Exploring PCSK9 Genetic Impact on Lipoprotein(a) via Dual Approaches: Association and Mendelian Randomization
Abstract
:1. Introduction
2. Results
2.1. Hyperlipoproteinemia(a) and Plasma Levels of Lipid
2.2. PCSK9 Genotypes and Plasma Levels of Lipid
2.3. PCSK9 Genotypes and Plasma Levels of Lp(a)
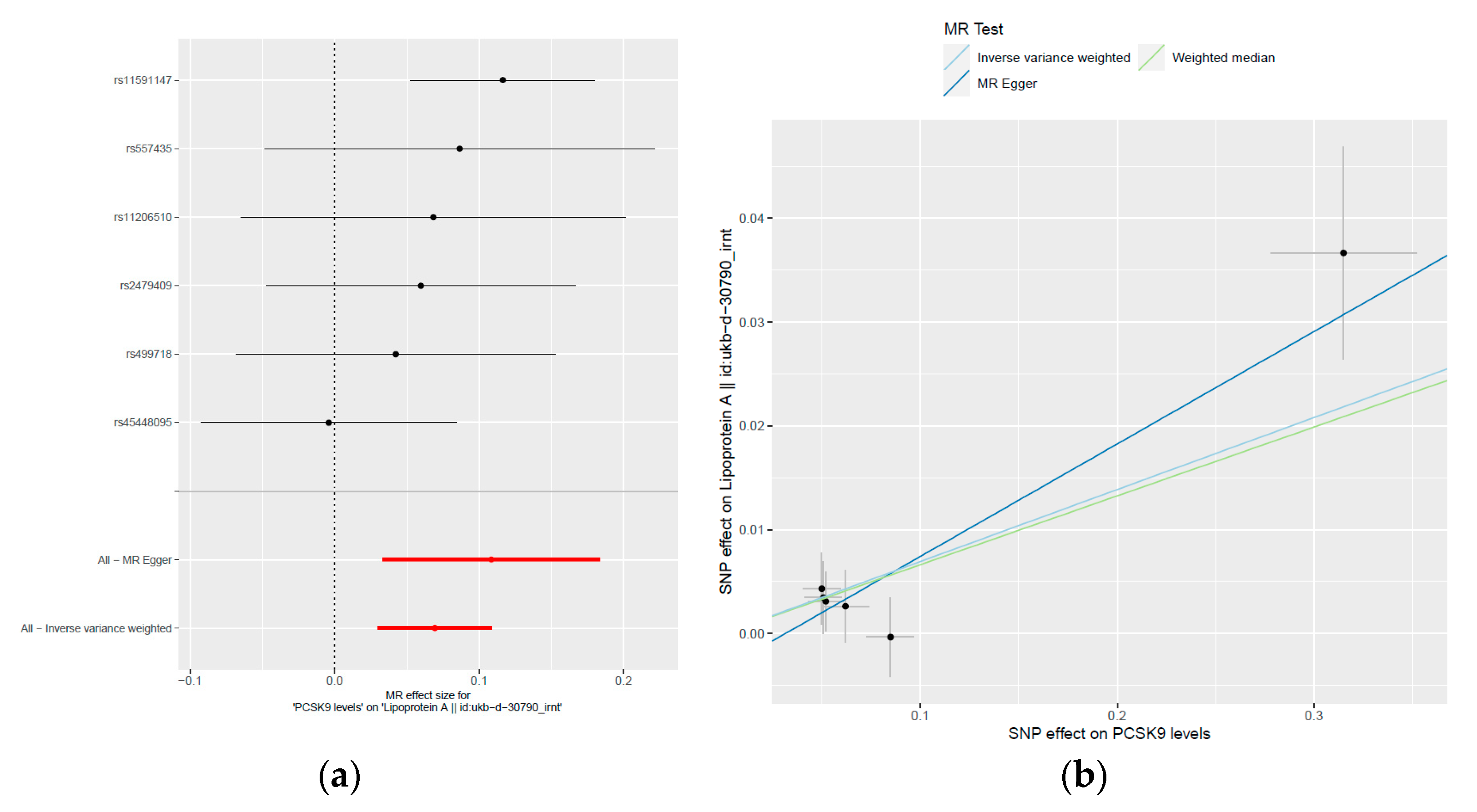
2.4. MR Study
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Chemistry
4.3. Genomic DNA Extraction and Genotyping
4.4. MR Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef]
- Sundararaman, S.S.; Doring, Y.; van der Vorst, E.P.C. PCSK9: A Multi-Faceted Protein That Is Involved in Cardiovascular Biology. Biomedicines 2021, 9, 793. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef]
- Kostner, K.M.; Kostner, G.M. Lipoprotein (a): A historical appraisal. J. Lipid Res. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Kostner, G.M.; Gavish, D.; Leopold, B.; Bolzano, K.; Weintraub, M.S.; Breslow, J.L. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation 1989, 80, 1313–1319. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Ballantyne, C.M. Existing and emerging strategies to lower Lipoprotein(a). Atherosclerosis 2022, 349, 110–122. [Google Scholar] [CrossRef]
- Scartezini, M.; Hubbart, C.; Whittall, R.A.; Cooper, J.A.; Neil, A.H.; Humphries, S.E. The PCSK9 gene R46L variant is associated with lower plasma lipid levels and cardiovascular risk in healthy U.K. men. Clin. Sci. 2007, 113, 435–441. [Google Scholar] [CrossRef]
- Qiu, C.; Zeng, P.; Li, X.; Zhang, Z.; Pan, B.; Peng, Z.Y.F.; Li, Y.; Ma, Y.; Leng, Y.; Chen, R. What is the impact of PCSK9 rs505151and rs11591147 polymorphisms on serum lipids level and cardiovascular risk: A meta-analysis. Lipids Health Dis. 2017, 16, 111. [Google Scholar] [CrossRef]
- Hsu, L.A.; Teng, M.S.; Wu, S.; Chou, H.H.; Ko, Y.L. Common and Rare PCSK9 Variants Associated with Low-Density Lipoprotein Cholesterol Levels and the Risk of Diabetes Mellitus: A Mendelian Randomization Study. Int. J. Mol. Sci. 2022, 23, 10418. [Google Scholar] [CrossRef]
- Chen, S.N.; Ballantyne, C.M.; Gotto, A.M., Jr.; Tan, Y.; Willerson, J.T.; Marian, A.J. A common PCSK9 haplotype, encompassing the E670G coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J. Am. Coll. Cardiol. 2005, 45, 1611–1619. [Google Scholar] [CrossRef]
- Davey, S.G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Sourlas, A.; Mallarkey, G.; Silverio, D.; Ynoa, D.Y.; Montan, P.D.; Guzman, E.; Garcia, M.J. Therapeutic management of hyperlipoproteinemia (a). Drugs Context 2019, 8, 212609. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.A.; Teng, M.S.; Ko, Y.L.; Chang, C.J.; Wu, S.; Wang, C.L.; Hu, C.F. The PCSK9 gene E670G polymorphism affects low-density lipoprotein cholesterol levels but is not a risk factor for coronary artery disease in ethnic Chinese in Taiwan. Clin. Chem. Lab. Med. 2009, 47, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.; Schlegel, V.; Teren, A.; Horn, K.; Kirsten, H.; Bluecher, C.; Kratzsch, J.; Loeffler, M.; Thiery, J.; Burkhardt, R.; et al. Genetic Regulation of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Plasma Levels and Its Impact on Atherosclerotic Vascular Disease Phenotypes. Circ. Genom. Precis. Med. 2018, 11, e001992. [Google Scholar] [CrossRef]
- Chernogubova, E.; Strawbridge, R.; Mahdessian, H.; Mälarstig, A.; Krapivner, S.; Gigante, B.; Hellénius, M.L.; de Faire, U.; Franco-Cereceda, A.; Syvänen, A.C.; et al. Common and low-frequency genetic variants in the PCSK9 locus influence circulating PCSK9 levels. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1526–1534. [Google Scholar] [CrossRef]
- Theusch, E.; Medina, M.W.; Rotter, J.I.; Krauss, R.M. Ancestry and other genetic associations with plasma PCSK9 response to simvastatin. Pharmacogenet. Genom. 2014, 24, 492–500. [Google Scholar] [CrossRef]
- Cui, J.; Qiu, Y.; Kang, N.; Lu, J.; Zheng, L. Correlations of PCSK9 and LDLR gene polymorphisms as well as serum PCSK9 levels with atherosclerosis and lipid metabolism in maintenance hemodialysis patients. J. Clin. Pharmacol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Said, M.A.; Yeung, M.W.; van de Vegte, Y.J.; Benjamins, J.W.; Dullaart, R.P.F.; Ruotsalainen, S.; Ripatti, S.; Natarajan, P.; Juarez-Orozco, L.E.; Verweij, N.; et al. Genome-Wide Association Study and Identification of a Protective Missense Variant on Lipoprotein(a) Concentration: Protective Missense Variant on Lipoprotein(a) Concentration-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1792–1800. [Google Scholar] [CrossRef]
- Hamamura, H.; Adachi, H.; Enomoto, M.; Fukami, A.; Nakamura, S.; Nohara, Y.; Morikawa, N.; Sakaue, A.; Toyomasu, K.; Yamamoto, M.; et al. Serum Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) is Independently Associated with Insulin Resistance, Triglycerides, Lipoprotein(a) Levels but not Low-Density Lipoprotein Cholesterol Levels in a General Population. J. Atheroscler. Thromb. 2021, 28, 329–337. [Google Scholar] [CrossRef]
- Tavori, H.; Christian, D.; Minnier, J.; Plubell, D.; Shapiro, M.D.; Yeang, C.; Giunzioni, I.; Croyal, M.; Duell, P.B.; Lambert, G.; et al. PCSK9 Association with Lipoprotein(a). Circ. Res. 2016, 119, 29–35. [Google Scholar] [CrossRef]
- Romagnuolo, R.; Scipione, C.A.; Boffa, M.B.; Marcovina, S.M.; Seidah, N.G.; Koschinsky, M.L. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J. Biol. Chem. 2015, 290, 11649–11662. [Google Scholar] [CrossRef]
- Korneva, V.A.; Kuznetsova, T.Y.; Julius, U. Modern Approaches to Lower Lipoprotein(a) Concentrations and Consequences for Cardiovascular Diseases. Biomedicines 2021, 9, 1271. [Google Scholar] [CrossRef]
- Katsiki, N.; Vrablik, M.; Banach, M.; Gouni-Berthold, I. Inclisiran, Low-Density Lipoprotein Cholesterol and Lipoprotein(a). Pharmaceuticals 2023, 16, 577. [Google Scholar] [CrossRef]
- Chemello, K.; Beeské, S.; Trang Tran, T.T.; Blanchard, V.; Villard, E.F.; Poirier, B.; Le Bail, J.C.; Dargazanli, G.; Ho-Van-Guimbal, S.; Boulay, D.; et al. Lipoprotein(a) Cellular Uptake Ex Vivo and Hepatic Capture In Vivo Is Insensitive to PCSK9 Inhibition with Alirocumab. JACC Basic. Transl. Sci. 2020, 5, 549–557. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Wade, K.; Laurin, C.; Elsworth, B.; Burgess, S.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. MR-Base: A platform for systematic causal inference across the phenome using billions of genetic associations. bioRxiv 2016. [Google Scholar] [CrossRef]

| Lp(a) < 9.4 mg/dL | Lp(a) 9.4~30 mg/dL | Lp(a) > 30 mg/dL | p | |
|---|---|---|---|---|
| (n = 262) | (n = 233) | (n = 119) | ||
| Age, years | 45 ± 10 | 47 ± 11 | 47 ± 10 | 0.078 |
| Male, (%) | 54.2 | 53.6 | 52.5 | 0.953 |
| Smoker, (%) | 25.6 | 25.5 | 21.7 | 0.676 |
| Hypertension, (%) | 11.8 | 13.2 | 10.8 | 0.795 |
| Diabetes, (%) | 1.9 | 2.6 | 4.2 | 0.435 |
| Body mass index, kg/m2 | 24.5 ± 3.7 | 24.3 ± 3.3 | 24.0 ± 3.3 | 0.488 |
| Cholesterol, mg/dL | 192 ± 36 | 199 ± 37 | 207 ± 37 | 0.001 |
| LDL-cholesterol, mg/dL | 109 ± 30 | 117 ± 33 | 127 ± 35 | <0.001 |
| HDL-cholesterol, mg/dL | 55 ± 15 | 55 ± 15 | 55 ± 13 | 0.938 |
| Triglyceride, mg/dL | 153 ± 148 | 139 ± 91 | 124 ± 83 | 0.269 |
| Lp(a) < 9.4 mg/dL | Lp(a) 9.4~30 mg/dL | Lp(a) > 30 mg/dL | Total | p | |
|---|---|---|---|---|---|
| (n = 262) | (n = 233) | (n = 119) | (n = 614) | ||
| Genotype | 0.044 | ||||
| AA | 219 (83.6%) | 212 (91.0%) | 110 (92.4%) | 541 (88.1%) | |
| AG | 42 (16.0%) | 21 (9.0%) | 9 (7.6%) | 72 (11.7%) | |
| GG | 1 (0.4%) | 1 (0.2%) | |||
| AG+GG vs. AA | 43 (16.4%) | 21 (9.0%) | 9 (7.6%) | 0.011 | |
| Allele | |||||
| G, % | 8.4 | 4.5 | 3.8 | 6.0 | 0.010 |
| Average Risk | Highest Risk | |||
|---|---|---|---|---|
| OR (95%) | p | OR (95%) | p | |
| Model 1 | ||||
| PCSK9 E670G AG+GG vs. AA | 0.50 (0.29–0.87) | 0.015 | 0.40 (0.19–0.86) | 0.019 |
| Model 2 | ||||
| PCSK9 E670G AG+GG vs. AA | 0.53 (0.30–0.93) | 0.026 | 0.46 (0.21–0.99) | 0.047 |
| Exposure (PCSK9 Levels) | Outcome (Lipoprotein(a) Levels) | Two Sample IV Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrumental SNP | Gene | Effect Allele/Other Allele | β | SE | p Value | β | SE | p Value | β | SE | p Value |
| rs11206510 | PCSK9 | T/C | −0.051 | 0.009 | 8.98 × 10−8 | −0.0035 | 0.0034 | 0.315 | 0.068 | 0.068 | 0.31493 |
| rs11591147 | PCSK9 | G/T | −0.315 | 0.037 | 1.94 × 10−17 | −0.0366 | 0.0102 | 3 × 10−4 | 0.116 | 0.032 | 0.000339 |
| rs2479409 | PCSK9 | G/A | −0.052 | 0.009 | 7.41 × 10−9 | −0.0031 | 0.0028 | 0.274 | 0.060 | 0.054 | 0.273589 |
| rs45448095 | PCSK9 | C/T | −0.085 | 0.012 | 4.40 × 10−12 | 0.0003 | 0.0038 | 0.928 | −0.004 | 0.045 | 0.92752 |
| rs499718 | PCSK9 | T/C | 0.062 | 0.012 | 7.81 × 10−8 | 0.0026 | 0.0035 | 0.453 | 0.042 | 0.056 | 0.453332 |
| rs557435 | PCSK9 | A/G | 0.050 | 0.010 | 2.22 × 10−7 | 0.0043 | 0.0034 | 0.209 | 0.087 | 0.069 | 0.209168 |
| MR Method | Number of SNPs | β | SE | Association p Value | Cochran’s Q Statistic | Heterogeneity p Value |
|---|---|---|---|---|---|---|
| Inverse-variance weighted | 6 | 0.06933 | 0.02007 | 0.0005528 | 5.079 | 0.4063 |
| MR-Egger | 6 | 0.1083 | 0.0383 | 0.04741 | 3.658 | 0.4063 |
| Weighted median | 6 | 0.06628 | 0.02888 | 0.02176 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Hsu, L.-A.; Ko, Y.-L. Exploring PCSK9 Genetic Impact on Lipoprotein(a) via Dual Approaches: Association and Mendelian Randomization. Int. J. Mol. Sci. 2023, 24, 14668. https://doi.org/10.3390/ijms241914668
Chang Y-C, Hsu L-A, Ko Y-L. Exploring PCSK9 Genetic Impact on Lipoprotein(a) via Dual Approaches: Association and Mendelian Randomization. International Journal of Molecular Sciences. 2023; 24(19):14668. https://doi.org/10.3390/ijms241914668
Chicago/Turabian StyleChang, Ya-Ching, Lung-An Hsu, and Yu-Lin Ko. 2023. "Exploring PCSK9 Genetic Impact on Lipoprotein(a) via Dual Approaches: Association and Mendelian Randomization" International Journal of Molecular Sciences 24, no. 19: 14668. https://doi.org/10.3390/ijms241914668





