H or C diet from 10 weeks of age until 28 d.p.c. to obtain F1 feto-placental unit | Blood biochemical parameters (n = 12): 🡭 total cholesterol in H vs. C (p < 0.05) 🡭 of FA in H vs. C (p < 0.01) 🡭 MUFA in H vs. C (p < 0.05) 🡭 PUFA in H vs. C (p < 0.001) | Blood biochemical parameters (n =11): - - - 🡭 PUFA in H vs. C (p < 0.01) |
| Hepatic FA profiles (n = 6): 🡭 of FA storage in H vs. C (p < 0.001) 🡭 (p < 0.05) PUFA (p < 0.001) storage in H vs. C | Hepatic FA profiles (n = 6): 🡭 of FA storage in H vs. C (p < 0.01) 🡭 (p < 0.05) PUFA (p < 0.001) storage in H vs. C | |
| Placental lipid profiles (n = 5): 🡭 of total cholesteryl esters in H vs. C (p < 0.01) 🡭 of triacylglycerol in H vs. C (p < 0.05) FA placental membranes: - - - FA placental storage: 🡭 of total FA in H vs. C (p < 0.01) - - 🡭 of total PUFA in H vs. C (p < 0.01) | Placental lipid profiles (n = 5): 🡭 of total cholesteryl esters in H vs. C (p < 0.01) - FA placental membranes: 🡭 of FA (p < 0.01), SFA (p < 0.01), MUFA (p < 0.01) and PUFA (p < 0.01) in H females compared to H males 🡭 of PUFA in H vs. C (p < 0.01) FA placental storage: 🡭 of total FA in H vs. C (p < 0.05) 🡭 of total FA (p < 0.05), SFA (p < 0.05), MUFA (p < 0.05) and PUFA (p < 0.01) in H females compared to H males 🡭 of total PUFA in H vs. C (p < 0.01) | |
| Postnatal period | Gonadal phenotype at 37 weeks of age (n = 8H-H and 7C-C) * Lighter testes and epididymes in H-H vs. C-C (p = 0.03 and 0.015, respectively) No significant differences in sperm concentration, sperm DNA integrity and sperm membrane composition 🡶 of plasma-free testosterone concentrations in H-H vs. C-C (p = 0.05) | Gonadal phenotype at adulthood (n = 7C-Caw, 14H-Caw, 9H-Caw, 8H-Haw) # 🡶 of width of the ovaries and ovarian longitudinal surface in H-Haw vs. H-Caw group (p < 0.05) 🡶 of ovarian longitudinal surface in H-Haw vs. C-Caw group (p = 0.03) No significant differences in the number of primordial, primary and secondary follicles between groups. 🡭 of atretic follicle number in H-Caw, C-Haw (p < 0.001) and H-Haw (p < 0.01) ovaries compared to the C-Caw group No significant differences of the number of atretic follicles between H-Haw, H-Caw and C-Haw groups Positive correlation between follicular atresia and plasma total cholesterol (p = 0.03) or triglyceride concentration (p = 0.01) Negative correlation between follicular atresia and plasma testosterone (p = 0.02) |
| Postnatal period | Cardiometabolic phenotype (n = 26) $ At 1 month of age (weaning period), 🡭 of total cholesterol concentration in C-H vs. H-H group (p < 0.05) 🡭 of total cholesterol concentration in C-H and H-H vs. C-C and H-C groups (p < 0.05) 🡭 plasma triglyceride concentrations in H-H vs. H-C and C-C groups (p < 0.05) At 3 months of age, of weight 🡭 of the H-H and H-C vs. C-C and C-H groups (p < 0.0001) 🡭 of weight in C-H g vs. C-C offspring (p < 0.05) At 6 months of age, 🡭 H-H and H-C offspring vs. C-C and C-H groups (p < 0.05) At 6 months of age, all offspring were exposed to the H diet for 3 weeks. At the end of the challenge, 🡭—systolic, diastolic, and mean blood pressure in C-C, C-H, and H-C offspring (p < 0.01) 🡭 mean arterial pressure in H-H animals (p < 0.05) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau-Ralliard, D.; Chavatte-Palmer, P.; Couturier-Tarrade, A. The Effect of Maternal Exposure to a Diet High in Fats and Cholesterol on the Placental Function and Phenotype of the Offspring in a Rabbit Model: A Summary Review of About 15 Years of Research. Int. J. Mol. Sci. 2023, 24, 14547. https://doi.org/10.3390/ijms241914547
Rousseau-Ralliard D, Chavatte-Palmer P, Couturier-Tarrade A. The Effect of Maternal Exposure to a Diet High in Fats and Cholesterol on the Placental Function and Phenotype of the Offspring in a Rabbit Model: A Summary Review of About 15 Years of Research. International Journal of Molecular Sciences. 2023; 24(19):14547. https://doi.org/10.3390/ijms241914547
Chicago/Turabian StyleRousseau-Ralliard, Delphine, Pascale Chavatte-Palmer, and Anne Couturier-Tarrade. 2023. "The Effect of Maternal Exposure to a Diet High in Fats and Cholesterol on the Placental Function and Phenotype of the Offspring in a Rabbit Model: A Summary Review of About 15 Years of Research" International Journal of Molecular Sciences 24, no. 19: 14547. https://doi.org/10.3390/ijms241914547





