Alleviative Effect of Iodine Pretreatment on the Stress of Saccharina japonica (Phaeophyceae, Laminariales) Caused by Cadmium and Its Molecular Basis Revealed by Comparative Transcriptomic Analysis
Abstract
:1. Introduction
2. Results
2.1. Effects of Cadmium and Iodine on Growth of S. japonica Sporophytes
2.2. Quantification of Iodine and Cadmium in Saccharina japonica Thalli
2.3. Trend Analysis
2.4. Analysis of the Differentially Expressed Genes (DEGs)
2.5. DEGs Related to Iodine Metabolism
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
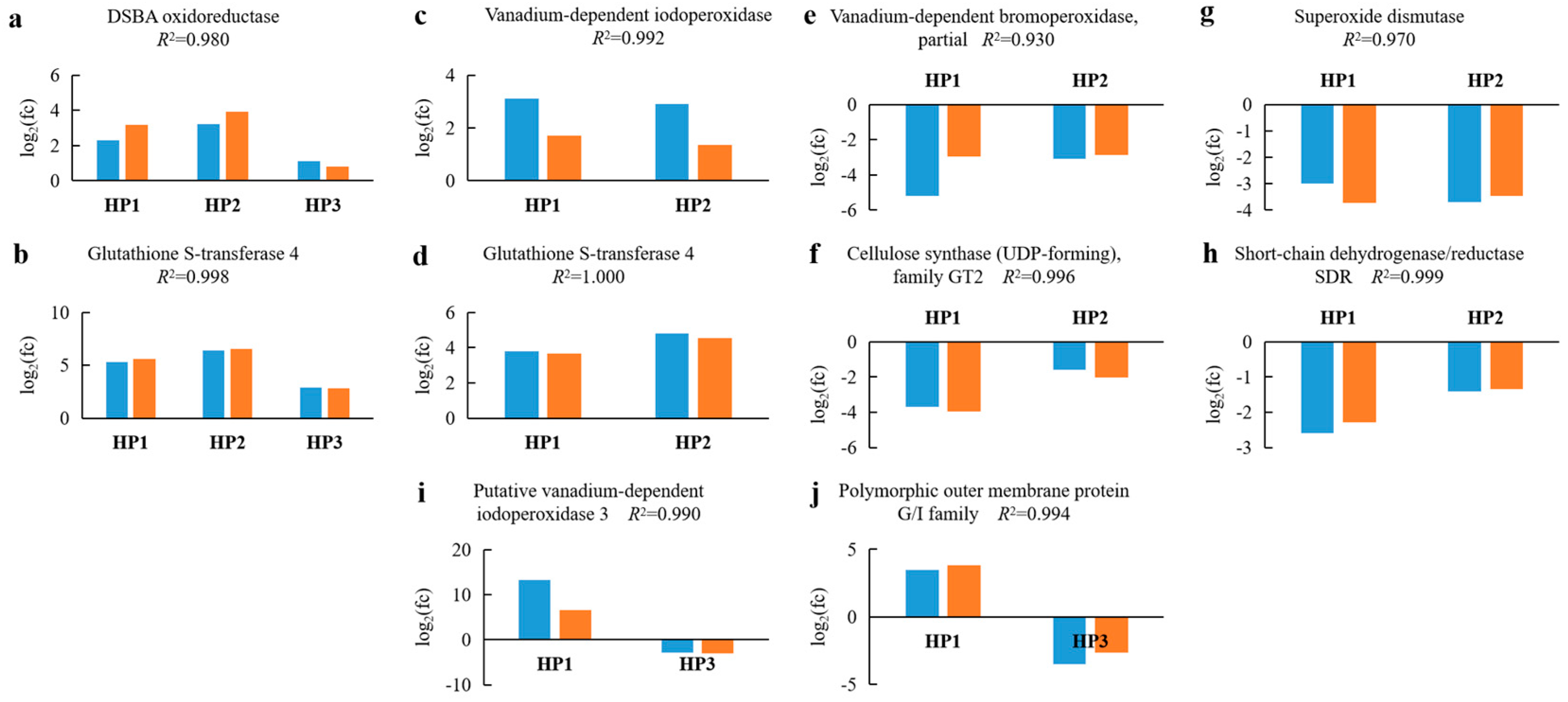
2.7. Validation of DEGs by Reverse Transcription Quantitative PCR (RT-qPCR)
3. Discussion
4. Materials and Methods
4.1. Algal Materials, Cultivation, and Treatment
4.2. Iodine and Cadmium Quantitative Analysis
4.3. RNA Preparation, cDNA Library Construction, and Sequencing
4.4. Alignment and Annotation
4.5. Differential Expression Analysis and GO Enrichment Analysis
4.6. WGCNA Analysis
4.7. Validation of DEGs by RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitschke, U.; Walsh, P.; McDaid, J.; Stengel, D.B. Variability in iodine in temperate seaweeds and iodine accumulation kinetics of Fucus vesiculosus and Laminaria digitata (Phaeophyceae, Ochrophyta). J. Phycol. 2018, 54, 114–125. [Google Scholar] [CrossRef]
- Carpenter, L.J.; MacDonald, S.M.; Shaw, M.D.; Kumar, R.; Saunders, R.W.; Parthipan, R.; Wilson, J.; Plane, J.M.C. Atmospheric iodine levels influenced by sea surface emissions of inorganic iodine. Nat. Geosci. 2013, 6, 108–111. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; Mcfiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef]
- China Agriculture Department. China Fishery Statistical Yearbook; Compiled by Fishery Bureau of China Agriculture Department; China Agriculture Press: Beijing, China, 2022. (In Chinese) [Google Scholar]
- Klettner, A. Fucoidan as a potential therapeutic for major blinding diseases-a hypothesis. Mar. Drugs 2016, 14, 31. [Google Scholar] [CrossRef]
- Tseng, C.K. Algal biotechnology industries and research activities in China. J. Appl. Phycol. 2001, 13, 375–380. [Google Scholar] [CrossRef]
- Yan, Z.M.; Jiang, N.E.; Fang, Z.X. Responses to iodine of the female gametophytes of Laminaria japonica. J. Shandong Col. Oceanol. 1981, 11, 86–89. (In Chinese) [Google Scholar]
- Gu, R.T.; Lin, H.Y.; Zhou, Y.; Song, X.Y.; Xu, S.C.; Yue, S.D.; Zhang, Y.; Xu, S.; Zhang, X.M. Programmed responses of different life-stages of the seagrass Ruppia sinensis to copper and cadmium exposure. J. Hazard. Mater. 2021, 403, 123875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Xu, T.; Zou, H.X.; Pang, Q.Y. Comparative proteomic analysis provides insight into cadmium stress responses in brown algae Sargassum fusiforme. Aquat. Toxicol. 2015, 163, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kremer, B.P.; Markham, J.W. Primary Metabolic Effects of Cadmium in the Brown Alga, Laminaria saccharina. Z. Pflanzenphysiol. 1982, 108, 125–130. [Google Scholar] [CrossRef]
- Lee, M.Y.; Shin, H.W. Cadmium-induced changes in antioxidant enzymes from the marine alga Nannochloropsis oculata. J. Appl. Phycol. 2003, 15, 13–19. [Google Scholar] [CrossRef]
- Shi, W.G.; Zhang, Y.H.; Chen, S.L.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Küpper, F.C.; Miller, E.P.; Andrews, S.J.; Hughes, C.; Carpenter, L.J.; Meyer-Klaucke, W.; Toyama, C.; Muramatsu, Y.; Feiters, M.C.; Carrano, C.J. Emission of volatile halogenated compounds, speciation and localization of bromine and iodine in the brown algal genome model Ectocarpus siliculosus. J. Biol. Inorg. Chem. 2018, 23, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Vilter, H. Vanadium-dependent haloperoxidases. Met. Ions Biol. Syst. 1995, 31, 325–362. [Google Scholar] [PubMed]
- Liang, X.Y.; Wang, X.M.; Chi, S.; Wu, S.X.; Sun, J.; Liu, C.; Chen, S.P.; Yu, J.; Liu, T. Analysis of Saccharina japonica transcriptome using the high-throughput DNA sequencing technique and its vanadium-dependent haloperoxidase gene. Acta Oceanol. Sin. 2014, 33, 27–36. [Google Scholar] [CrossRef]
- Kovacik, J.; Micalizzi, G.; Dresler, S.; Babula, P.; Hladky, J.; Chemodanov, A.; Mondello, L. Metabolic responses of Ulva compressa to single and combined heavy metals. Chemosphere 2018, 213, 384–394. [Google Scholar] [CrossRef]
- Kovacik, J.; Klejdus, B.; Babula, P.; Hedbavny, J. Age affects not only metabolome but also metal toxicity in Scenedesmus quadricauda cultures. J. Hazard. Mater. 2016, 306, 58–66. [Google Scholar] [CrossRef]
- Stengel, D.B.; Nitschke, U. Iodine contributes to osmotic acclimatisation in the kelp Laminaria digitata (Phaeophyceae). Planta 2014, 239, 521–530. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; Leblanc, C.; Toyama, C.; Uchida, Y.; Maskrey, B.H.; Robinson, J.; Verhaeghe, E.F.; Malin, G.; Luther, G.W., III; et al. In vivo speciation studies and antioxidant properties of bromine in Laminaria digitata reinforce the significance of iodine accumulation for kelps. J. Exp. Bot. 2013, 64, 2653–2664. [Google Scholar] [CrossRef]
- Su, L.; Shan, T.F.; Li, J.; Gao, S.Q.; Pang, S.J.; Leng, X.F.; Zhang, Y.; Zhang, M.F.; Gao, H.T. Characterization of the novel hybrid cultivar E25 of Saccharina japonica in the northern farming region of China. J. Appl. Phycol. 2021, 33, 4011–4021. [Google Scholar] [CrossRef]
- Wang, X.M.; Shan, T.F.; Pang, S.J. Phytoremediation potential of Saccharina japonica and Sargassum horneri (phaeophyceae): Biosorption study of strontium. Bull. Environ. Contam. Toxicol. 2018, 101, 501–505. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Yao, J.T.; Wang, X.L.; Guo, H.; Duan, D.L. Transcriptome sequencing and comparative analysis of Saccharina japonica (Laminariales, Phaeophyceae) under blue light induction. PLoS ONE 2012, 7, e39704. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
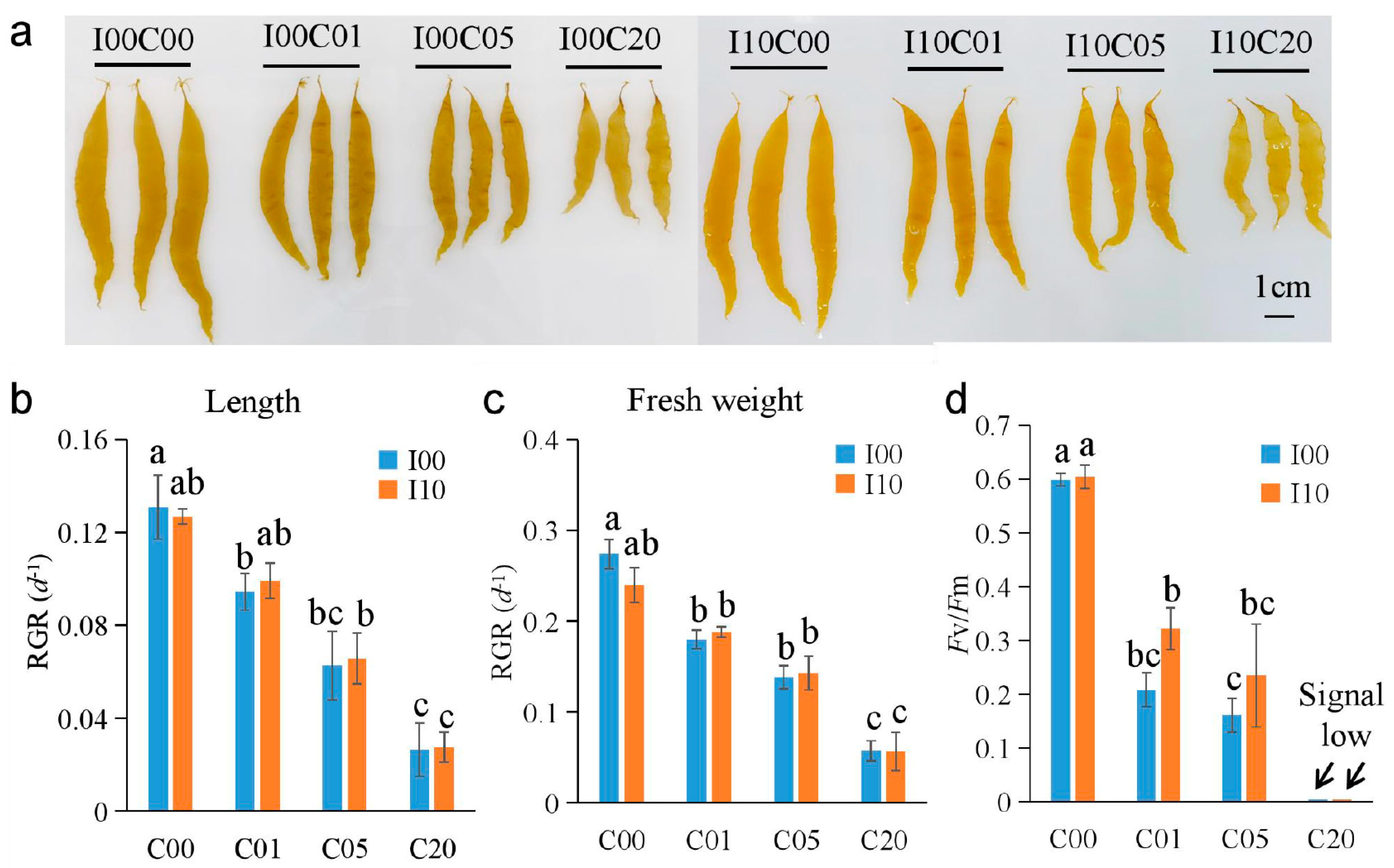
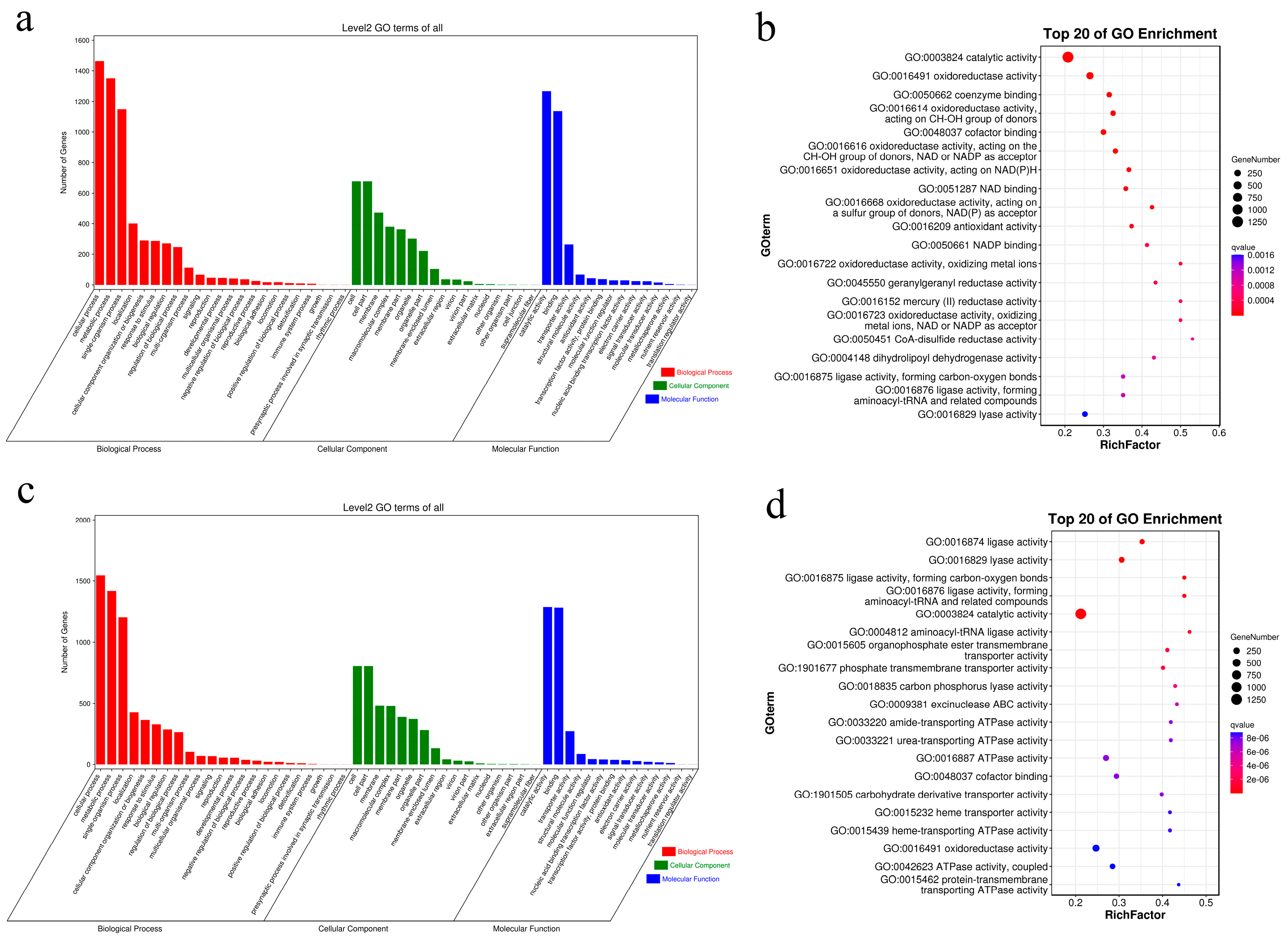

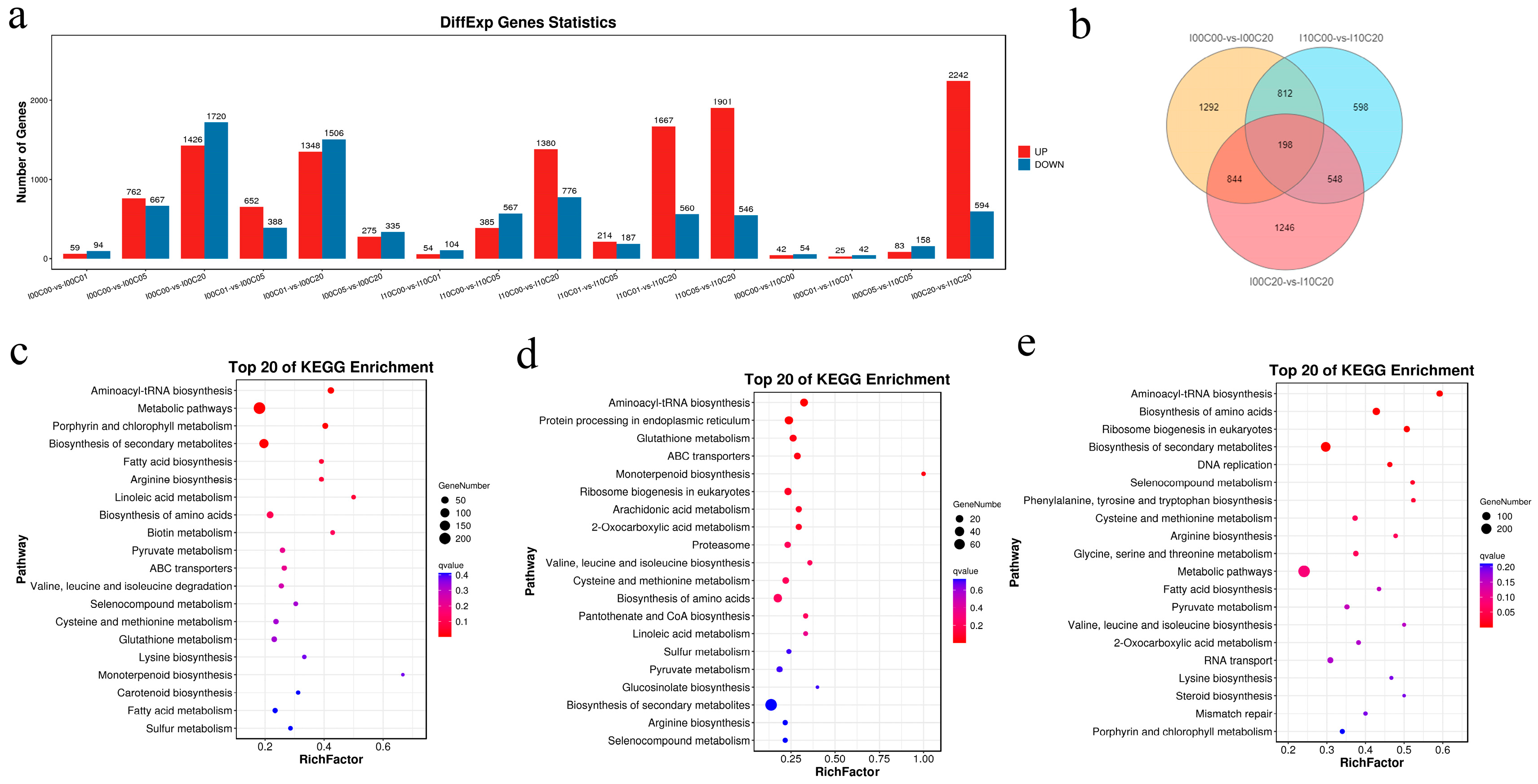
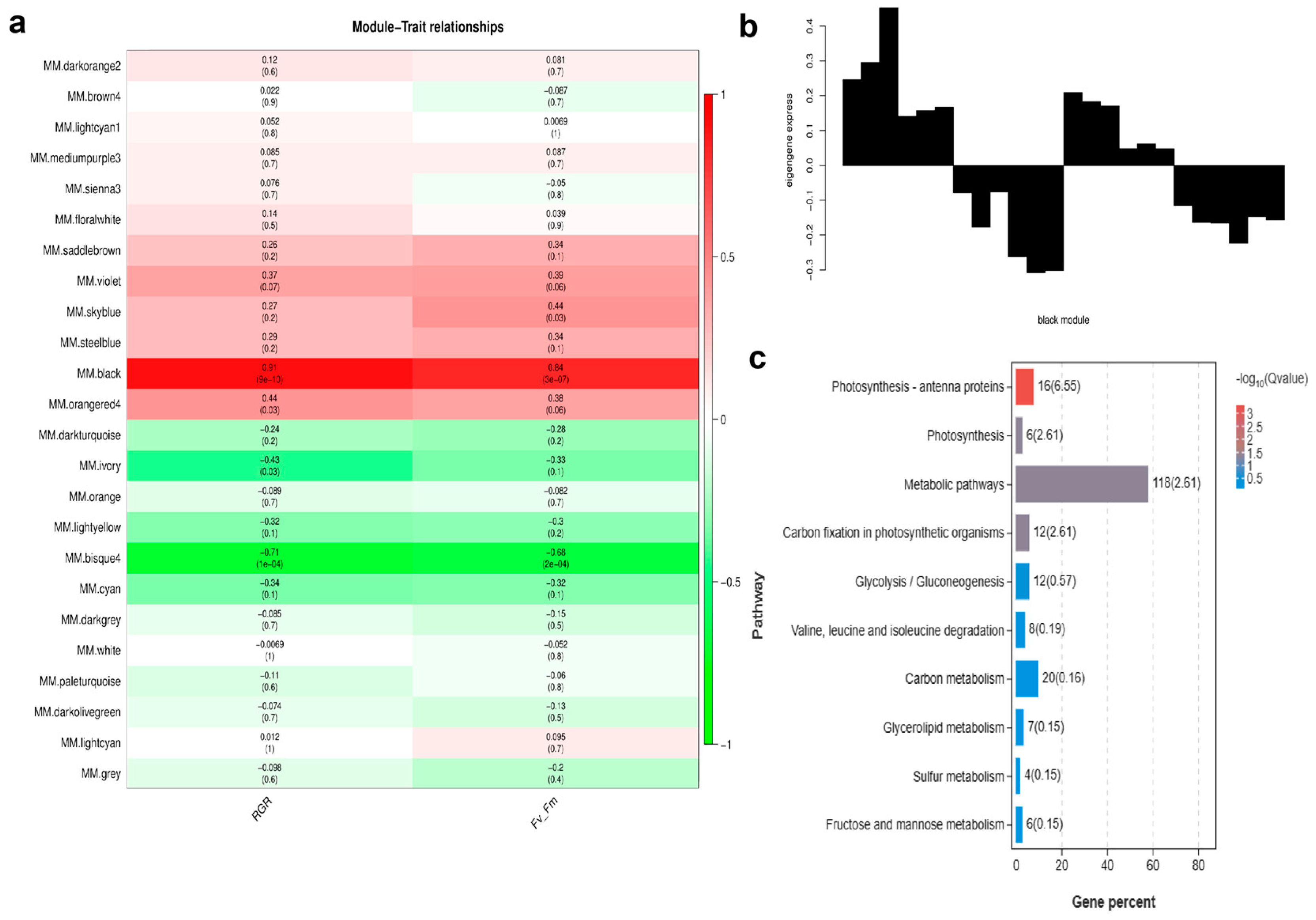
| Group | Iodine Content (mg kg−1) | Cadmium Content (mg kg−1) |
|---|---|---|
| I00C00 | 457.73 ± 9.28 a | 0.33 ± 0.02 a |
| I00C01 | 454.20 ± 10.14 a | 51.49 ± 1.48 c |
| I00C05 | 466.67 ± 13.36 a | 148.01 ± 2.15 d |
| I00C20 | 490.87 ± 6.07 a | 425.21 ± 4.84 e |
| I10C00 | 1531.50 ± 19.99 b | 9.28 ± 0.32 b |
| I10C01 | 1552.70 ± 37.64 b | 48.08 ± 2.26 c |
| I10C05 | 1809.00 ± 12.13 c | 158.76 ± 4.64 d |
| I10C20 | 2236.07 ± 24.00 d | 434.00 ± 4.64 e |
| Gene ID | Gene Description | log2(fc) | ||
|---|---|---|---|---|
| I00C20/I00C00 | I10C20/I10C00 | I10C20/I00C20 | ||
| SJ07392 | vBPO, partial | −5.2 | −3.1 | NS |
| SJ07391 | vBPO, partial | −3.0 | −4.9 | NS |
| SJ10798 | vBPO | −1.5 | NS | 1.2 |
| SJ10789 | vBPO, partial | 2.5 | NS | −2.0 |
| MSTRG.19135 | vBPO2 | 11.1 | NS | NS |
| SJ01273 | vIPO1 | −1.7 | NS | NS |
| SJ15636 | vIPO | 2.6 | 2.3 | NS |
| SJ15637 | vIPO | 3.1 | 2.9 | NS |
| SJ15629 | vIPO | 3.5 | 3.8 | NS |
| SJ10612 | vIPO1 | 8.4 | NS | −3.7 |
| SJ02183 | vIPO1 | NS | −9.4 | NS |
| SJ02174 | vIPO1 | NS | −2.6 | NS |
| SJ15639 | vIPO | NS | NS | 2.3 |
| SJ17646 | Putative vIPO3 | −1.5 | NS | NS |
| SJ12376 | Putative vIPO3 | 13.2 | NS | −2.8 |
| SJ12374 | Putative vIPO3 | NS | 1.3 | 1.6 |
| SJ12375 | Putative vIPO3 | NS | 2.2 | 2.5 |
| SJ17646 | Putative vIPO3 | NS | NS | 2.4 |
| Iodine Pretreatment | Concentration of Added Cadmium | Group Codes |
|---|---|---|
| No iodine pretreatment | 0 mg L−1 | I00C00 |
| 1 mg L−1 | I00C01 | |
| 5 mg L−1 | I00C05 | |
| 20 mg L−1 | I00C20 | |
| 10 mg L−1 iodine pretreatment for 48 h | 0 mg L−1 | I10C00 |
| 1 mg L−1 | I10C01 | |
| 5 mg L−1 | I10C05 | |
| 20 mg L−1 | I10C20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shan, T.; Pang, S. Alleviative Effect of Iodine Pretreatment on the Stress of Saccharina japonica (Phaeophyceae, Laminariales) Caused by Cadmium and Its Molecular Basis Revealed by Comparative Transcriptomic Analysis. Int. J. Mol. Sci. 2023, 24, 14825. https://doi.org/10.3390/ijms241914825
Wang X, Shan T, Pang S. Alleviative Effect of Iodine Pretreatment on the Stress of Saccharina japonica (Phaeophyceae, Laminariales) Caused by Cadmium and Its Molecular Basis Revealed by Comparative Transcriptomic Analysis. International Journal of Molecular Sciences. 2023; 24(19):14825. https://doi.org/10.3390/ijms241914825
Chicago/Turabian StyleWang, Xuemei, Tifeng Shan, and Shaojun Pang. 2023. "Alleviative Effect of Iodine Pretreatment on the Stress of Saccharina japonica (Phaeophyceae, Laminariales) Caused by Cadmium and Its Molecular Basis Revealed by Comparative Transcriptomic Analysis" International Journal of Molecular Sciences 24, no. 19: 14825. https://doi.org/10.3390/ijms241914825






