Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway
Abstract
:1. Introduction
2. Results
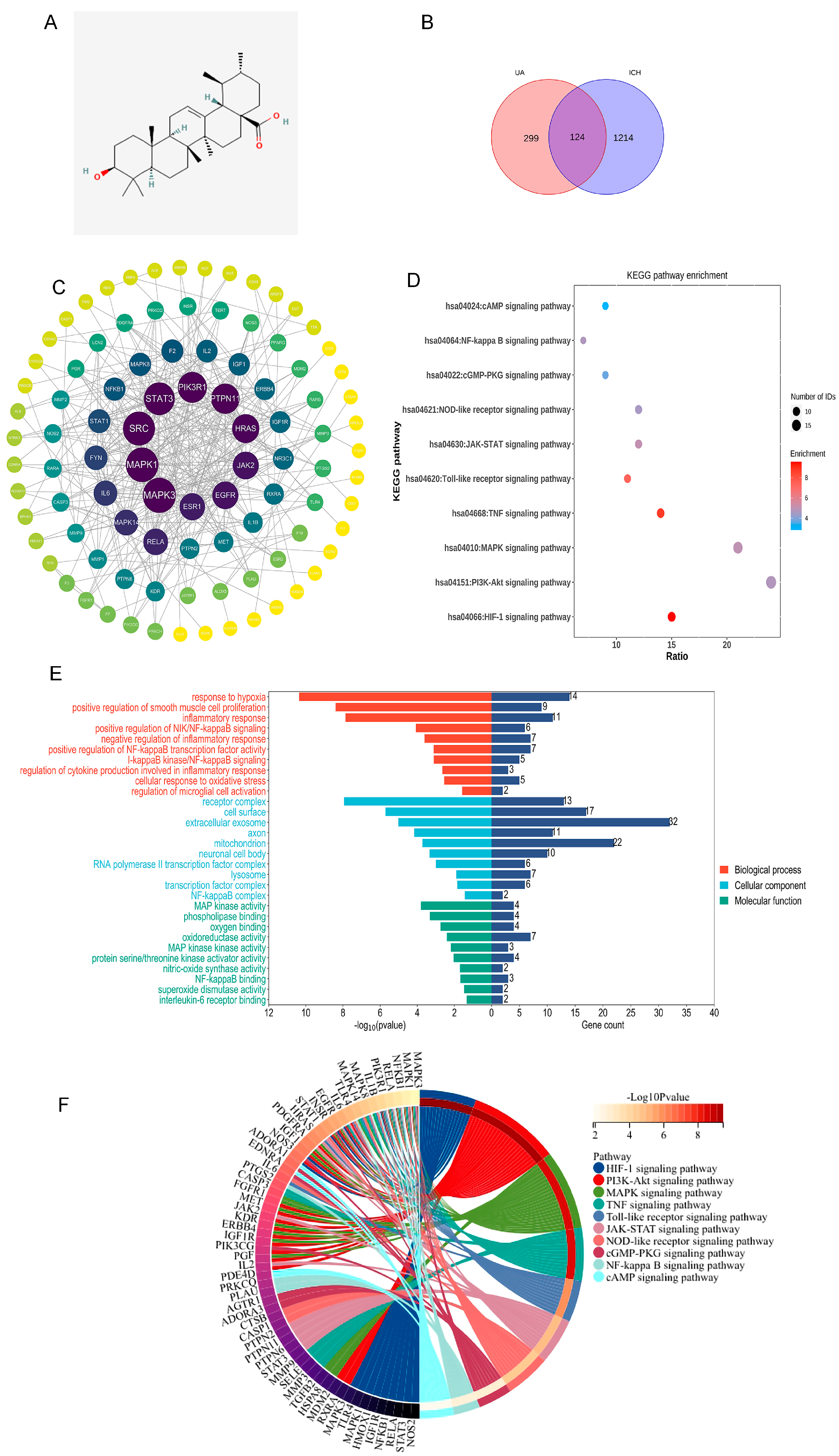
2.1. Network Analysis Suggests That Ursolic Acid May Treat Cerebral Hemorrhage via Multiple Targets
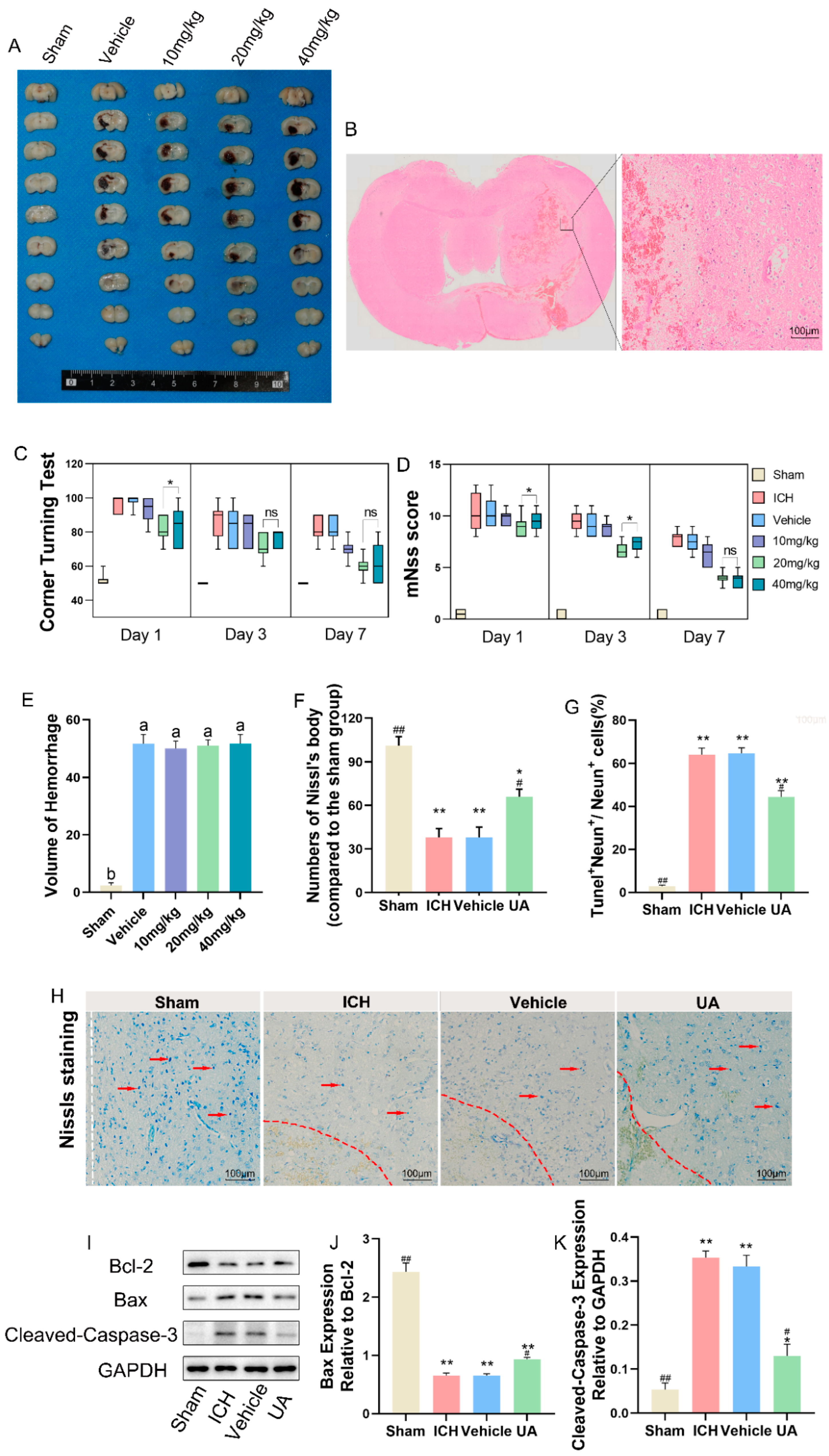
2.2. Ursolic Acid Improves Neurological Deficits in Rats with Experimental Cerebral Hemorrhage
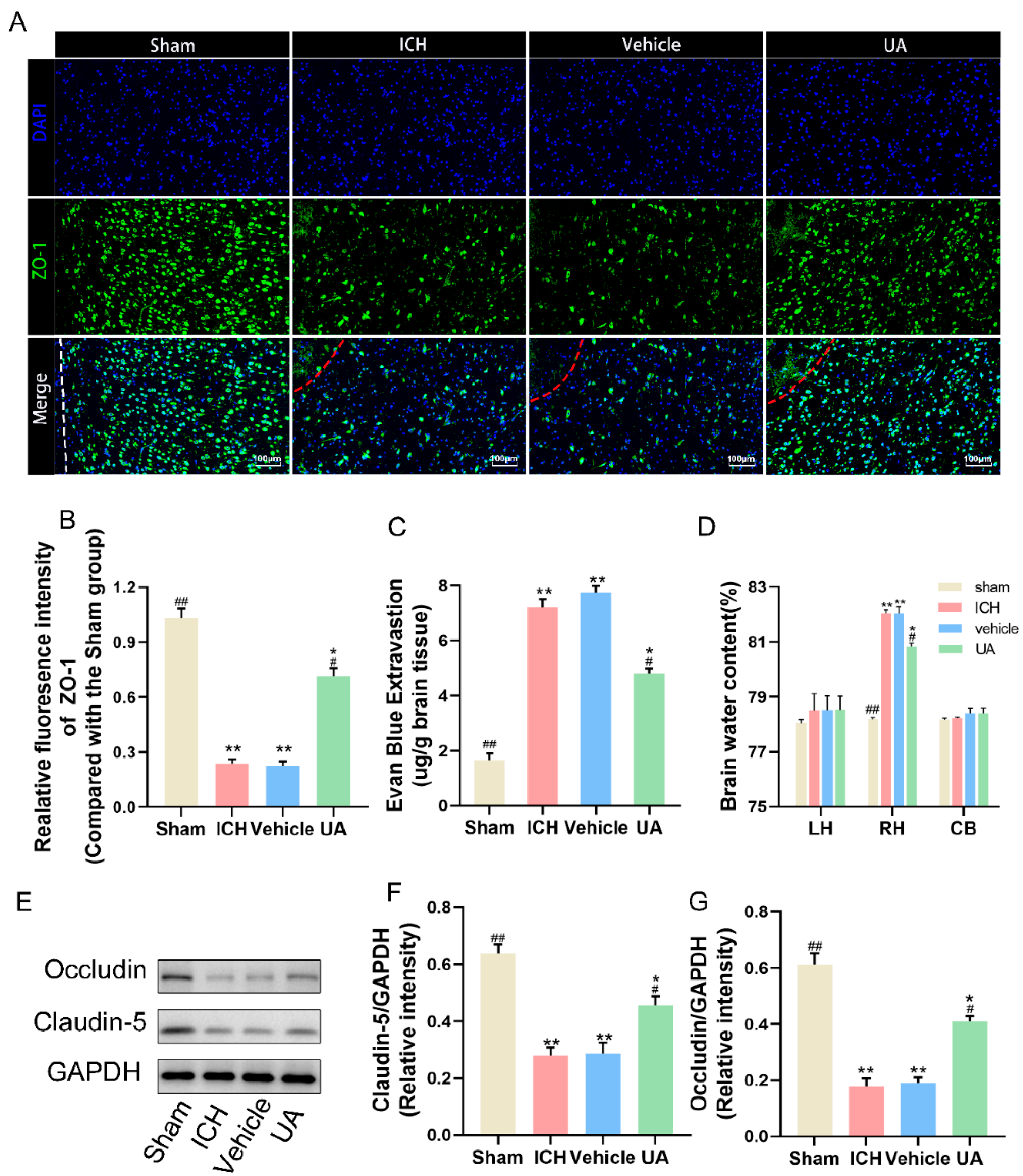
2.3. Ursolic Acid Protects the Blood–Brain Barrier
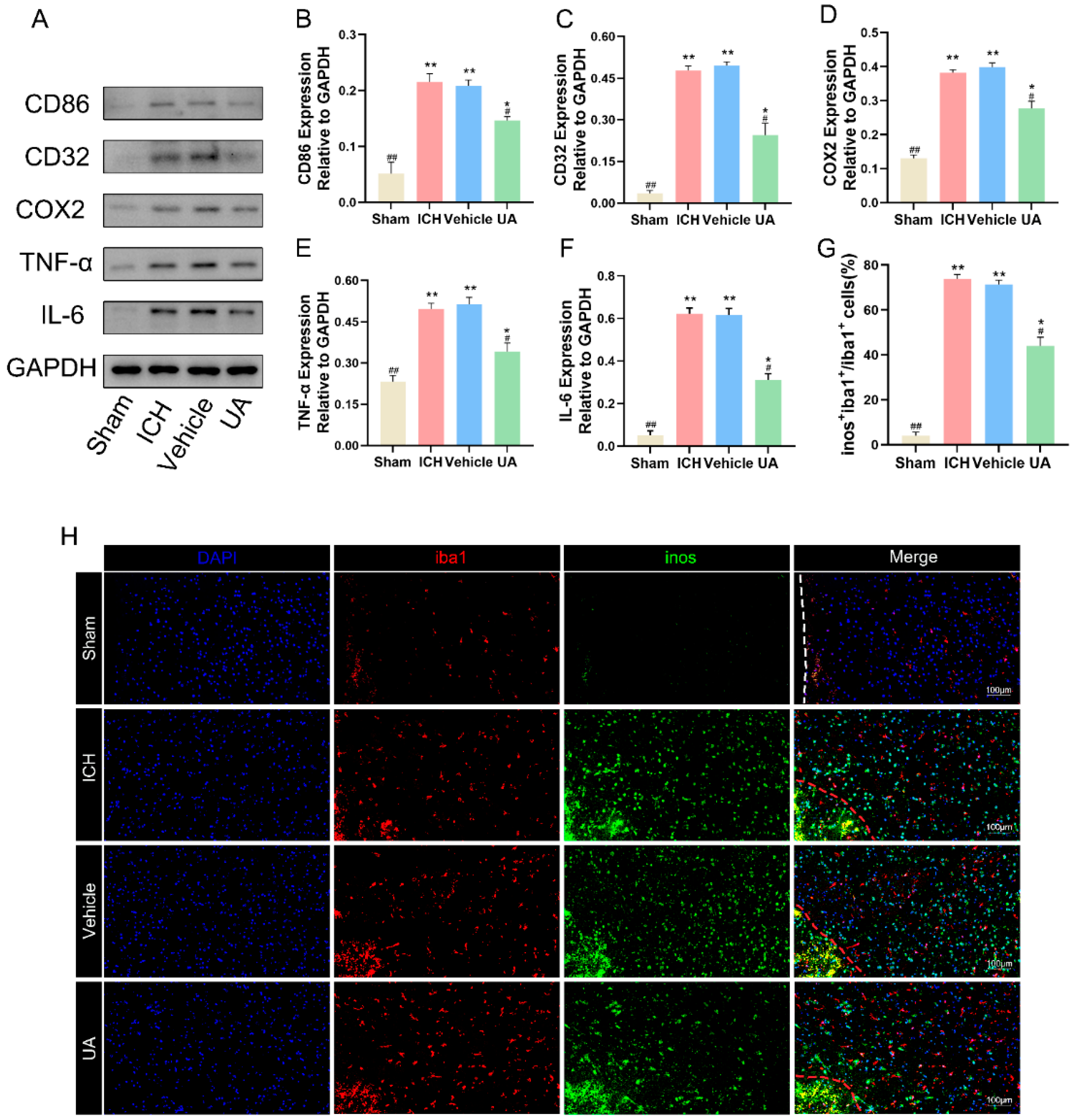
2.4. Ursolic Acid Inhibits the Inflammatory Response and Decreases Microglial M1 Polarization
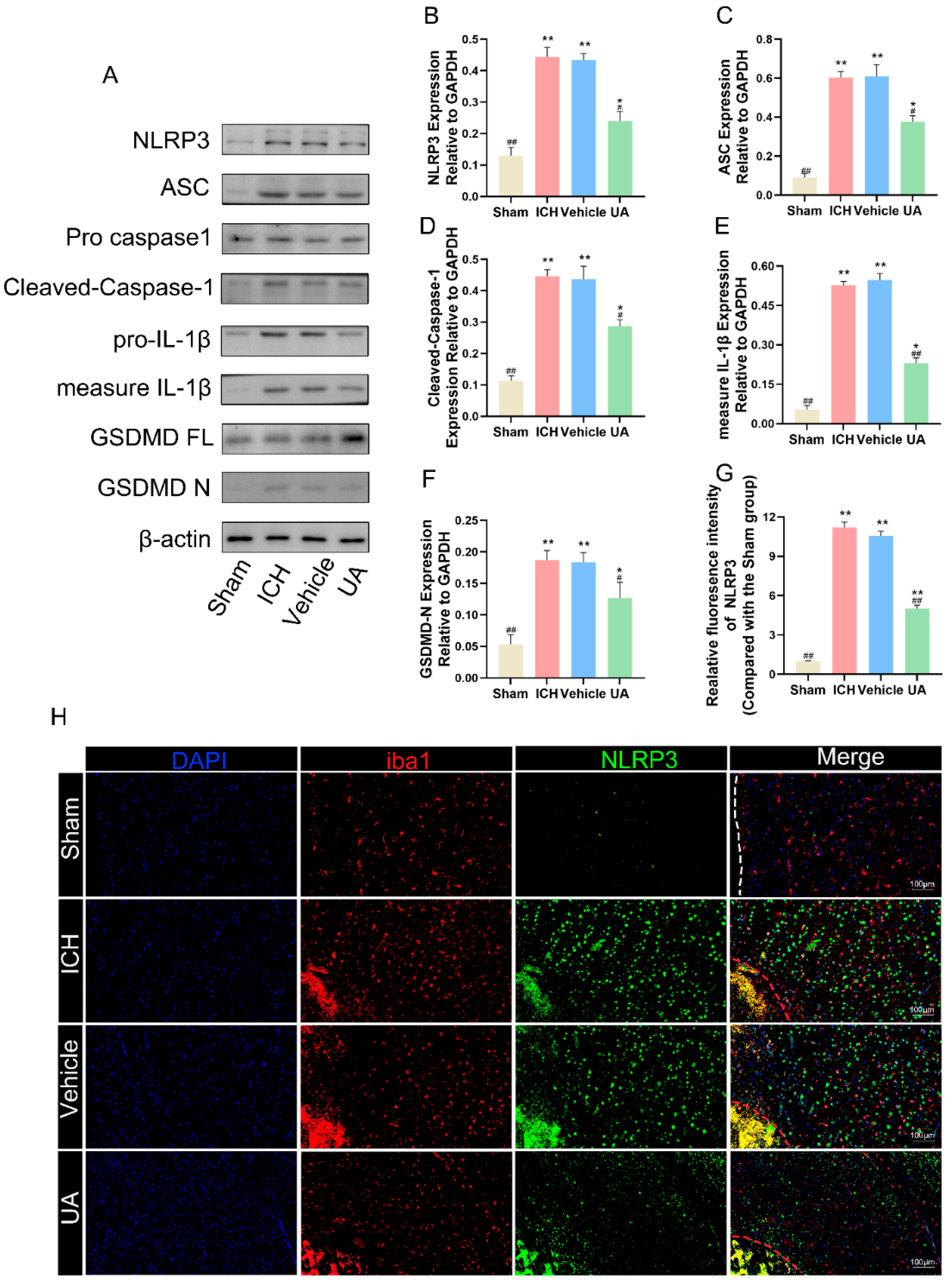
2.5. Ursolic Acid Treatment Reduces Cerebral Hemorrhage-Induced Microglial Cell Pyroptosis In Vivo
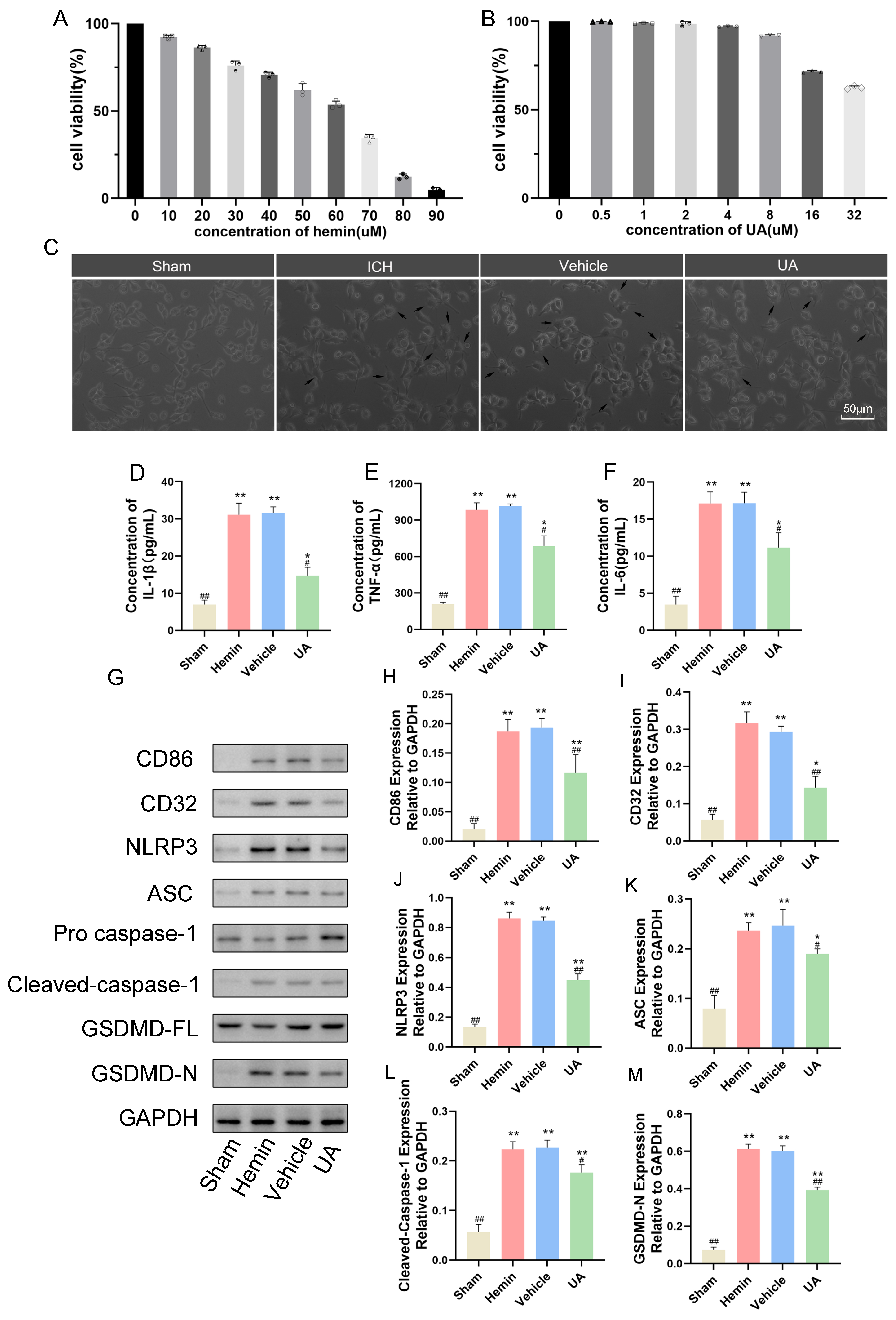
2.6. Ursolic Acid Inhibits the Microglial Cell Inflammatory Response and Pyroptosis Stimulated by Hemin In Vitro
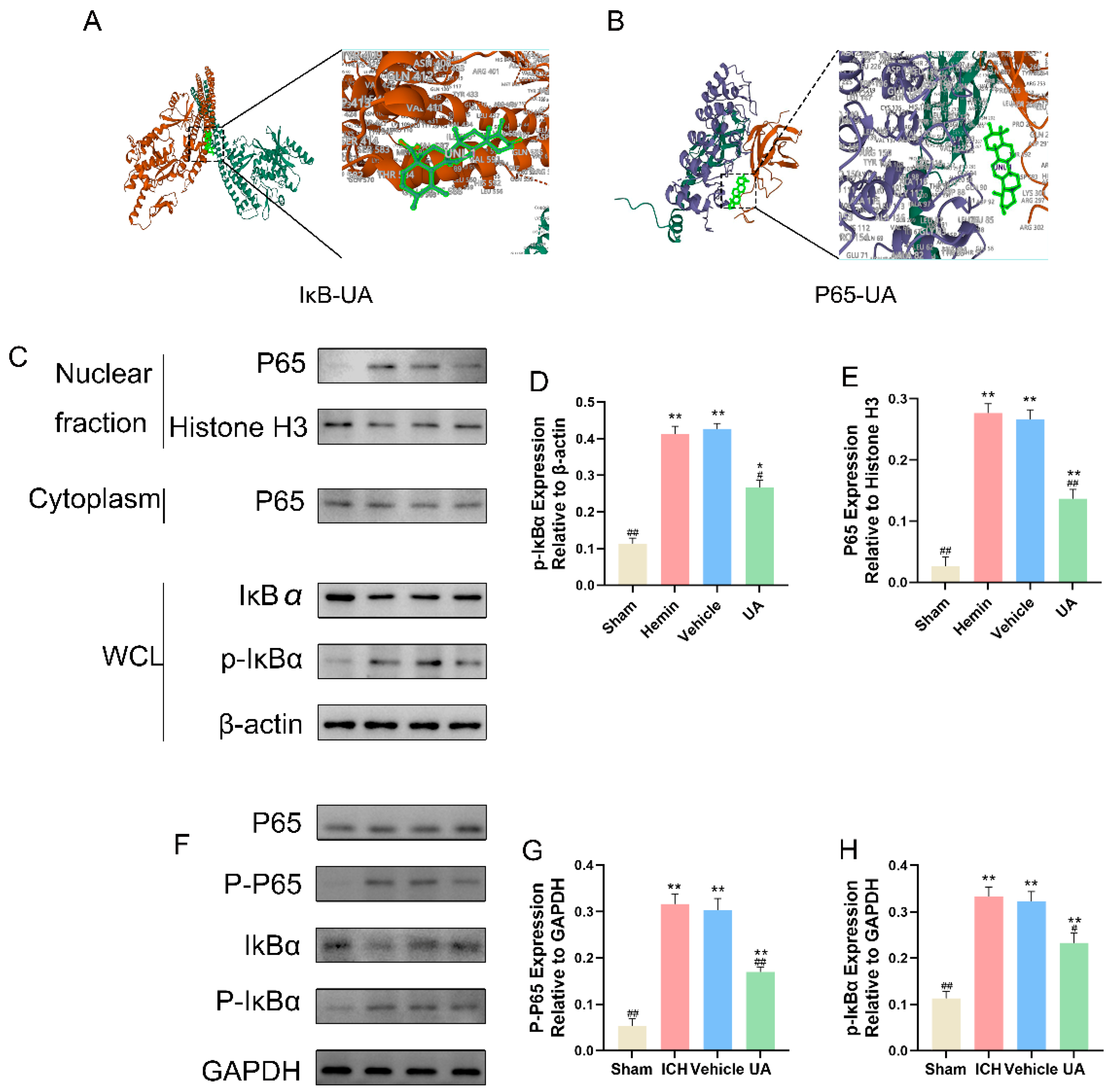
2.7. UA Attenuates Microglial Focal Death after Cerebral Hemorrhage by Inhibiting the NF-κB Pathway
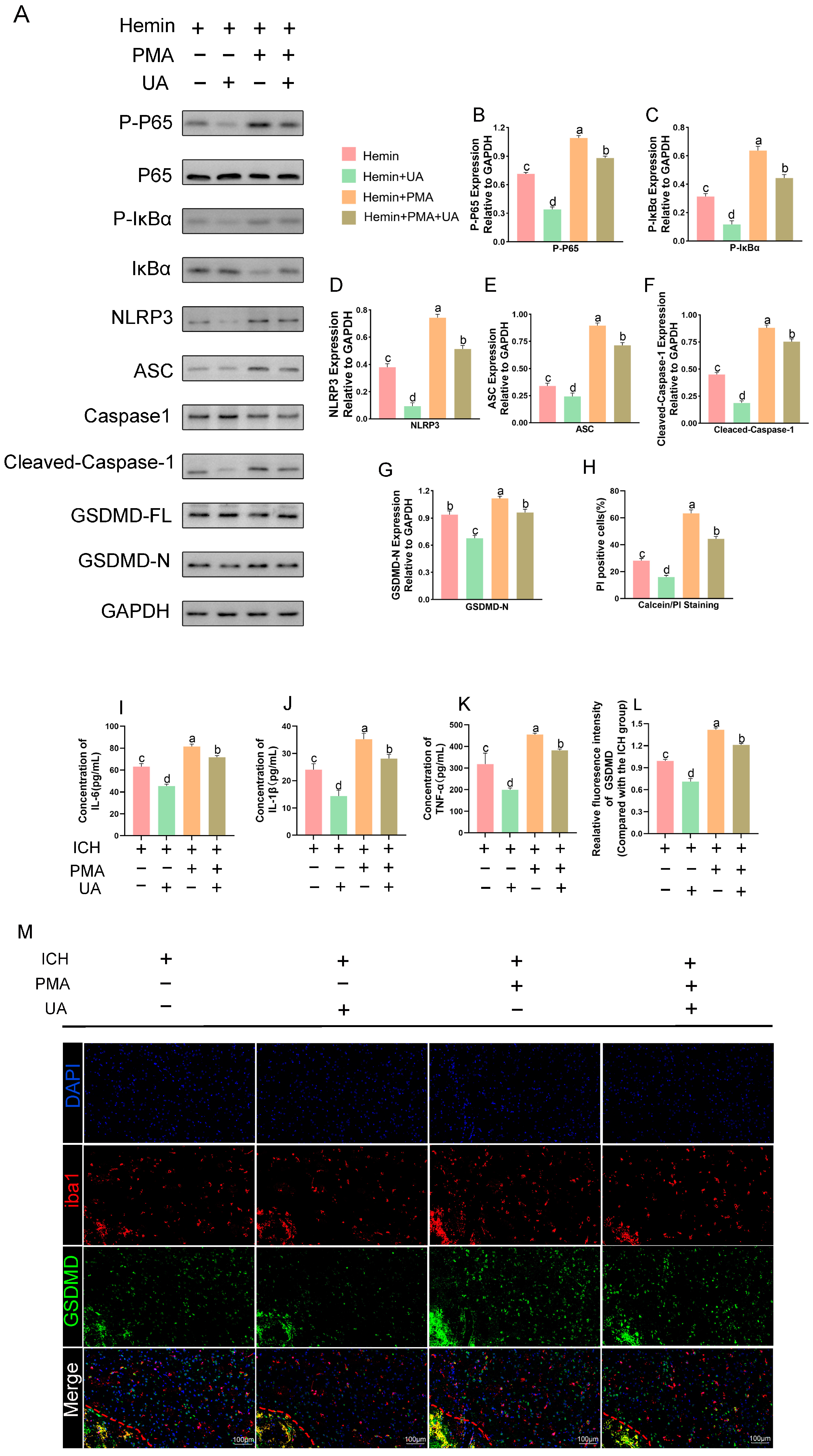
2.8. PMA Abrogates the Role of UA in Attenuating Microglial Pyroptosis
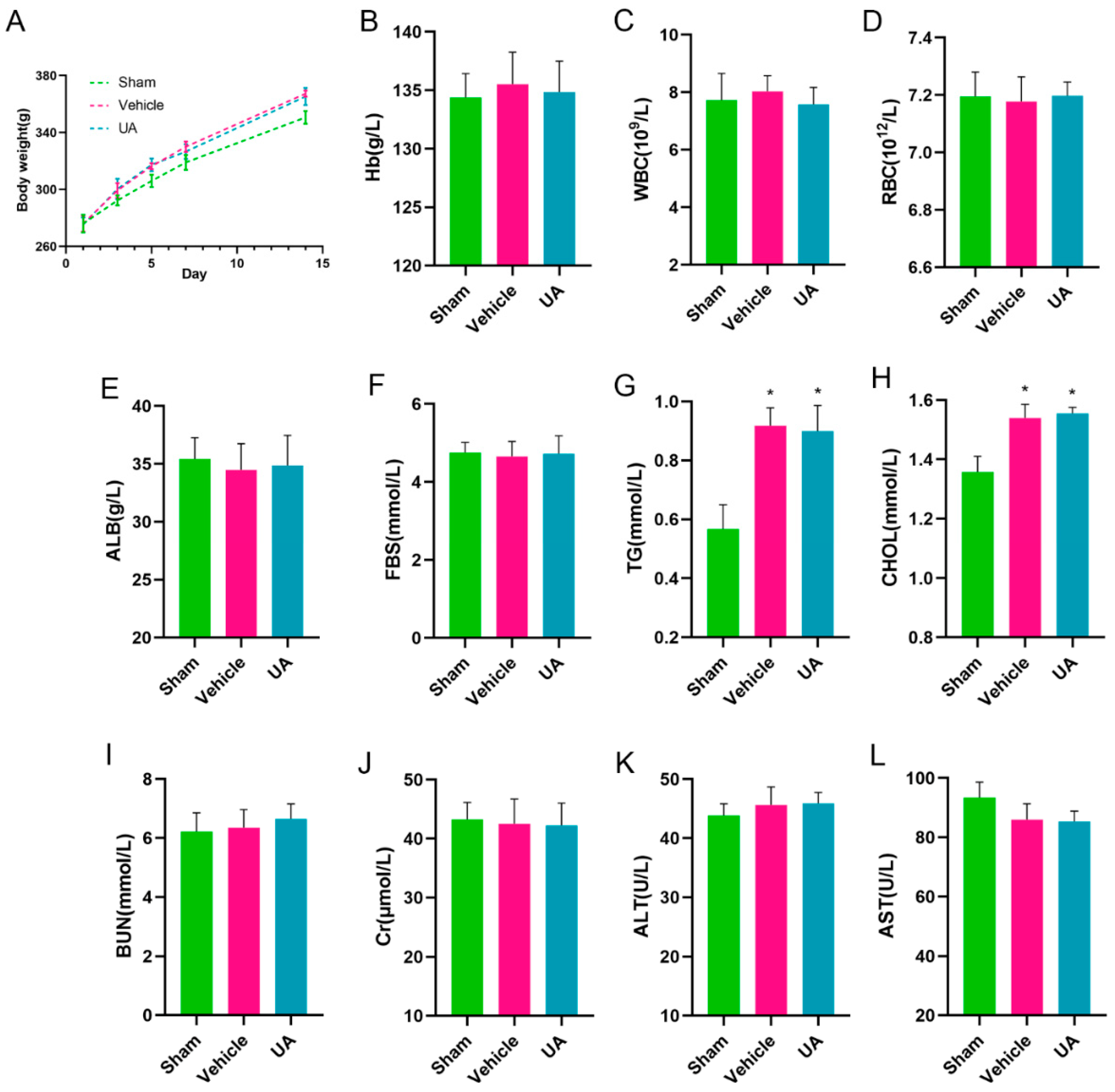
2.9. In Vivo Biosafety Assessment of Ursolic Acid
3. Discussion
4. Materials and Methods
4.1. Analysis of the Overlap of Ursolic Acid Therapeutic Targets and Cerebral Hemorrhage Markers
4.1.1. Acquisition of Therapeutic Targets for Cerebral Hemorrhage
4.1.2. Ursolic Acid Therapeutic Target Prediction
4.1.3. Ursolic Acid Therapeutic Targets and Cerebral Hemorrhage Marker Overlap
4.1.4. Protein–Protein Interaction Network Construction
4.1.5. Enrichment Analysis of Genes Overlapping Cerebral Hemorrhage and Ursolic Acid
4.2. Reagents
4.3. Animal Experiments
4.3.1. Animals
4.3.2. Modeling of Cerebral Hemorrhage
4.3.3. Neurologic function assessment
4.3.4. Paraffin Section Production
4.3.5. Measurement of Brain Water Content
4.3.6. TUNEL Staining
4.3.7. Nissl Staining
4.3.8. Immunofluorescence Staining
4.3.9. In Vivo Biosafety Assessment of Ursolic Acid
4.4. Cellular Experiments
4.4.1. BV2 Cell Culture and Processing
4.4.2. Cell Counting kit-8 (CCK-8) Assay
4.4.3. Calcein/PI Staining
4.5. Western Blotting for Protein Expression
4.6. Enzyme Linked Immunosorbent Assay (ELISA)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, L.; Hu, X.; Song, L.; Chen, X.; Ouyang, M.; Billot, L.; Li, Q.; Malavera, A.; Li, X.; Muñoz-Venturelli, P.; et al. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): An international, stepped wedge cluster randomised controlled trial. Lancet 2023, 402, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., III; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. American Heart Association/American Stroke, 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, C.; Jin, Y.; Wang, Y.; Ma, X.; Li, J.; Guo, S.; Yang, J.; Niu, J.; Liang, X. Induced neural stem cells suppressed neuroinflammation by inhibiting the microglial pyroptotic pathway in intracerebral hemorrhage rats. iScience 2023, 26, 107022. [Google Scholar] [CrossRef]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Ohashi, S.N.; DeLong, J.H.; Kozberg, M.G.; Mazur-Hart, D.J.; van Veluw, S.J.; Alkayed, N.J.; Sansing, L.H. Role of Inflammatory Processes in Hemorrhagic Stroke. Stroke 2023, 54, 605–619. [Google Scholar] [CrossRef]
- Ding, Z.; Zhong, Z.; Wang, J.; Zhang, R.; Shao, J.; Li, Y.; Wu, G.; Tu, H.; Yuan, W.; Sun, H.; et al. Inhibition of Dectin-1 Alleviates Neuroinflammatory Injury by Attenuating NLRP3 Inflammasome-Mediated Pyroptosis After Intracerebral Hemorrhage in Mice: Preliminary Study Results. J. Inflamm. Res. 2022, 15, 5917–5933. [Google Scholar] [CrossRef]
- Xue, M.; Yong, V.W. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol. 2020, 19, 1023–1032. [Google Scholar] [CrossRef]
- Xiong, X.-Y.; Liu, L.; Yang, Q.-W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef]
- Ye, F.; Yang, J.; Holste, K.G.; Koduri, S.; Hua, Y.; Keep, R.F.; Garton, H.J.; Xi, G. Characteristics of activation of monocyte-derived macrophages versus microglia after mouse experimental intracerebral hemorrhage. J. Cereb. Blood Flow. Metab. 2023, 43, 1475–1489. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Hu, J.; Zhao, Q.; Lv, B.; Ma, W.; Wang, W.; Yin, H.; Hao, Q.; Zhou, C.; et al. NOD1/RIP2 signalling enhances the microglia-driven inflammatory response and undergoes crosstalk with inflammatory cytokines to exacerbate brain damage following intracerebral haemorrhage in mice. J. Neuroinflamm. 2020, 17, 364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Q.; Hao, S.; Chen, S. The hallmark and crosstalk of immune cells after intracerebral hemorrhage: Immunotherapy perspectives. Front. Neurosci. 2022, 16, 1117999. [Google Scholar] [CrossRef]
- Jing, C.; Bian, L.; Wang, M.; Keep, R.F.; Xi, G.; Hua, Y. Enhancement of Hematoma Clearance With CD47 Blocking Antibody in Experimental Intracerebral Hemorrhage. Stroke 2019, 50, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, L.; Wang, X.; Jiang, R.; Bai, Q.; Wang, G. Microglia: A Double-Edged Sword in Intracerebral Hemorrhage from Basic Mechanisms to Clinical Research. Front. Immunol. 2021, 12, 675660. [Google Scholar] [CrossRef]
- Namdeo, P.; Gidwani, B.; Tiwari, S.; Jain, V.; Joshi, V.; Shukla, S.S.; Pandey, R.K.; Vyas, A. Therapeutic potential and novel formulations of ursolic acid and its derivatives: An updated review. J. Sci. Food Agric. 2023, 103, 4275–4292. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, Q.; Shi, A.; Wang, N.; Wang, L.; Wei, Y. Oleanolic acid and ursolic acid: Therapeutic potential in neurodegenerative diseases, neuropsychiatric diseases and other brain disorders. Nutr. Neurosci. 2023, 26, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Luan, M.; Wang, H.; Wang, J.; Zhang, X.; Zhao, F.; Liu, Z. Advances in Anti-inflammatory Activity, Mechanism and Therapeutic Application of Ursolic Acid. Mini Rev. Med. Chem. 2022, 22, 422–436. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.M.; González-Ortiz, M.; Martínez-Abundis, E.; Acuña Ortega, N. Effect of Ursolic Acid on Metabolic Syndrome, Insulin Sensitivity, and Inflammation. J. Med. Food 2017, 20, 882–886. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, Z.; Zheng, J.; Shi, J.; Cui, C. Ursolic acid ameliorates traumatic brain injury in mice by regulating microRNA-141-mediated PDCD4/PI3K/AKT signaling pathway. Int. Immunopharmacol. 2023, 120, 110258. [Google Scholar] [CrossRef]
- Zhang, T.; Su, J.; Guo, B.; Zhu, T.; Wang, K.; Li, X. Ursolic acid alleviates early brain injury after experimental subarachnoid hemorrhage by suppressing TLR4-mediated inflammatory pathway. Int. Immunopharmacol. 2014, 23, 585–591. [Google Scholar] [CrossRef]
- Zhang, T.; Su, J.; Wang, K.; Zhu, T.; Li, X. Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci. Lett. 2014, 579, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Deng, S.; Liu, F.; He, Z. Ursolic Acid Ameliorates Inflammation in Cerebral Ischemia and Reperfusion Injury Possibly via High Mobility Group Box 1/Toll-Like Receptor 4/NFkappaB Pathway. Front. Neurol. 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Sun, Y.; Liu, S.; Yang, S.; Chen, C.; Zhang, Z.; Chu, S.; Yang, Y.; Pei, G.; Lin, M.; et al. Targeting pyroptosis as a preventive and therapeutic approach for stroke. Cell Death Discov. 2023, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Pharmacological Inhibition of the NLRP3 Inflammasome: Structure, Molecular Activation, and Inhibitor-NLRP3 Interaction. Pharmacol. Rev. 2023, 75, 487–520. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, T.; Luo, C.; Cai, J.; Zhou, X.; Xiao, X.; Liu, S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020, 104, 6129–6140. [Google Scholar] [CrossRef]
- Tu, W.J.; Wang, L.D. Special Writing Group of China Stroke Surveillance, China stroke surveillance report 2021. Mil. Med. Res. 2023, 10, 33. [Google Scholar]
- Hemphill, J.C., III; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, M.; Yong, V.W. Central Nervous System Tissue Regeneration after Intracerebral Hemorrhage: The Next Frontier. Cells 2021, 10, 2513. [Google Scholar] [CrossRef]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef]
- Li, Z.; Khan, S.; Liu, Y.; Wei, R.; Yong, V.W.; Xue, M. Therapeutic strategies for intracerebral hemorrhage. Front. Neurol. 2022, 13, 1032343. [Google Scholar] [CrossRef]
- Yang, G.; Fan, X.; Mazhar, M.; Guo, W.; Zou, Y.; Dechsupa, N.; Wang, L. Neuroinflammation of microglia polarization in intracerebral hemorrhage and its potential targets for intervention. Front. Mol. Neurosci. 2022, 15, 1013706. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dai, W.; Zheng, Y.; Qiao, W.; Chen, W.; Peng, L.; Zhou, H.; Zhao, T.; Liu, H.; Zheng, F.; et al. Mechanism and Regulation of Microglia Polarization in Intracerebral Hemorrhage. Molecules 2022, 27, 7080. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Gao, L.; Cheng, H. Inflammatory Profiles of the Interleukin Family and Network in Cerebral Hemorrhage. Cell. Mol. Neurobiol. 2018, 38, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- Gu, L.; Sun, M.; Li, R.; Tao, Y.; Luo, X.; Zhang, X.; Yuan, Y.; Xie, Z. Microglial pyroptosis: Therapeutic target in secondary brain injury following intracerebral hemorrhage. Front. Cell. Neurosci. 2022, 16, 971469. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ullevig, S.L.; Short, J.D.; Wang, L.; Ahn, Y.J.; Asmis, R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants 2021, 10, 1161. [Google Scholar] [CrossRef]
- Liu, H.; Ahmad, N.; Lv, B.; Li, C. Advances in production and structural derivatization of the promising molecule ursolic acid. Biotechnol. J. 2021, 16, e2000657. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.; Zhao, J.; Wang, H. A phase I trial to evaluate the multiple-dose safety and antitumor activity of ursolic acid liposomes in subjects with advanced solid tumors. Biomed. Res. Int. 2015, 2015, 809714. [Google Scholar] [CrossRef]
- Cao, D.; Li, B.; Cao, C.; Zhang, J.; Li, X.; Li, H.; Yu, Z.; Shen, H.; Ye, M. Caveolin-1 aggravates neurological deficits by activating neuroinflammation following experimental intracerebral hemorrhage in rats. Exp. Neurol. 2023, 368, 114508. [Google Scholar] [CrossRef]
- Yao, Z.-J.; Dong, J.; Che, Y.-J.; Zhu, M.-F.; Wen, M.; Wang, N.-N.; Wang, S.; Lu, A.-P.; Cao, D.-S. TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput. Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, H.; Guo, S.; Li, P.; Qin, S.; Shi, M.; Zeng, C. Effect and mechanism of edible oil co-digestion on the bioaccessibility and bioavailability of ursolic acid. Food Chem. 2023, 423, 136220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Z.; Chen, Y.; Wang, C.; Gong, P.; Jiang, R.; Liu, Q. Targeting the AKT-P53/CREB pathway with epicatechin for improved prognosis of traumatic brain injury. CNS Neurosci. Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, D.; Li, J.; Zhang, L.; Chen, J.; Wang, L.; Gao, Y. A comparative study of different doses of bone marrow-derived mesenchymal stem cells improve post-stroke neurological outcomes via intravenous transplantation. Brain Res. 2023, 1798, 148161. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, X.; Pang, Y.; Cheng, S.; Liu, J. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat. Commun. 2019, 10, 5783. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Yang, X.; Fu, Z.; Ji, X.; Shi, Y.; Zhong, J.; Hu, W.; Ye, Y.; Wang, Z.; et al. Oral zero-valent-molybdenum nanodots for inflammatory bowel disease therapy. Sci. Adv. 2022, 8, eabp9882. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, Y.; Ma, Y.; Lin, B.; Cao, L.; Wang, B.; Zhang, Z.; Yu, H.; Li, J.; Huang, M.; et al. Targeted photodynamic therapy of cancer using a novel gallium (III) tris (ethoxycarbonyl) corrole conjugated-mAb directed against cancer/testis antigens 83. Cancer Med. 2018, 7, 3057–3065. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, P.; Li, Z.; Hua, Q.; Song, P.; Gao, L.; Zhou, L.; Cai, Q. Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway. Int. J. Mol. Sci. 2023, 24, 14771. https://doi.org/10.3390/ijms241914771
Lei P, Li Z, Hua Q, Song P, Gao L, Zhou L, Cai Q. Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway. International Journal of Molecular Sciences. 2023; 24(19):14771. https://doi.org/10.3390/ijms241914771
Chicago/Turabian StyleLei, Pan, Zhiyang Li, Qiuwei Hua, Ping Song, Lun Gao, Long Zhou, and Qiang Cai. 2023. "Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway" International Journal of Molecular Sciences 24, no. 19: 14771. https://doi.org/10.3390/ijms241914771
APA StyleLei, P., Li, Z., Hua, Q., Song, P., Gao, L., Zhou, L., & Cai, Q. (2023). Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway. International Journal of Molecular Sciences, 24(19), 14771. https://doi.org/10.3390/ijms241914771






