bFGF-like Activity Supported Tissue Regeneration, Modulated Neuroinflammation, and Rebalanced Ca2+ Homeostasis following Spinal Cord Injury
Abstract
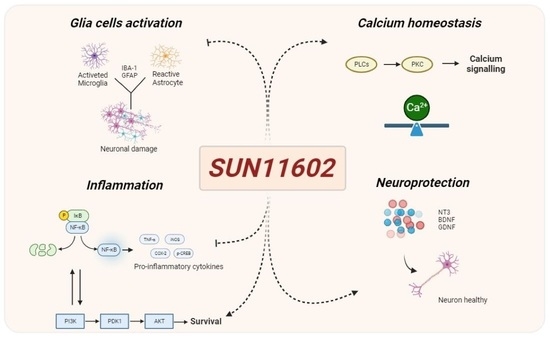
:1. Introduction
2. Results
2.1. SUN11602 Treatment Counteracted Motor Deficits and Tissue Damage, and It Reduced the MPO Activity following SCI
2.2. SUN11602 Treatment Lessened Neuronal Demyelination following SCI
2.3. SUN11602 Treatment Reduced Mast Cell Infiltration following an SCI
2.4. The Effect of SUN11602 Treatment on Glial Cell Activation following an SCI
2.5. SUN11602 Treatment Exerted an Anti-Inflammatory Effect by Modulating NF-κB and Its Interplay with PI3K/AKT Axis and p38 MAPK following an SCI
2.6. SUN11602 Administration Successfully Restored Ca2+-Homeostasis following an SCI
2.7. SUN11602 Treatment Modulated Neurotrophic Factor Levels following an SCI through CREB Induction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. SCI Surgical Procedure
4.4. Experimental Groups
4.5. BMS Open-Field Score
4.6. Histological Evaluation
4.7. Luxol Fast Blue (LFB) Staining
4.8. Toluidine Blue Staining
4.9. The Immunolocalization of NT-3 in Spinal Cord Tissues
4.10. The Immunofluorescence Staining of GFAP and IBA-1 in Spinal Cord Tissues
4.11. The Western Blot Analysis of BDNF, GDNF, IkBa, NF-kB, PI3K, p-p38, p-AKT, p-CREB, Calbindin-D28K, and Anti-S100b in Spinal Cord Tissues
4.12. Calpain Activity
4.13. MPO Assay
4.14. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCI | Spinal cord injury |
| GFAP | Glial fibrillary acidic protein |
| IBA-1 | ionized calcium binding adaptor molecule 1 |
| TNF-α | Tumor necrosis factor alpha |
| IL-1β | Interleukin-1 beta |
| IFN-γ | Interferon–gamma |
| S100-β | S100 calcium-binding protein B |
| CNS | Central Nervous System |
| bFGF | Basic fibroblast growth factor |
| NF-κB | Nuclear factor kappa B |
| WHO | World Health Organization |
| FGFRs | Fibroblast growth factor receptors |
| IκB-α | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| Calb1 | Calbindin-D28k |
| PD | Parkinson disease |
| MPO | Myeloperoxidase |
| BMS | Basso Mouse Scale |
| H&E | Hematoxylin and eosin |
| LFB | Luxol Fast Blue |
| MCs | Mast Cells |
| PI3K | Phosphoinositide 3-kinase |
| AKT | AKT serine/threonine kinase 1/Protein kinase B |
| MAPK | Mitogen-activated protein kinases |
| COX-2 | Cyclooxygenase 2 |
| iNOS | Inducible nitric oxide synthase |
| p38 | p38 mitogen-activated protein kinases |
| PKCδ | Protein kinase C delta type |
| ELISA | enzyme-linked immunosorbent assay |
| BDNF | Brain-derived neurotrophic factor |
| GDNF | Glial cell line-derived neurotrophic facto |
| NT-3 | Neurotrophin-3 |
| CREB | cAMP response element-binding protein |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| IgG | Immunoglobulin G |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene difluoride |
References
- Snider, S.; Cavalli, A.; Colombo, F.; Gallotti, A.L.; Quattrini, A.; Salvatore, L.; Madaghiele, M.; Terreni, M.R.; Sannino, A.; Mortini, P. A novel composite type I collagen scaffold with micropatterned porosity regulates the entrance of phagocytes in a severe model of spinal cord injury. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1040–1053. [Google Scholar] [CrossRef]
- World Health Organization; International Society of Citriculture. International Perspectives on Spinal Cord Injury; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Eli, I.; Lerner, D.P.; Ghogawala, Z. Acute Traumatic Spinal Cord Injury. Neurol. Clin. 2021, 39, 471–488. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Wilson, J.R.; Harrop, J.S.; Kwon, B.K.; Tetreault, L.A.; Arnold, P.M.; Singh, J.M.; Hawryluk, G.; Dettori, J.R. Efficacy and Safety of Methylprednisolone Sodium Succinate in Acute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7, 116S–137S. [Google Scholar] [CrossRef]
- Wang, W.; Zuo, B.; Liu, H.; Cui, L. Intermittent injection of methylprednisolone sodium succinate in the treatment of cervical spinal cord injury complicated with incomplete paraplegia. Pak. J. Med. Sci. 2019, 35, 141. [Google Scholar] [CrossRef]
- Ito, Y.; Sugimoto, Y.; Tomioka, M.; Kai, N.; Tanaka, M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: A prospective study about neurological recovery and early complications. Spine 2009, 34, 2121–2124. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.i.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Kawai, M.; Nagoshi, N.; Okano, H.; Nakamura, M. A review of regenerative therapy for spinal cord injury using human iPS cells. N. Am. Spine Soc. J. (NASSJ) 2022, 13, 100184. [Google Scholar] [CrossRef]
- Jin, Y.; Song, Y.; Lin, J.; Liu, T.; Li, G.; Lai, B.; Gu, Y.; Chen, G.; Xing, L. Role of inflammation in neurological damage and regeneration following spinal cord injury and its therapeutic implications. Burn. Trauma 2023, 11, tkac054. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment imbalance of spinal cord injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef]
- Freyermuth-Trujillo, X.; Segura-Uribe, J.J.; Salgado-Ceballos, H.; Orozco-Barrios, C.E.; Coyoy-Salgado, A. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells 2022, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Bova, V.; Casili, G.; Repici, A.; Lanza, M.; Giuffrida, R.; Colarossi, C.; Mare, M.; Cuzzocrea, S.; Esposito, E.; et al. Role of Basic Fibroblast Growth Factor in Cancer: Biological Activity, Targeted Therapies, and Prognostic Value. Cells 2023, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Bogousslavsky, J.; Victor, S.J.; Salinas, E.O.; Pallay, A.; Donnan, G.A.; Fieschi, C.; Kaste, M.; Orgogozo, J.M.; Chamorro, A.; Desmet, A.; et al. Fiblast (trafermin) in acute stroke: Results of the European-Australian phase II/III safety and efficacy trial. Cerebrovasc. Dis. 2002, 14, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Tao, Q.; Li, G.; Xiang, L.; Zheng, X.; Zhang, T.; Wu, C.; Li, D. Fibroblast Growth Factor 2 Attenuates Renal Ischemia-Reperfusion Injury via Inhibition of Endoplasmic Reticulum Stress. Front. Cell Dev. Biol. 2020, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Dai, D.; He, X.; Zhu, S.; Yao, Y.; Gao, H.; Wang, J.; Qu, F.; Qiu, J.; Wang, H.; et al. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity 2015, 43, 488–501. [Google Scholar] [CrossRef]
- Shi, Y.J.; Shi, M.; Xiao, L.J.; Li, L.; Zou, L.H.; Li, C.Y.; Zhang, Q.J.; Zhou, L.F.; Ji, X.C.; Huang, H.; et al. Inhibitive Effects of FGF2/FGFR1 Pathway on Astrocyte-Mediated Inflammation in vivo and in vitro After Infrasound Exposure. Front. Neurosci. 2018, 12, 582. [Google Scholar] [CrossRef]
- Ardizzone, A.; Bova, V.; Casili, G.; Filippone, A.; Campolo, M.; Lanza, M.; Esposito, E.; Paterniti, I. SUN11602, a bFGF mimetic, modulated neuroinflammation, apoptosis and calcium-binding proteins in an in vivo model of MPTP-induced nigrostriatal degeneration. J. Neuroinflammation 2022, 19, 107. [Google Scholar] [CrossRef]
- Ogino, R.; Murayama, N.; Noshita, T.; Takemoto, N.; Toba, T.; Oka, T.; Narii, N.; Yoshida, S.; Ueno, N.; Inoue, T. SUN11602 has basic fibroblast growth factor-like activity and attenuates neuronal damage and cognitive deficits in a rat model of Alzheimer’s disease induced by amyloid beta and excitatory amino acids. Brain Res. 2014, 1585, 159–166. [Google Scholar] [CrossRef]
- Murayama, N.; Noshita, T.; Ogino, R.; Masuda, T.; Kadoshima, T.; Oka, T.; Ueno, N.; Takemoto, N.; Toba, T.; Ueno, S.; et al. SUN11602-induced hyperexpression of calbindin D-28k is pivotal for the survival of hippocampal neurons under neurotoxic conditions. Brain Res. 2015, 1594, 71–81. [Google Scholar] [CrossRef]
- Kubota, K.; Saiwai, H.; Kumamaru, H.; Maeda, T.; Ohkawa, Y.; Aratani, Y.; Nagano, T.; Iwamoto, Y.; Okada, S. Myeloperoxidase exacerbates secondary injury by generating highly reactive oxygen species and mediating neutrophil recruitment in experimental spinal cord injury. Spine 2012, 37, 1363–1369. [Google Scholar] [CrossRef]
- Casili, G.; Campolo, M.; Lanza, M.; Filippone, A.; Scuderi, S.; Messina, S.; Ardizzone, A.; Esposito, E.; Paterniti, I. Role of ABT888, a Novel Poly(ADP-Ribose) Polymerase (PARP) Inhibitor in Countering Autophagy and Apoptotic Processes Associated to Spinal Cord Injury. Mol. Neurobiol. 2020, 57, 4394–4407. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Schumacher, A.M.; Snaidero, N.; Ullrich Gavilanes, E.M.; Neziraj, T.; Kocsis-Jutka, V.; Engels, D.; Jurgens, T.; Wagner, I.; Weidinger, J.D.F.; et al. Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat. Neurosci. 2021, 24, 355–367. [Google Scholar] [CrossRef]
- Peluso, J.J.; Pappalardo, A.; Fernandez, G. Basic fibroblast growth factor maintains calcium homeostasis and granulosa cell viability by stimulating calcium efflux via a PKC delta-dependent pathway. Endocrinology 2001, 142, 4203–4211. [Google Scholar] [CrossRef] [PubMed]
- Murayama, N.; Kadoshima, T.; Takemoto, N.; Kodama, S.; Toba, T.; Ogino, R.; Noshita, T.; Oka, T.; Ueno, S.; Kuroda, M.; et al. SUN11602, a novel aniline compound, mimics the neuroprotective mechanisms of basic fibroblast growth factor. ACS Chem. Neurosci. 2013, 4, 266–276. [Google Scholar] [CrossRef]
- Fuentes, C.T.; Pazzaglia, M.; Longo, M.R.; Scivoletto, G.; Haggard, P. Body image distortions following spinal cord injury. J. Neurol. Neurosurg. Psychiatry 2013, 84, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Abu-Baker, N.N.; Al-Zyoud, N.m.H.; Alshraifeen, A. Quality of life and self-care ability among individuals with spinal cord injury. Clin. Nurs. Res. 2021, 30, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, F.; Cheng, F.; Ying, L.; Wang, C.; Shi, K.; Wang, J.; Xia, K.; Gong, Z.; Huang, X. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis. 2020, 11, 439. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Yousefifard, M.; Azizi, Y.; Zadegan, S.A.; Sajadi, K.; Sharif-Alhoseini, M.; Shakouri-Motlagh, A.; Mokhatab, M.; Rezvan, M.; Shokraneh, F. Axonal degeneration and demyelination following traumatic spinal cord injury: A systematic review and meta-analysis. J. Chem. Neuroanat. 2019, 97, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Oudega, M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012, 349, 269–288. [Google Scholar] [CrossRef]
- Nelissen, S.; Lemmens, E.; Geurts, N.; Kramer, P.; Maurer, M.; Hendriks, J.; Hendrix, S. The role of mast cells in neuroinflammation. Acta Neuropathol. 2013, 125, 637–650. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Mast cells, glia and neuroinflammation: Partners in crime? Immunology 2014, 141, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Hachem, L.D.; Fehlings, M.G. Pathophysiology of spinal cord injury. Neurosurg. Clin. 2021, 32, 305–313. [Google Scholar] [CrossRef]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of secondary spinal cord injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Fritz, Z.; Sulakhiya, K.; Theis, T.; Berthiaume, F. Transcriptional factors and protein biomarkers as target therapeutics in traumatic spinal cord and brain injury. Curr. Neuropharmacol. 2020, 18, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Wang, Y.; Wang, H.; Huang, C.; Li, J. Endoplasmic reticulum stress-induced hepatic stellate cell apoptosis through calcium-mediated JNK/P38 MAPK and Calpain/Caspase-12 pathways. Mol. Cell. Biochem. 2014, 394, 1–12. [Google Scholar] [CrossRef]
- Asih, P.R.; Prikas, E.; Stefanoska, K.; Tan, A.R.P.; Ahel, H.I.; Ittner, A. Functions of p38 MAP Kinases in the Central Nervous System. Front. Mol. Neurosci. 2020, 13, 570586. [Google Scholar] [CrossRef]
- Kasuya, Y.; Umezawa, H.; Hatano, M. Stress-Activated Protein Kinases in Spinal Cord Injury: Focus on Roles of p38. Int. J. Mol. Sci. 2018, 19, 867. [Google Scholar] [CrossRef]
- Sama, D.M.; Norris, C.M. Calcium dysregulation and neuroinflammation: Discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res. Rev. 2013, 12, 982–995. [Google Scholar] [CrossRef]
- Jiang, H.; Guroff, G. Actions of the neurotrophins on calcium uptake. J. Neurosci. Res. 1997, 50, 355–360. [Google Scholar] [CrossRef]
- Finkbeiner, S. Calcium regulation of the brain-derived neurotrophic factor gene. Cell. Mol. Life Sci. 2000, 57, 394–401. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Iyanoye, A.; Ay, B.; Mantilla, C.B.; Pabelick, C.M. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L447–L456. [Google Scholar] [CrossRef] [PubMed]
- Boyce, V.S.; Mendell, L.M. Neurotrophic factors in spinal cord injury. Neurotrophic Factors 2014, 220, 443–460. [Google Scholar]
- Thorne, R.G.; Frey, W.H. Delivery of neurotrophic factors to the central nervous system: Pharmacokinetic considerations. Clin. Pharmacokinet. 2001, 40, 907–946. [Google Scholar] [CrossRef] [PubMed]
- Corbett, G.T.; Roy, A.; Pahan, K. Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of cAMP-response element-binding protein (CREB): Implications for Alzheimer disease therapy. J. Biol. Chem. 2013, 288, 8299–8312. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]
- Ardizzone, A.; Filippone, A.; Mannino, D.; Scuderi, S.A.; Casili, G.; Lanza, M.; Cucinotta, L.; Campolo, M.; Esposito, E. Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-kappaB/Nrf2/SIRT1 Signaling Pathways. J. Clin. Med. 2022, 11, 4301. [Google Scholar] [CrossRef]
- Campolo, M.; Casili, G.; Lanza, M.; Filippone, A.; Cordaro, M.; Ardizzone, A.; Scuderi, S.A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. The inhibition of mammalian target of rapamycin (mTOR) in improving inflammatory response after traumatic brain injury. J. Cell. Mol. Med. 2021, 25, 7855–7866. [Google Scholar] [CrossRef]
- Casili, G.; Lanza, M.; Campolo, M.; Messina, S.; Scuderi, S.; Ardizzone, A.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Therapeutic potential of flavonoids in the treatment of chronic venous insufficiency. Vasc. Pharmacol. 2021, 137, 106825. [Google Scholar] [CrossRef]
- Filippone, A.; Casili, G.; Ardizzone, A.; Lanza, M.; Mannino, D.; Paterniti, I.; Esposito, E.; Campolo, M. Inhibition of Prolyl Oligopeptidase Prevents Consequences of Reperfusion following Intestinal Ischemia. Biomedicines 2021, 9, 1354. [Google Scholar] [CrossRef]
- Singh, A.; Verma, P.; Raju, A.; Mohanakumar, K.P. Nimodipine attenuates the parkinsonian neurotoxin, MPTP-induced changes in the calcium binding proteins, calpain and calbindin. J. Chem. Neuroanat. 2019, 95, 89–94. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.C.; Mota-Filipe, H.; Paul, A.; Cuzzocrea, S.; Abdelrahman, M.; Harwood, S.; Plevin, R.; Chatterjee, P.K.; Yaqoob, M.M.; Thiemermann, C. Calpain inhibitor I reduces the activation of nuclear factor-kappaB and organ injury/dysfunction in hemorrhagic shock. FASEB J. 2001, 15, 171–186. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardizzone, A.; Bova, V.; Casili, G.; Filippone, A.; Lanza, M.; Repici, A.; Esposito, E.; Paterniti, I. bFGF-like Activity Supported Tissue Regeneration, Modulated Neuroinflammation, and Rebalanced Ca2+ Homeostasis following Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 14654. https://doi.org/10.3390/ijms241914654
Ardizzone A, Bova V, Casili G, Filippone A, Lanza M, Repici A, Esposito E, Paterniti I. bFGF-like Activity Supported Tissue Regeneration, Modulated Neuroinflammation, and Rebalanced Ca2+ Homeostasis following Spinal Cord Injury. International Journal of Molecular Sciences. 2023; 24(19):14654. https://doi.org/10.3390/ijms241914654
Chicago/Turabian StyleArdizzone, Alessio, Valentina Bova, Giovanna Casili, Alessia Filippone, Marika Lanza, Alberto Repici, Emanuela Esposito, and Irene Paterniti. 2023. "bFGF-like Activity Supported Tissue Regeneration, Modulated Neuroinflammation, and Rebalanced Ca2+ Homeostasis following Spinal Cord Injury" International Journal of Molecular Sciences 24, no. 19: 14654. https://doi.org/10.3390/ijms241914654