Mucoadhesive Mesoporous Silica Particles as Versatile Carriers for Doxorubicin Delivery in Cancer Therapy
Abstract
:1. Introduction
2. Results
2.1. FT-IR Spectroscopy of MS and DOX-Loaded MSPs
2.2. DLS and Zeta Potential Analyses
2.3. TEM Morphology of the MSPs before and after Loading of DOX
2.4. Release of DOX from MS Samples
2.5. The Evaluation of Cytotoxicity of DOX-Loaded MS Samples
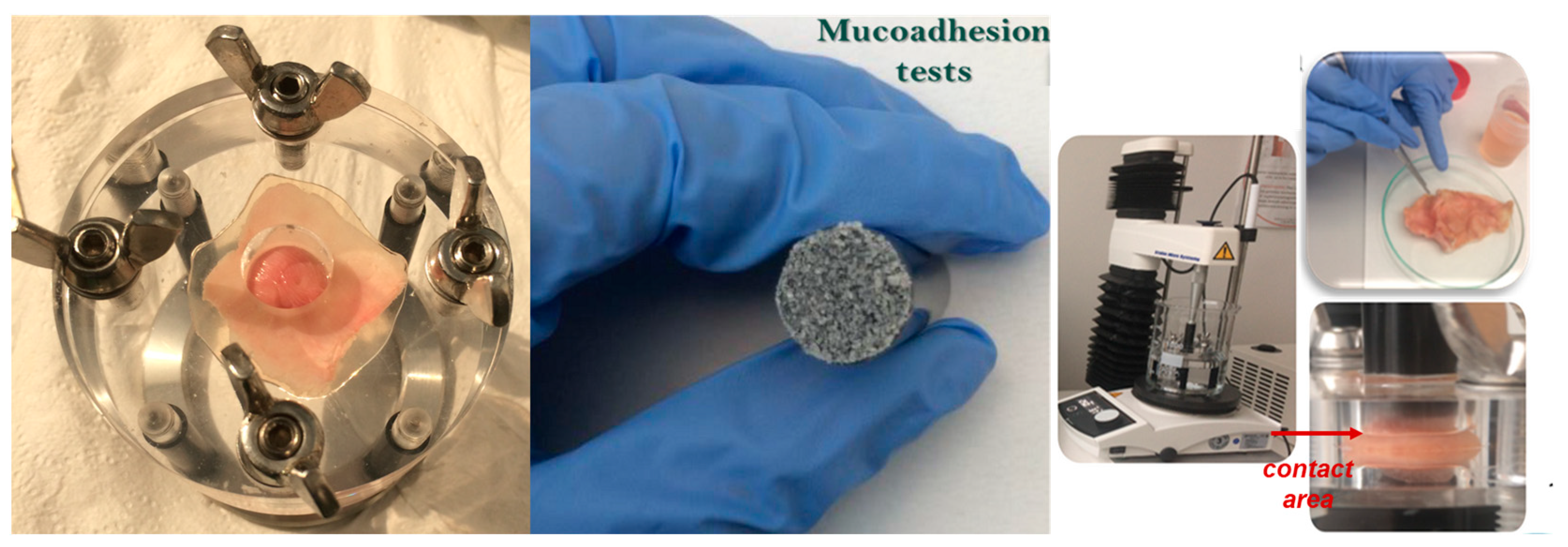
2.6. Evaluation of Bio- and Mucoadhesion of the Functionalized MS
2.7. The Antimicrobial Screening of the Functionalized MS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of MSPs with Various Surface Groups (M1-M5)
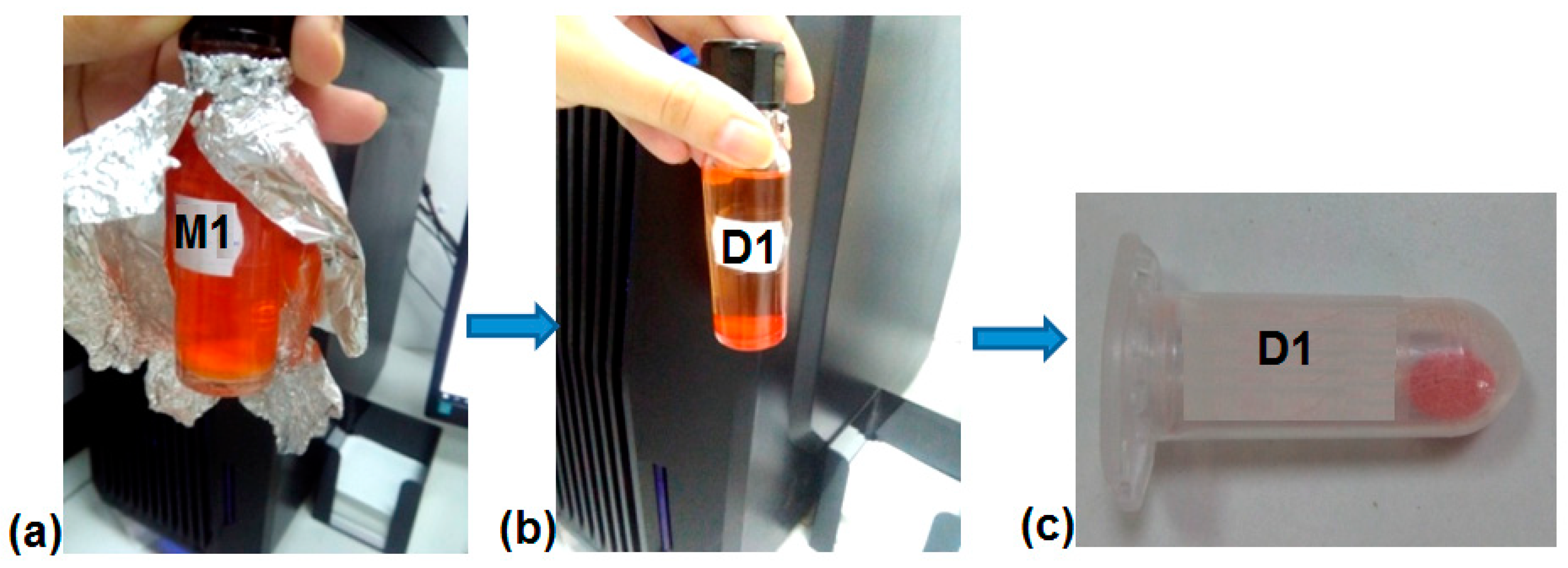
4.3. DOX Loading in MS (Samples D1-D5)
4.4. DOX Release Studies
4.5. Characterization Techniques
4.6. Statistical Analysis Was Used to Study the Differences among Means of the Obtained Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica Nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Song, H.; Yu, C. Silica-based nanoparticles for biomedical applications: From nanocarriers to biomodulators. Acc. Chem. Res. 2020, 53, 1545–1556. [Google Scholar] [CrossRef]
- Zivojević, K.; Mladenović, M.; Djisalov, M.; Mundzic, M.; Ruiz-Hernandez, E.; Gadjanski, I.; Knězević, N.Z. Advanced mesoporous silica nanocarriers in cancer theranostics and gene editing applications. J. Control. Release 2021, 337, 193–211. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Wang, K.; Lu, J.; Li, J.; Gao, Y.; Mao, Y.; Zhao, Q.; Wang, S. Current trends in smart mesoporous silica-based nanovehicles for photoactivated cancer therapy. J. Control. Release 2021, 339, 445–472. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Lu, J.; Lin, Y.; Feng, S.; Luo, X.; Di, D.; Wang, S.; Zhao, Q. Recent trends of mesoporous silica-based nanoplatforms for nanodynamic therapies. Coord. Chem. Rev. 2022, 469, 214687. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticlesfor drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Poonia, N.; Lather, V.; Pandita, D. Mesoporous silica nanoparticles: A smart nanosystemfor management of breast cancer. Drug Discov. Today 2018, 23, 2. [Google Scholar] [CrossRef]
- Craig, M.; Jenner, A.L.; Namgung, B.; Lee, L.P.; Goldman, A. Engineering in Medicine to Address the Challenge of Cancer Drug Resistance: From Micro- and Nanotechnologies to Computational and Mathematical Modeling. Chem. Rev. 2021, 121, 3352–3389. [Google Scholar] [CrossRef]
- Feng, S.; Lu, J.; Wang, K.; Di, D.; Shi, Z.; Zhao, Q.; Wang, S. Advances in smart mesoporous carbon nanoplatforms for photothermal–enhanced synergistic cancer therapy. Chem. Eng. J. 2022, 435, 134886. [Google Scholar] [CrossRef]
- Zaharudin, N.S.; Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A.; Jumbri, K. Functionalized mesoporous silica nanoparticles templated by pyridinium ionic liquid for hydrophilic and hydrophobic drug release application. J. Saudi Chem. Soc. 2020, 24, 289–302. [Google Scholar] [CrossRef]
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface functionalized mesoporous silica nanoparticles for intravitreal application of tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033. [Google Scholar] [CrossRef]
- Chen, C.-C.; Fa, Y.-C.; Kuo, Y.-Y.; Liu, Y.-C.; Lin, C.-Y.; Wang, X.-H.; Lu, Y.-H.; Chiang, Y.-H.; Yang, C.-M.; Wu, L.-C.; et al. Thiolated Mesoporous Silica Nanoparticles as an Immunoadjuvant to Enhance Efficacy of Intravesical Chemotherapy for Bladder Cancer. Adv. Sci. 2023, 10, 2204643. [Google Scholar] [CrossRef]
- Aitah, K.A.; Hassan, H.A.; Ammar, N.M.; Baker, D.H.A.; Higazy, I.M.; Shaker, O.G.; Elsayed, A.A.A.; Hassan, A.M.E. Novel delivery system with a dual–trigger release of savory essential oil by mesoporous silica nanospheres and its possible targets in leukemia cancer cells: In vitro study. Cancer Nanotechnol. 2023, 14, 3. [Google Scholar]
- Peng, H.; Xu, Z.; Wang, Y.; Feng, N.; Yang, W.; Tang, J. Biomimetic Mesoporous Silica Nanoparticles for Enhanced Blood Circulation and Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 7849–7857. [Google Scholar] [CrossRef]
- Lebold, T.; Jung, C.; Michaelis, J.; Brauchle, C. Nanostructured Silica Materials as Drug-Delivery Systems for Doxorubicin: Single Molecule and Cellular Studies. Nano Lett. 2009, 9, 2877–2883. [Google Scholar] [CrossRef]
- Giret, S.; Man, M.W.C.; Carcel, C. Mesoporous-Silica-Functionalized Nanoparticles for Drug Delivery. Chem. Eur. J. 2015, 21, 13850–13865. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Q.; Dai, L.; Shen, X.; Chen, W.; Cai, K. Phenylboronic acid-modified hollow silica nanoparticles for dual-responsive delivery of doxorubicin for targeted tumor therapy. Regen. Biomater. 2017, 4, 111–124. [Google Scholar] [CrossRef]
- Aquib, M.; Farooq, M.A.; Banerjee, P.; Akhtar, F.; Filli, M.S.; Boakye-Yiadom, K.O.; Kesse, S.; Raza, F.; Maviah, M.B.J.; Mavlyanova, R.; et al. Targeted and stimuli–responsive mesoporous silica nanoparticles for drug delivery and theranostic use. J. Biomed. Mater. Res. 2019, 107A, 2643–2666. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, W.; Liu, R.; Zhang, J.; Zhang, D.; Li, Z.; Luan, Y. Rational Design of MOF Nanocarrier-Based Co-Delivery System of Doxorubicin Hydrochloride/Verapamil Hydrochloride for Overcoming Multidrug Resistance with Efficient Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 14, 19687–19697. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Qian, G.; Ito, A. Synergistical chemotherapy and cancer immunotherapy using dual drug-delivering and immunopotentiating mesoporous silica. Appl. Mater. Today 2019, 16, 102–111. [Google Scholar] [CrossRef]
- Hajebi, S.; Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Hybrid and hollow Poly(N,N-dimethylaminoethyl methacrylate) nanogels as stimuli responsive carriers for controlled release of doxorubicin. Polymer 2019, 180, 121716. [Google Scholar] [CrossRef]
- Liu, C.G.; Han, Y.H.; Zhang, J.T.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Rerouting Engineered Metal-dependent Shapes of Mesoporous Silica Nanocontainers to Biodegradable Janus-type (Sphero-ellipsoid) Nanoreactors for Chemodynamic therapy. Chem. Eng. J. 2019, 370, 1188–1199. [Google Scholar] [CrossRef]
- Yang, S.; Song, S.; Han, K.; Wu, X.; Chen, L.; Hu, Y.; Wang, J.; Liu, B. Characterization, in vitro evaluation and comparative study on the cellular internalization of mesoporous silica nanoparticle-supported lipid bilayers. Microporous Mesoporous Mater. 2019, 284, 212–224. [Google Scholar] [CrossRef]
- Huh, S.; Wiench, J.W.; Yoo, J.-C.; Pruski, M.; Lin, V.S.-Y. Organic Functionalization and Morphology Control of Mesoporous Silicas via a Co-Condensation Synthesis Method. Chem. Mater. 2003, 15, 4247–4256. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef]
- Bansal, R.; Singh, R.; Kaur, K. Quantitative analysis of doxorubicin hydrochloride and arterolane maleate by mid IR spectroscopy using transmission and reflectance modes. BMC Chem. 2021, 15, 27. [Google Scholar] [CrossRef]
- Sikora, A.; Shard, A.G.; Minelli, C. Size and ζ-Potential Measurement of Silica Nanoparticles in Serum Using Tunable Resistive Pulse Sensing. Langmuir 2016, 32, 2216–2224. [Google Scholar] [CrossRef]
- Tuoriniemi, J.; Johnsson, A.-C.J.H.; Holmberg, J.P.; Gustafsson, S.; Gallego-Urrea, J.A.; Olsson, E.; Pettersson, J.B.C.; Hassellöv, M. Intermethod comparison of the particle size distributions of colloidal silica nanoparticles. Sci. Technol. Adv. Mater. 2014, 15, 035009. [Google Scholar] [CrossRef]
- Lim, J.K.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendre, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Mazumdar, S.; Chitkara, D.; Mittal, A. Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers. Acta Pharm. Sin. B 2021, 11, 903–924. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Björk, E.M.; Söderlind, F.; Odén, M. Tuning the Shape of Mesoporous Silica Particles by Alterations in Parameter Space: From Rods to Platelets. Langmuir 2013, 29, 13551–13561. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-P.; Mou, C.-Y. Structural and Morphological Control of Cationic Surfactant-Templated Mesoporous Silica. Acc. Chem. Res. 2002, 35, 927–935. [Google Scholar] [CrossRef]
- Zhan, S.; Wang, J.; Wang, W.; Cui, L.; Zhao, Q. Preparation and in vitro release kinetics of nitrendipine-loaded PLLA–PEG–PLLA microparticles by supercritical solution impregnation process. RSC Adv. 2019, 9, 16167. [Google Scholar] [CrossRef]
- Choi, E.; Lim, D.-K.; Kim, S. Hydrolytic surface erosion of mesoporous silica nanoparticles for efficient intracellular delivery of cytochrome C. J. Colloid Interface Sci. 2020, 560, 416–425. [Google Scholar] [CrossRef]
- Golla, K.; Bhaskar, C.; Ahmed, F.; Kondapi, A.K. A Target-Specific Oral Formulation of Doxorubicin- Protein Nanoparticles: Efficacy and Safety in Hepatocellular Cancer. J. Cancer 2013, 4, 644–652. [Google Scholar] [CrossRef]
- Hernandez-Montoto, A.; Gorbe, M.; Llopis-Lorente, A.; Terres, J.M.; Montes, R.; Cao-Milan, R.; Dıaz de Grenu, B.; Alfonso, M.; Orzaez, M.; Dolores Marcos, M.; et al. A NIR light-triggered drug delivery system using core–shell gold nanostars–mesoporous silica nanoparticles based on multiphoton absorption photo-dissociation of 2-nitrobenzyl PEG. Chem. Commun. 2019, 55, 9039–9042. [Google Scholar] [CrossRef]
- Dong, J.; Yao, X.; Sun, S.; Zhong, Y.; Qiana, C.; Yang, D. In vivo targeting of breast cancer with a vasculature-specific GQDs/hMSN nanoplatform. RSC Adv. 2019, 9, 11576. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, H.; Xu, S.; Zhi, C.; Nakanishi, H.; Gao, X.D. Folate-conjugated, mesoporous silica functionalized boron nitride nanospheres for targeted delivery of doxorubicin. Mater. Sci. Eng. C 2019, 96, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Heggannavar, B.; Vijeth, S.; Kariduraganavar, M.Y. Development of dual drug loaded PLGA based mesoporous silica nanoparticles and their conjugation with Angiopep-2 to treat glioma. J. Drug Deliv. Sci. Technol. 2019, 53, 101157. [Google Scholar] [CrossRef]
- Kuanga, Y.; Chenb, H.; Chen, Z.; Wan, L.; Liu, J.; Xu, Z.; Chen, X.; Jiang, B.; Li, C. Poly(amino acid)/ZnO/mesoporous silica nanoparticle based complex drug delivery system with a charge-reversal property for cancer therapy. Colloids Surf. B 2019, 181, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pishnamazi, M.; Hafizi, H.; Pishnamazi, M.; Marjani, A.; Shirazian, S.; Walker, G.M. Controlled release evaluation of paracetamol loaded amine functionalized mesoporous silica KCC1 compared to microcrystalline cellulose based tablets. Sci. Rep. 2021, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Li, L.; Guo, Z.; Song, J.; Yang, X.; Wan, G.; Li, R.; Wang, Y. Leukocyte/platelet hybrid membrane-camouflaged dendritic large pore mesoporous silica nanoparticles co-loaded with photo/chemotherapeutic agents for triple negative breast cancer combination treatment. Bioact. Mater. 2021, 6, 3865–3878. [Google Scholar] [CrossRef]
- Sun, P.; Leidner, A.; Weigel, S.; Weidler, P.G.; Heissler, S.; Scharnweber, T.; Niemeyer, C.M. Biopebble Containers: DNA-Directed Surface Assembly of Mesoporous Silica Nanoparticles for Cell Studies. Small 2019, 15, 1900083. [Google Scholar] [CrossRef]
- Vaz-Ramos, J.; Cordeiro, R.; Castro, M.M.C.A.; Geraldes, C.F.G.C.; Costa, B.F.O.; Faneca, H.; Durães, L. Supercritically dried superparamagnetic mesoporous silica nanoparticles for cancer theranostics. Mater. Sci. Eng. C 2020, 115, 111124. [Google Scholar] [CrossRef]
- Tang, S.; Huang, X.; Chen, X.; Zheng, N. Hollow Mesoporous ZirconiaNanocapsules for Drug Delivery. Adv. Funct. Mater. 2010, 20, 2442–2447. [Google Scholar] [CrossRef]
- Chen, C.; Wu, C.; Yu, J.; Zhu, X.; Wu, Y.; Liu, J.; Zhang, Y. Photodynamic-based combinatorial cancer therapy strategies: Tuning the properties of nanoplatform according to oncotherapy needs. Coord. Chem. Rev. 2022, 461, 214495. [Google Scholar] [CrossRef]
- Vo, U.V.; Nguyen, C.K.; Nguyen, V.C.; Tran, T.V.; Thi, B.Y.T.; Nguyen, D.H. Gelatin-poly (ethylene glycol) methyl ether-functionalized porous Nanosilica for efficient doxorubicin delivery. J. Polym. Res. 2019, 26, 6. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous silica nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Côté, M.F.; Gaudreault, R.C.; Fortin, M.A.; Kleitz, F. Size-Controlled Functionalized Mesoporous Silica Nanoparticles for Tunable Drug Release and Enhanced Anti-Tumoral Activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, C.; Liu, Y.; Chen, F.; Zheng, Z.; Wang, X. Enzyme-/Redox-Responsive Mesoporous Silica Nanoparticles Based on Functionalized Dopamine as Nanocarriers for Cancer Therapy. ACS Omega 2019, 4, 6097–6105. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: A review. Microporous Mesoporous Mater. 2016, 236, 141–157. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Butnarasu, C.; Petrini, P.; Bracotti, F.; Visai, L.; Guagliano, G.; Pla, A.F.; Sansone, E.; Petrillo, S.; Visentin, S. Mucosomes: Intrinsically Mucoadhesive Glycosylated Mucin Nanoparticles as Multi-Drug Delivery Platform. Adv. Healthcare Mater. 2022, 11, 2200340. [Google Scholar] [CrossRef]
- Mansfield, E.D.H.; de la Rosa, V.R.; Kowalczyk, R.M.; Grillo, I.; Hoogenboom, R.; Sillence, K.; Hole, P.; Williams, A.C.; Khutoryanskiy, V.V. Side chain variations radically alter the diffusion of poly(2-alkyl-2-oxazoline) functionalised nanoparticles through a mucosal barrier. Biomater. Sci. 2016, 4, 1318. [Google Scholar] [CrossRef]
- Zhang, Q.; Neoh, K.G.; Xu, L.; Lu, S.; Kang, E.T.; Mahendran, R.; Chiong, E. Functionalized Mesoporous Silica Nanoparticles with Mucoadhesive and Sustained Drug Release Properties for Potential Bladder Cancer Therapy. Langmuir 2014, 30, 6151–6161. [Google Scholar] [CrossRef]
- Müller, L.; Rosenbaum, C.; Rump, A.; Grimm, M.; Klammt, F.; Kleinwort, A.; Busemann, A.; Weitschies, W. Determination of Mucoadhesion of Polyvinyl Alcohol Films to Human Intestinal Tissue. Pharmaceutics 2023, 15, 1740. [Google Scholar] [CrossRef]
- Preisig, D.; Roth, R.; Tognola, S.; Varum, F.J.O.; Bravo, R.; Cetinkaya, Y.; Huwyler, J.; Puchkov, M. Mucoadhesive microparticles for local treatment of gastrointestinal diseases. Eur. J. Pharm. Biopharm. 2016, 105, 156–165. [Google Scholar] [CrossRef]
- Huang, R.; Shen, Y.-W.; Guan, Y.-Y.; Jiang, Y.-X.; Wu, Y.; Rahman, K.; Zhang, L.-J.; Liu, H.-J.; Luan, X. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, D.; Díez, J.; Barba, I.I.; Colilla, M.; Vallet-Regí, M. Amine-Functionalized Mesoporous Silica Nanoparticles: A New Nanoantibiotic for Bone Infection Treatment. Biomed. Glasses 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2022, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: The evolution of culture media in clinical microbiology. New Microbes New Infect. 2020, 34, 100622. [Google Scholar] [CrossRef]
- Phuong, P.T.; Oliver, S.; He, J.; Wong, E.H.H.; Mathers, R.T.; Boyer, C. Effect of Hydrophobic Groups on Antimicrobial and Hemolytic Activity: Developing a Predictive Tool for Ternary Antimicrobial Polymers. Biomacromolecules 2020, 21, 5241–5255. [Google Scholar] [CrossRef]







| Sample | M1 | D1 | M2 | D2 | M3 | D3 | M4 | D4 | M5 | D5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Size DLS (nm) | 825.2 ± 0.3–1280.3 ± 1.2 | 615.3 ± 0.4–825.1 ± 2.2 | 712.4 ± 1.6–955.2 ± 2.5 | 396.5 ± 4.5–712.4 ± 0.9 | 396.2 ± 4.2–531.6 ± 2.2 | 1484.2 ± 12.3–2305.8 ± 23.5 | 295.3 ± 0.8–825.7 ± 0.4 | 396.4 ± 2.2–712.4 ± 0.9 | 255.2 ± 1.1–458.7 ± 0.6 | 342.4 ± 2.2–615.6 ± 2.5 |
| PDI * | 0.216 ± 0.11 | 0.305 ± 0.08 | 0.546 ± 0.12 | 0.614 ± 0.04 | 0.478 ± 0.13 | 0.397 ± 0.09 | 0.641 ± 0.21 | 0.611 ± 0.13 | 0.572 ± 0.15 | 0.603 ± 0.22 |
| Size by TEM (nm) | >500 | >500 | >500 | >500 | 200–400 | 200 | 200–400 | 200 | 200–400 | 200 |
| Zeta potential (mV) | 2.98 ± 0.62 | 11.10 ± 3.72 | 38.00 ± 5.54 | −6.10 ± 1.47 | −9.29 ± 2.88 | −11.8 ± 0.47 | −16.0 ± 2.7 | −10.7 ± 1.1 | −10.8 ± 1.31 | −9.81 ± 1.82 |
| Kinetic Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Higuchi | Korsmeyer–Peppas | Peppas–Sahlin | ||||||
| pH 7.4 | |||||||||
| KH | R2 | n | K | R2 | n | K1 | K2 | R2 | |
| D1 | 0.1495 | 0.9814 | 0.4238 | 0.2718 | 0.9858 | 0.2479 | 0.2340 | 0.1207 | 0.9842 |
| D2 | 0.1517 | 0.9573 | 0.3907 | 0.3570 | 0.9676 | 0.2360 | 0.3491 | 0.1332 | 0.9652 |
| D3 | 0.0349 | 0.9550 | 0.5402 | 0.0304 | 0.9814 | 0.5266 | 0.0281 | 0.0011 | 0.9814 |
| D4 | 0.0338 | 0.9726 | 0.6346 | 0.0098 | 0.9511 | 0.6330 | 0.0099 | 1.35 × 10−5 | 0.9839 |
| D5 | 0.1672 | 0.9647 | 0.5485 | 0.1141 | 0.9633 | 0.3337 | 0.1670 | 0.0325 | 0.9597 |
| pH 5 | |||||||||
| KH | R2 | n | K | R2 | n | K1 | K2 | R2 | |
| D1 | 0.1958 | 0.9866 | 0.4314 | 0.3352 | 0.9890 | 0.2667 | 0.3735 | 0.1041 | 0.9879 |
| D2 | 0.1739 | 0.9822 | 0.4498 | 0.2576 | 0.9852 | 0.2835 | 0.3353 | 0.0662 | 0.9830 |
| D3 | 0.0406 | 0.9781 | 0.3657 | 0.1150 | 0.9828 | 0.1633 | 0.4368 | 0.0287 | 0.9828 |
| D4 | 0.0383 | 0.9927 | 0.3674 | 0.0969 | 0.9948 | 0.2139 | 0.0093 | 0.0653 | 0.9932 |
| D5 | 0.1541 | 0.9570 | 0.8301 | 0.0114 | 0.9752 | 0.8281 | 0.0101 | 1.58 × 10−6 | 0.9660 |
| pH 1.5 | |||||||||
| KH | R2 | n | K | R2 | n | K1 | K2 | R2 | |
| D1 | 0.2091 | 0.9927 | 0.3961 | 0.4720 | 0.9974 | 0.2599 | 0.6213 | 0.0955 | 0.9969 |
| D2 | 0.1858 | 0.9907 | 0.4180 | 0.3531 | 0.9951 | 0.2459 | 0.2332 | 0.1620 | 0.9935 |
| D3 | 0.0413 | 0.9017 | 0.2542 | 0.2826 | 0.9579 | 0.1567 | 0.3676 | 0.0663 | 0.9543 |
| D4 | 0.0458 | 0.9728 | 0.2863 | 0.2433 | 0.9888 | 0.1819 | 0.2715 | 0.0663 | 0.9890 |
| D5 | 0.1523 | 0.9666 | 0.7957 | 0.0148 | 0.9802 | 0.7921 | 0.0148 | 6.17 × 10−7 | 0.9561 |
| IC50 (μg/mL) | SIa | ||||
|---|---|---|---|---|---|
| MS Samples | HeLa | MCF-7 | HGF | SI1b | SI2c |
| D1 | 136.5 ± 0.2 (0.90 ± 0.2 μg/mL DOX) | 73.98 ± 0.1 (0.49 ± 0.2 μg/mL DOX) | 60.06 ± 0.2 (0.40 ± 0.2 μg/mL DOX) | 0.44 | 0.81 |
| D3 | 18.95 ± 0.2 (0.59 ± 0.1 μg/mL DOX) | 10.84 ± 0.2 (0.33 ± 0.1 μg/mL DOX) | - | - | - |
| D5 | 43.66 ± 0.3 (1.56 ± 0.1 μg/mL DOX) | 35.54 ± 0.3 (1.27 ± 0.1 μg/mL DOX) | 24.24 ± 0.2 (0.86 ± 0.2 μg/mL DOX) | 0.55 | 0.68 |
| Sample | Mucoadhesion on Different Tissue Mucosae | |||||||
|---|---|---|---|---|---|---|---|---|
| Detachment Force (N) (×10−2) | Work of Adhesion (mJ) (×10−2) | |||||||
| Stomach | Small Intestine | Large Intestine | Colon | Stomach | Small Intestine | Large Intestine | Colon | |
| M1 | 4.34 ± 0.07 | 7.09 ± 0.39 | 4.61 ± 0.19 | 8.82 ± 1.28 | 0.99 ± 0.39 | 0.62 ± 0.37 | 0.55 ± 0.05 | 1.34 ± 0.88 |
| M2 | 3.85 ± 0.05 | 16.50 ± 2.87 | 5.19 ± 0.19 | 8.79 ± 2.16 | 0.85 ± 0.04 | 3.17 ± 0.86 | 0.80 ± 0.02 | 1.20 ± 0.71 |
| M3 | 4.02 ± 0.16 | 5.88 ± 0.19 | 6.01 ± 0.28 | 5.94 ± 0.24 | 0.80 ± 0.04 | 0.69 ± 0.02 | 0.97 ± 0.05 | 0.56 ± 0.10 |
| M4 | 3.98 ± 0.28 | 9.08 ± 2.33 | 5.32 ± 0.14 | 8.17 ± 1.24 | 0.72 ± 0.02 | 2.57 ± 1.11 | 0.96 ± 0.03 | 1.08 ± 0.98 |
| M5 | 4.15 ± 0.03 | 5.68 ± 0.01 | 4.61 ± 0.17 | 10.42 ± 0.55 | 0.86 ± 0.04 | 0.38 ± 0.12 | 0.80 ± 0.006 | 3.39 ± 0.25 |
| Sample | MIC a (µg/mL) | ||||
|---|---|---|---|---|---|
| Fungi | Bacteria | ||||
| Aspergillus fumigatus | Penicillium frequentans | Fusarium | Bacillus sp. | Pseudomonas sp. | |
| M1 | 0.38 ± 0.17 | 0.38 ± 0.11 | 0.38 ± 0.09 | 2.00 ± 0.02 | 2.00 ± 0.22 |
| M3 | 0.75 ± 0.09 | 0.75 ± 0.12 | 0.75 ± 0.07 | 3.00 ± 1.11 | 3.00 ± 1.12 |
| M5 | >32 | >32 | >32 | >256 | >256 |
| Caspofungin b | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.20 ± 0.02 | - | - |
| Kanamycin b | - | - | - | 3.00 ± 0.02 | 3.00 ± 0.02 |
| Sample | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| Surface group | -(CH2)3NH2 | -(CH2)3NH2 | -OH | -OH | -CH3 |
| Pore size (nm) | 3.33 ± 0.01 | 3.02 ± 0.02 | 3.06 ± 0.01 | 3.04 ± 0.01 | 2.49 ± 0.06 |
| BET area (m2/g) | 722.08 ± 7.32 | 621.11 ± 15.24 | 1001.01 ± 12.43 | 936.20 ± 4.22 | 943.13 ± 0.74 |
| Samples | EE, % | LC μg/mg |
|---|---|---|
| M1 | 16.75 ± 0.04 | 6.70 ± 0.22 |
| M2 | 14.13 ± 0.11 | 5.65 ± 0.09 |
| M3 | 81.80 ± 0.12 | 32.70 ± 0.24 |
| M4 | 78.74 ± 0.01 | 31.49 ± 0.11 |
| M5 | 90.00 ± 0.02 | 36.00 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaltariov, M.-F.; Ciubotaru, B.-I.; Ghilan, A.; Peptanariu, D.; Ignat, M.; Iacob, M.; Vornicu, N.; Cazacu, M. Mucoadhesive Mesoporous Silica Particles as Versatile Carriers for Doxorubicin Delivery in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 14687. https://doi.org/10.3390/ijms241914687
Zaltariov M-F, Ciubotaru B-I, Ghilan A, Peptanariu D, Ignat M, Iacob M, Vornicu N, Cazacu M. Mucoadhesive Mesoporous Silica Particles as Versatile Carriers for Doxorubicin Delivery in Cancer Therapy. International Journal of Molecular Sciences. 2023; 24(19):14687. https://doi.org/10.3390/ijms241914687
Chicago/Turabian StyleZaltariov, Mirela-Fernanda, Bianca-Iulia Ciubotaru, Alina Ghilan, Dragos Peptanariu, Maria Ignat, Mihail Iacob, Nicoleta Vornicu, and Maria Cazacu. 2023. "Mucoadhesive Mesoporous Silica Particles as Versatile Carriers for Doxorubicin Delivery in Cancer Therapy" International Journal of Molecular Sciences 24, no. 19: 14687. https://doi.org/10.3390/ijms241914687
APA StyleZaltariov, M.-F., Ciubotaru, B.-I., Ghilan, A., Peptanariu, D., Ignat, M., Iacob, M., Vornicu, N., & Cazacu, M. (2023). Mucoadhesive Mesoporous Silica Particles as Versatile Carriers for Doxorubicin Delivery in Cancer Therapy. International Journal of Molecular Sciences, 24(19), 14687. https://doi.org/10.3390/ijms241914687





