Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Low Phosphorus Tolerance in Wheat Seedling
Abstract
:1. Introduction
2. Results
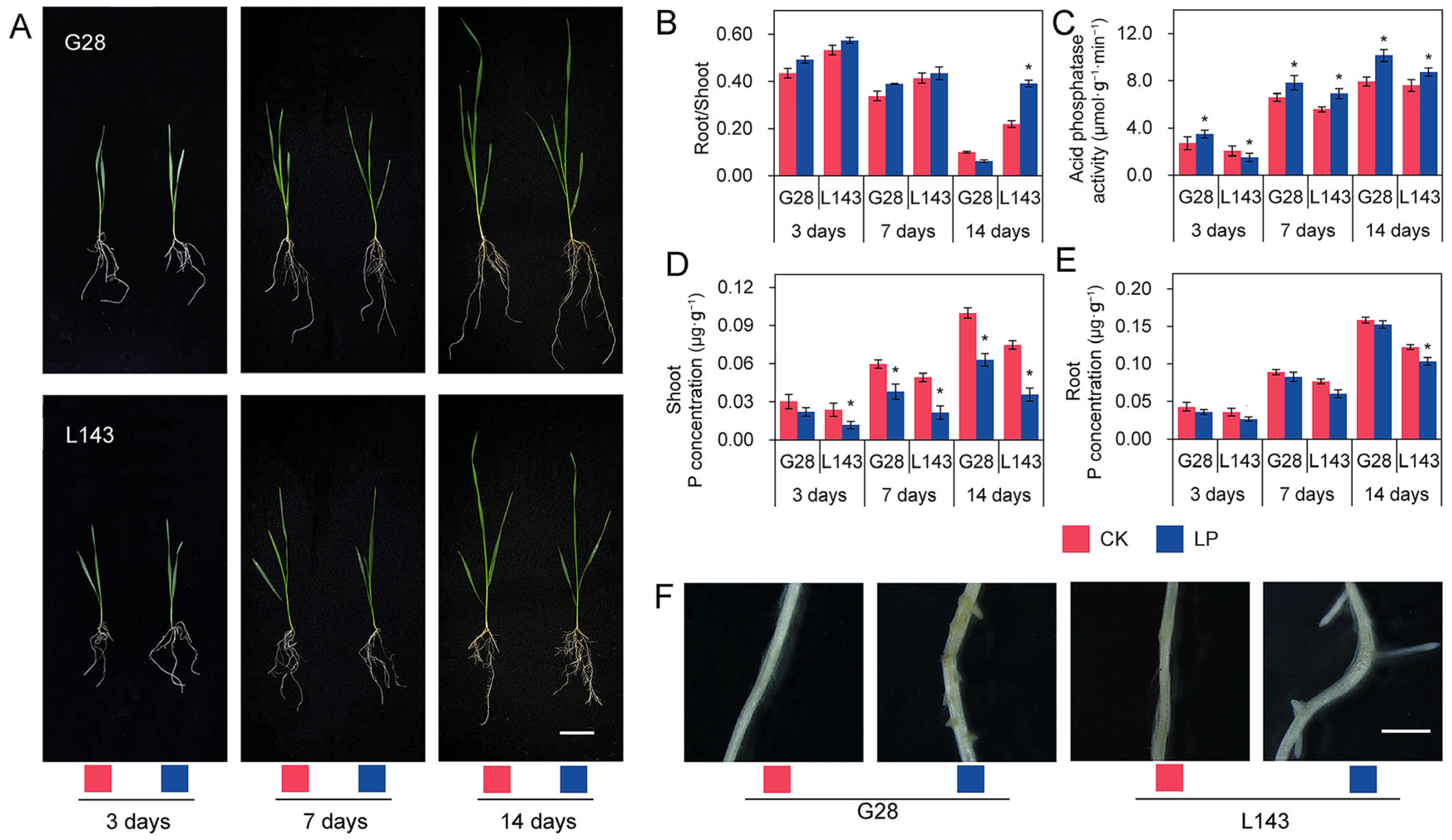
2.1. Phenotypic and Physiological Differences of Two Varieties under LP Stress
2.2. Metabolic Profile of Wheat in Response to LP Stress
2.3. The Response of the Known Metabolites to LP Stress in Wheat Root
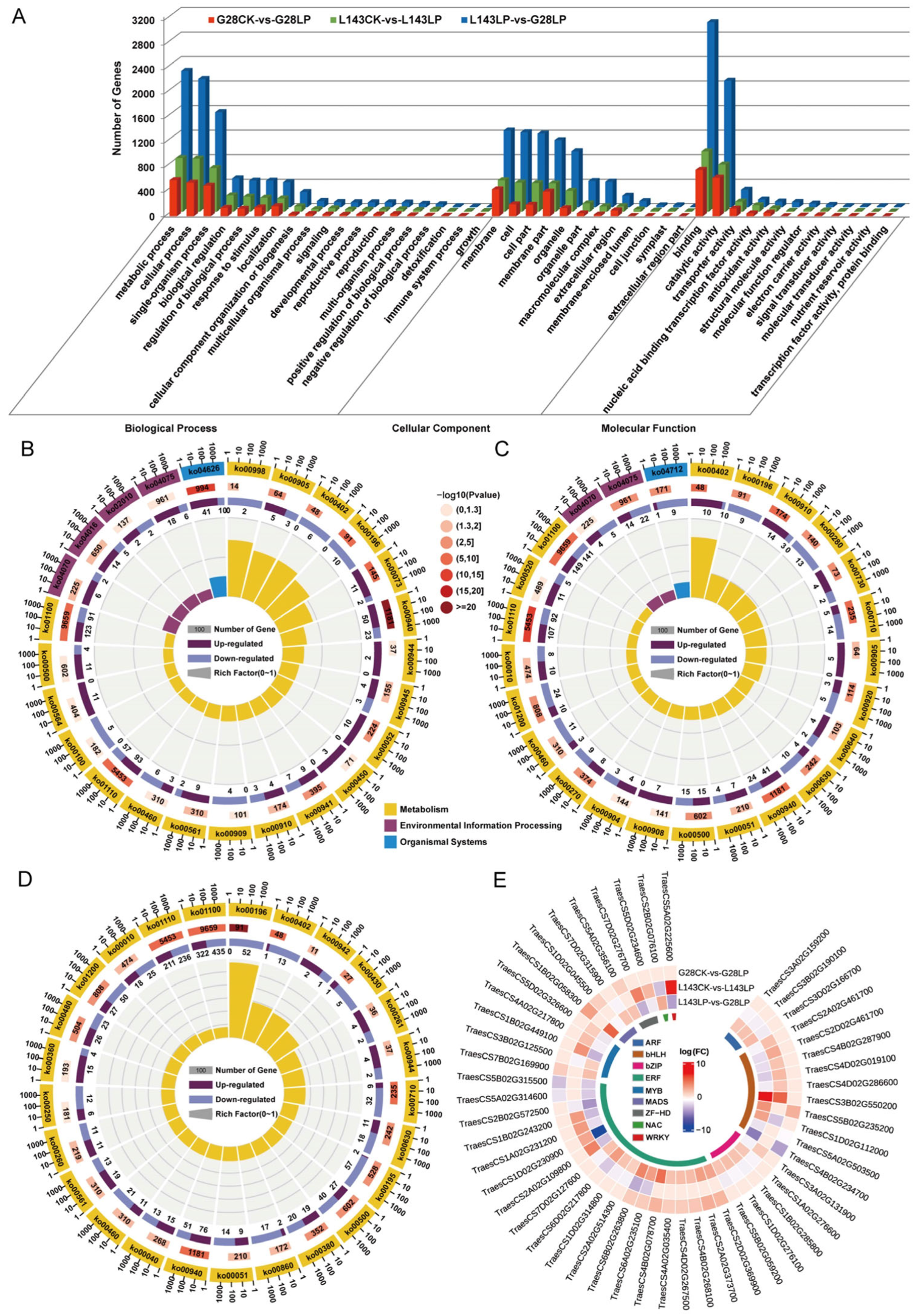
2.4. Transcriptome Identification of DEGs in G28 and L143 under LP Stress
2.5. GO and KEGG Analysis of DEGs in G28 and L143 under LP Stress
2.6. Identification and Analysis of TFs for Core DEGs
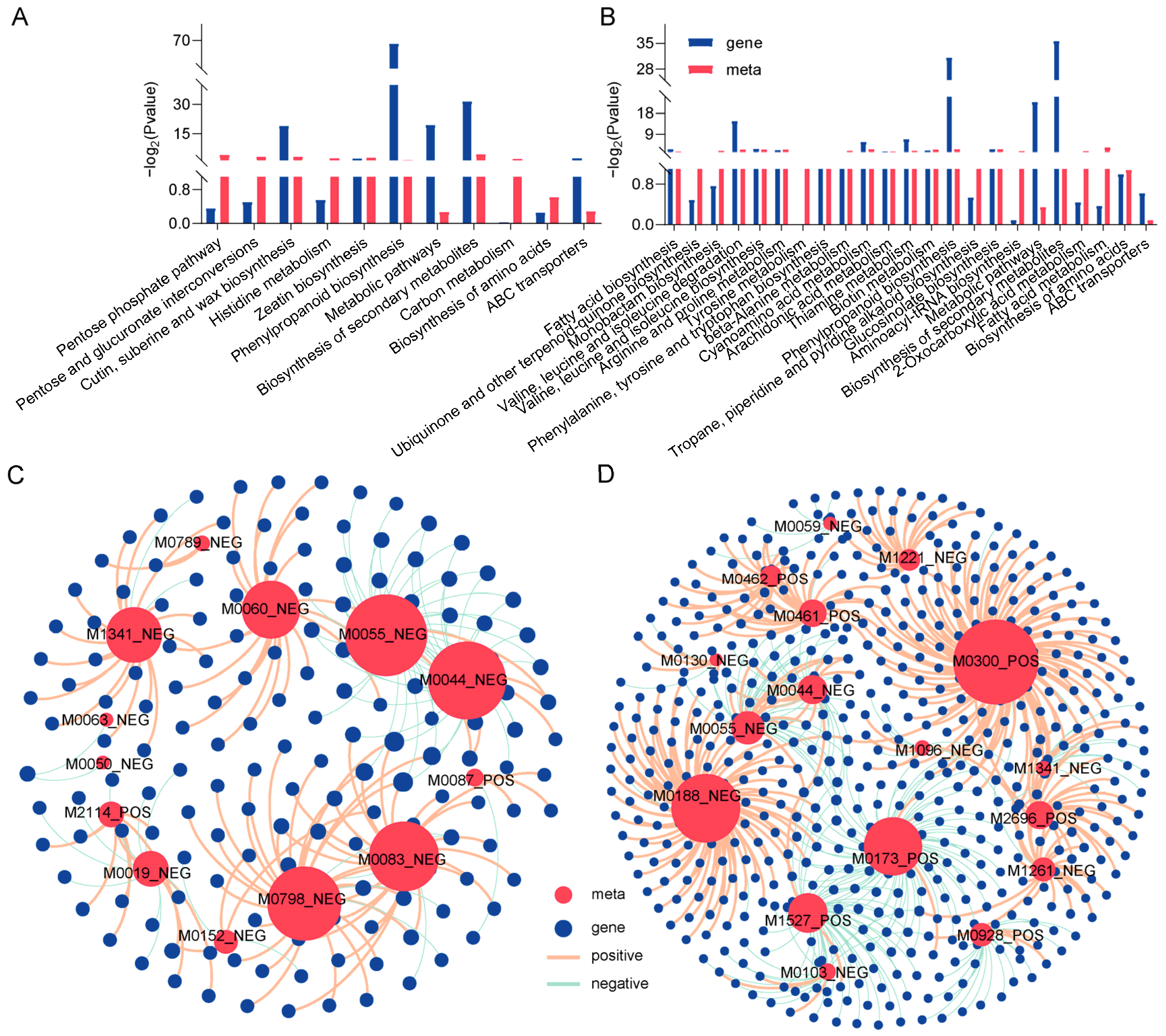
2.7. Integrated Transcriptomic and Metabolomic Analyses
2.8. qRT-PCR Verification of RNA-Seq Data
3. Discussion
3.1. Signaling in Wheat Root under Pi Starvation
3.2. Pi Transporters Play Essential Role in Pi Homeostasis in Wheat under LP Stress
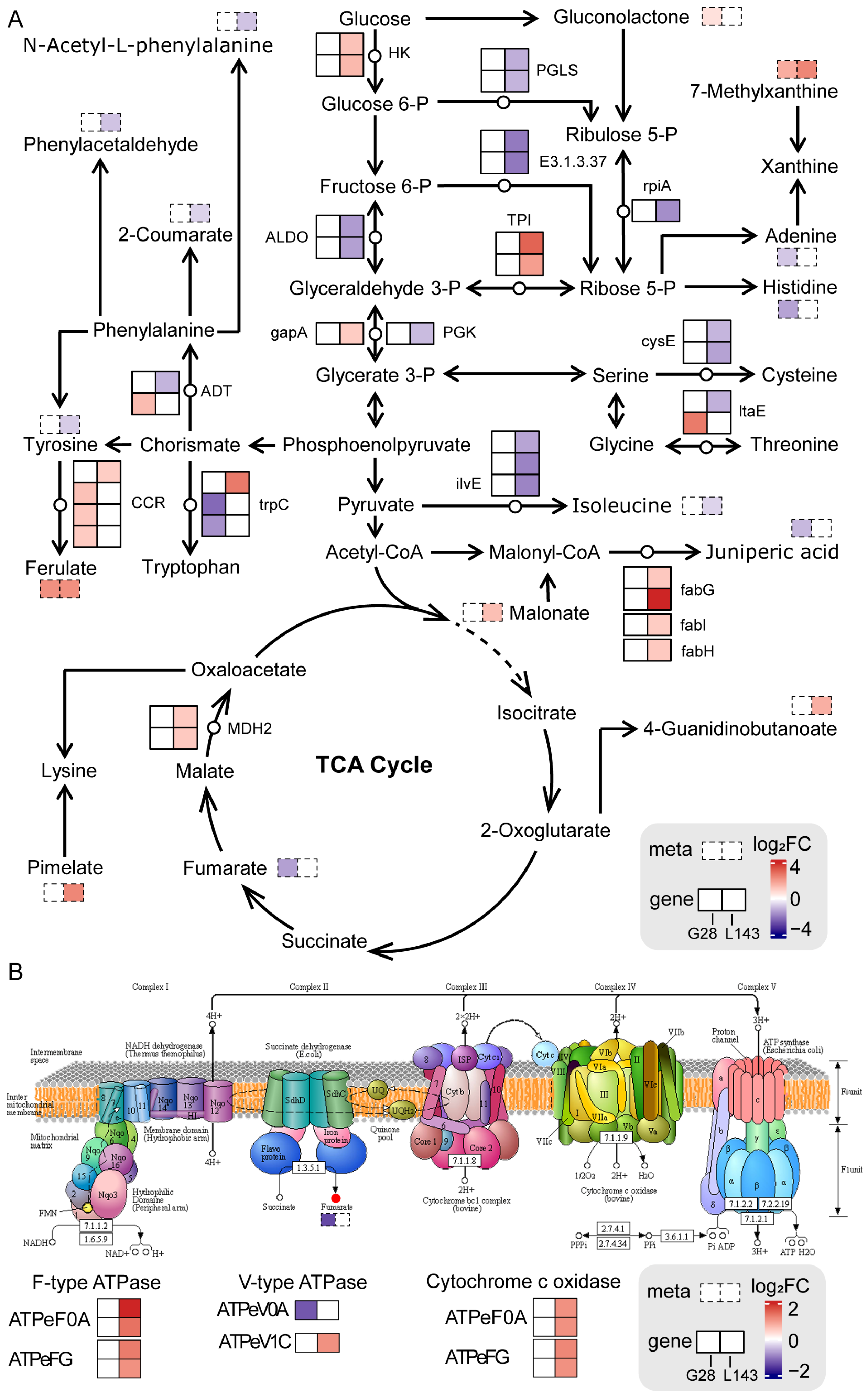
3.3. Sugar and Amino Acid Metabolism Were Significant for LP Response in Wheat
3.4. Oxidative Phosphorylation Was Critical under LP Stress in Wheat
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Phenotypic Physiology and Phosphorus Concentration
4.3. Transcriptome Sequencing and Metabolome Detection
4.4. Bioinformatics Analysis
4.5. Validation of Quantitative Real-Time PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Murray, T.D.; McIntosh, R.A.; Zhou, Y. Breeding new cultivars for sustainable wheat production. Crop J. 2019, 7, 715–717. [Google Scholar] [CrossRef]
- Wang, L.; Chen, F.; Zhang, F.; Mi, G. Two strategies for achieving higher yield under phosphorus deficiency in winter wheat grown in field conditions. Field Crops Res. 2010, 118, 36–42. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2020, 72, 199–223. [Google Scholar] [CrossRef]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ye, Z.; Chen, S. Engineered biochars for recovering phosphate and ammonium from wastewater: A review. Sci. Total Environ. 2021, 779, 146240. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Li, Z.; Xu, C.; Li, K.; Yan, S.; Qu, X.; Zhang, J. Phosphate starvation of maize inhibits lateral root formation and alters gene expression in the lateral root primordium zone. BMC Plant Biol. 2012, 12, 89. [Google Scholar] [CrossRef]
- Zhang, D.; Song, H.; Cheng, H.; Hao, D.; Wang, H.; Kan, G.; Jin, H.; Yu, D. The Acid Phosphatase-Encoding Gene GmACP1 Contributes to Soybean Tolerance to Low-Phosphorus Stress. PLos Genet. 2014, 10, e1004061. [Google Scholar] [CrossRef]
- Bhosale, R.; Giri, J.; Pandey, B.K.; Giehl, R.F.H.; Hartmann, A.; Traini, R.; Truskina, J.; Leftley, N.; Hanlon, M.; Swarup, K.; et al. A mechanistic framework for auxin dependent Arabidopsis root hair elongation to low external phosphate. Nat. Commun. 2018, 9, 1818. [Google Scholar] [CrossRef]
- Huang, K.; Ma, G.; Zhang, M.; Xiong, H.; Wu, H.; Zhao, C.; Liu, C.; Jia, H.; Chen, L.; Kjorven, J.O.; et al. The ARF7 and ARF19 transcription factors positively regulate PHOSPHATE STARVATION RESPONSE1 in Arabidopsis roots. Plant Physiol. 2018, 178, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Henao, J.E.; Vélez-Bermúdez, I.C.; Schmidt, W. The regulation and plasticity of root hair patterning and morphogenesis. Development 2016, 143, 1848–1858. [Google Scholar] [CrossRef]
- Song, L.; Yu, H.; Dong, J.; Che, X.; Jiao, Y.; Liu, D. The Molecular Mechanism of Ethylene-Mediated Root Hair Development Induced by Phosphate Starvation. PLoS Genet. 2016, 12, e1006194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, Y.; Wang, H.; Ma, X.; Yao, W.; Wang, H. Light and Ethylene Coordinately Regulate the Phosphate Starvation Response through Transcriptional Regulation of PHOSPHATE STARVATION RESPONSE1. Plant Cell 2017, 29, 2269–2284. [Google Scholar] [CrossRef]
- Navarro, C.; Mateo-Elizalde, C.; Mohan, T.C.; Sanchez-Bermejo, E.; Urrutia, O.; Fernandez-Muniz, M.N.; Garcia-Mina, J.M.; Munoz, R.; Paz-Ares, J.; Castrillo, G.; et al. Arsenite provides a selective signal that coordinates arsenate uptake and detoxification through the regulation of PHR1 stability in Arabidopsis. Mol. Plant 2021, 14, 1489–1507. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shao, H.; Huang, H.; Shen, Y.; Wang, L.; Wu, F.; Han, D.; Song, J.; Jia, H. Overexpression of the phosphate transporter gene OsPT8 improves the Pi and selenium contents in Nicotiana tabacum. Environ. Exp. Bot. 2017, 137, 158–165. [Google Scholar] [CrossRef]
- Ruan, W.; Guo, M.; Cai, L.; Hu, H.; Li, C.; Liu, Y.; Wu, Z.; Mao, C.; Yi, K.; Wu, P.; et al. Genetic manipulation of a high-affinity PHR1 target cis-element to improve phosphorous uptake in Oryza sativa L. Plant Mol. Biol. 2015, 87, 429–440. [Google Scholar] [CrossRef]
- Lei, M.; Liu, Y.; Zhang, B.; Zhao, Y.; Wang, X.; Zhou, Y.; Raghothama, K.G.; Liu, D. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiol. 2011, 156, 1116–1130. [Google Scholar] [CrossRef]
- Zhu, Q.; Kong, L.; Shan, Y.; Yao, X.; Zhang, H.; Xie, F.; Ao, X. Effect of biochar on grain yield and leaf photosynthetic physiology of soybean cultivars with different phosphorus efficiencies. J. Integr. Agric. 2019, 18, 2242–2254. [Google Scholar] [CrossRef]
- Ren, P.; Meng, Y.; Li, B.; Ma, X.; Si, E.; Lai, Y.; Wang, J.; Yao, L.; Yang, K.; Shang, X.; et al. Molecular Mechanisms of Acclimatization to Phosphorus Starvation and Recovery Underlying Full-Length Transcriptome Profiling in Barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 500. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Song, J.; Wu, W.; Li, K.; Zhang, J. Physiological and comparative proteome analyses reveal low-phosphate tolerance and enhanced photosynthesis in a maize mutant owing to reinforced inorganic phosphate recycling. BMC Plant Biol. 2016, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genom. Proteom. Bioinf. 2017, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, H.; Chu, S.; Li, H.; Chi, Y.; Triebwasser-Freese, D.; Lv, H.; Yu, D. Integrating QTL mapping and transcriptomics identifies candidate genes underlying QTLs associated with soybean tolerance to low-phosphorus stress. Plant Mol. Biol. 2017, 93, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant metabolomics in biotic and abiotic stress: A critical overview. Phytochem. Rev. 2022, 21, 503–524. [Google Scholar] [CrossRef]
- Xiao, J.F.; Zhou, B.; Ressom, H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. TrAC Trend Anal. Chem. 2012, 32, 1–14. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Huang, T.; Zhang, X.; Zhang, P.; Xie, H.; Liu, J.; Li, L.; Kong, Z.; Qin, P. Transcriptome and Metabolome Analyses Revealed the Response Mechanism of Quinoa Seedlings to Different Phosphorus Stresses. Int. J. Mol. Sci. 2022, 23, 4704. [Google Scholar] [CrossRef]
- Yuan, G.; Sun, D.; An, G.; Li, W.; Si, W.; Liu, J.; Zhu, Y. Transcriptomic and Metabolomic Analysis of the Effects of Exogenous Trehalose on Salt Tolerance in Watermelon (Citrullus lanatus). Cells 2022, 11, 2338. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, S.; Mo, X.; Guo, Q.; Li, Y.; Tian, J.; Liang, C. Proteomic Analysis Dissects Molecular Mechanisms Underlying Plant Responses to Phosphorus Deficiency. Cells 2022, 11, 651. [Google Scholar] [CrossRef]
- Miller, S.S.; Liu, J.; Allan, D.L.; Menzhuber, C.J.; Fedorova, M.; Vance, C.P. Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin. Plant Physiol. 2001, 127, 594–606. [Google Scholar] [CrossRef]
- Chien, P.S.; Chiang, C.P.; Leong, S.J.; Chiou, T.J. Sensing and signaling of phosphate ptarvation: From local to long distance. Plant Cell Physiol. 2018, 59, 1714–1722. [Google Scholar] [CrossRef]
- Cho, H.; Bouain, N.; Zheng, L.; Rouached, H. Plant resilience to phosphate limitation: Current knowledge and future challenges. Crit. Rev. Biotechnol. 2021, 41, 63–71. [Google Scholar] [CrossRef]
- Perez-Torres, C.A.; Lopez-Bucio, J.; Cruz-Ramirez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.M.; Wojciechowski, T.; Postma, J.A.; Brown, K.M.; Lynch, J.P. Ethylene modulates root cortical senescence in barley. Ann. Bot. 2018, 122, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Lopez, A.; Ibarra-Laclette, E.; Sanchez-Calderon, L.; Gutierrez-Alanis, D.; Herrera-Estrella, L. Global expression pattern comparison between low phosphorus insensitive 4 and WT Arabidopsis reveals an important role of reactive oxygen species and jasmonic acid in the root tip response to phosphate starvation. Plant Signal. Behav. 2011, 6, 382–392. [Google Scholar] [CrossRef]
- Kang, J.; Yu, H.; Tian, C.; Zhou, W.; Li, C.; Jiao, Y.; Liu, D. Suppression of photosynthetic gene expression in roots is required for sustained root growth under phosphate deficiency. Plant Physiol. 2014, 165, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Perez-Perez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Kim, M.C.; Chun, H.J.; Kang, S.; Park, H.C.; Shin, G.; Park, J.; Shen, M.; Hong, H.; Kim, W.Y.; et al. Regulation of miR399f transcription by AtMYB2 affects phosphate starvation responses in Arabidopsis. Plant Physiol. 2013, 161, 362–373. [Google Scholar] [CrossRef]
- Ruan, W.; Guo, M.; Wu, P.; Yi, K. Phosphate starvation induced OsPHR4 mediates Pi-signaling and homeostasis in rice. Plant. Mol. Biol. 2017, 93, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, C.; Qiao, L.; Zhao, J.; Han, R.; Wang, X.; Ge, C.; Zhang, W.; Zhang, S.; Qiao, L.; et al. The MYB transcription factor TaPHR3-A1 is involved in phosphate signaling and governs yield-related traits in bread wheat. J. Exp. Bot. 2020, 71, 5808–5822. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Xu, Q.; Kong, Y.; Wang, H.; Wu, W. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 2009, 21, 3554–3566. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, Y.; Zhang, W.H. OsWRKY74, a WRKY transcription factor, modulates tolerance to phosphate starvation in rice. J. Exp. Bot. 2016, 67, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Wu, Z.; Zhou, J.; Du, L.; Guo, L.; Wu, Y.; Wu, P. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol. 2005, 138, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Zhang, Y.; Wang, B.; Ran, Q.; Zhang, J. The bHLH family member ZmPTF1 regulates drought tolerance in maize by promoting root development and abscisic acid synthesis. J. Exp. Bot. 2019, 70, 5471–5486. [Google Scholar] [CrossRef]
- Han, Y.; Liu, N.; Li, C.; Wang, S.; Jia, L.; Zhang, R.; Li, H.; Tan, J.; Xue, H.; Zheng, W. TaMADS2-3D, a MADS transcription factor gene, regulates phosphate starvation responses in plants. Crop J. 2022, 10, 243–253. [Google Scholar] [CrossRef]
- Wang, D.; Lv, S.; Jiang, P.; Li, Y. Roles, Regulation, and Agricultural Application of Plant Phosphate Transporters. Front. Plant Sci. 2017, 8, 817. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Wang, L.; Guo, J.; Lu, K.; Song, R.; Zuo, J.; Chen, H.; Liu, W.C. Abscisic acid facilitates phosphate acquisition through the transcription factor ABA INSENSITIVE5 in Arabidopsis. Plant J. 2022, 111, 269–281. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Kong, Y.H.; Chen, Y.; Duan, J.Y.; Wu, W.H.; Chen, Y.F. Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1;1 expression in response to phosphate starvation. Plant Physiol. 2014, 164, 2020–2029. [Google Scholar] [CrossRef]
- Karthikeyan, A.S.; Varadarajan, D.K.; Jain, A.; Held, M.A.; Carpita, N.C.; Raghothama, K.G. Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 2007, 225, 907–918. [Google Scholar] [CrossRef]
- Dasgupta, K.; Khadilkar, A.S.; Sulpice, R.; Pant, B.; Scheible, W.R.; Fisahn, J.; Stitt, M.; Ayre, B.G. Expression of sucrose transporter cDNAs specifically in companion cells enhances phloem loading and long-distance transport of sucrose but leads to an inhibition of growth and the perception of a phosphate limitation. Plant Physiol. 2014, 165, 715–731. [Google Scholar] [CrossRef]
- Ulloa, M.; Nunes-Nesi, A.; da Fonseca-Pereira, P.; Poblete-Grant, P.; Reyes-Díaz, M.; Cartes, P. The effect of silicon supply on photosynthesis and carbohydrate metabolism in two wheat (Triticum aestivum L.) cultivars contrasting in response to phosphorus nutrition. Plant Physiol. Biochem. 2021, 169, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; White, P.J. Sugar signaling in root responses to low phosphorus availability. Plant Physiol. 2011, 156, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ganie, A.H.; Ahmad, A.; Pandey, R.; Aref, I.M.; Yousuf, P.Y.; Ahmad, S.; Iqbal, M. Metabolite profiling of low-P tolerant and low-P sensitive maize genotypes under phosphorus starvation and restoration conditions. PLoS ONE 2015, 10, e0129520. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Jin, J.; Christensen, M.J.; Gu, L.; Cheng, C.; Wang, J. Epichloe gansuensis Increases the Tolerance of Achnatherum inebrians to Low-P Stress by Modulating Amino Acids Metabolism and Phosphorus Utilization Efficiency. J. Fungi 2021, 7, 390. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Del-Saz, N.F.; Ribas-Carbo, M.; McDonald, A.E.; Lambers, H.; Fernie, A.R.; Florez-Sarasa, I. An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci. 2018, 23, 206–219. [Google Scholar] [CrossRef]
- Bryce, J.H.; Azcon-Bieto, J.; Wiskich, J.T.; Day, D.A. Adenylate control of respiration in plants: The contribution of rotenone-insensitive electron transport to ADP-limited oxygen consumption by soybean mitochondria. Physiol. Plant. 1990, 78, 105–111. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.; Li, C.; Ren, P.; Yao, L.; Li, B.; Meng, Y.; Ma, X.; Si, E.; Yang, K.; et al. Dynamic Responses of Barley Root Succinyl-Proteome to Short-Term Phosphate Starvation and Recovery. Front. Plant Sci. 2021, 12, 649147. [Google Scholar] [CrossRef]
- Rott, M.; Martins, N.F.; Thiele, W.; Lein, W.; Bock, R.; Kramer, D.M.; Schottler, M.A. ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 2011, 23, 304–321. [Google Scholar] [CrossRef]



| Metabolite ID | Metabolite Name | Compound ID | VIP Values | log2FC | p-Value |
|---|---|---|---|---|---|
| M0044_NEG | Purine | C00465 | 5.74/6.52 | 1.54/2.27 | 0.022/0.004 |
| M0055_NEG | 7-methylxanthine | C16353 | 5.72/6.55 | 1.63/2.42 | 0.024/0.004 |
| M0563_NEG | 4-morpholinecarboxamide | - | 5.06/4.91 | 2.15/2.76 | 0.004/0.000 |
| M0615_NEG | 13(S)-HpOTrE | - | 1.41/2.03 | 1.25/2.03 | 0.022/0.000 |
| M0631_POS | PMK ethyl glycidate | - | 1.62/1.19 | 2.70/1.43 | 0.019/0.022 |
| M1341_NEG | Ferulic acid | C01494 | 1.57/1.59 | 2.21/2.22 | 0.000/0.004 |
| M1560_POS | N-{[(2R,4S,5R)-5-ethyl-1-azabicyclo[2.2.2]oct-2-yl]methyl}-2-propanamine | - | 1.93/1.43 | 2.00/1.18 | 0.003/0.007 |
| M2125_POS | 4-amino-3-hydroxybenzoic acid | - | 1.64/1.20 | −1.29/−1.25 | 0.009/0.045 |
| M0019_NEG | L-histidine | C00135 | 2.16 | −1.37 | 0.0050 |
| M0043_NEG | beta-cellobiose | - | 3.35 | 2.07 | 0.0490 |
| M0050_NEG | D-lyxose | C00476 | 14.22 | 0.67 | 0.0478 |
| M0060_NEG | Gluconic acid lactone | C00198 | 1.38 | 0.59 | 0.0077 |
| M0063_NEG | Chlorhexidine gluconate | C08038 | 14.26 | 0.67 | 0.0482 |
| M0083_NEG | Fumaric acid | C00122 | 2.04 | −1.42 | 0.0062 |
| M0087_POS | Griseofulvin | C06686 | 1.41 | −1.27 | 0.0484 |
| M0094_POS | N3,N4-dimethylarginine | - | 1.28 | −1.44 | 0.0376 |
| M0152_NEG | Adenine | C00147 | 1.27 | −0.82 | 0.0082 |
| M0597_NEG | 15-deoxy-delta12,14-prostaglandin J2 | - | 1.03 | 2.55 | 0.0048 |
| M0632_POS | N4-acetylsulfamethazine | - | 1.23 | 2.74 | 0.0241 |
| M0662_NEG | Kukoamine B | - | 1.26 | 2.16 | 0.0258 |
| M0789_NEG | 16-Hydroxyhexadecanoic acid | C18218 | 3.99 | −1.06 | 0.0374 |
| M0798_NEG | alpha-eleostearic acid | C08315 | 1.31 | −0.82 | 0.0267 |
| M0877_NEG | Alisol A 24-acetate | - | 1.24 | −1.53 | 0.0390 |
| M0917_POS | Farnesyl acetate | - | 1.01 | −1.70 | 0.0034 |
| M1122_NEG | N-acetyl-d-alloisoleucine | - | 1.84 | −0.82 | 0.0306 |
| M1134_POS | 5-tetradecyloxy-2-furonic acid | - | 1.21 | 2.01 | 0.0040 |
| M1697_POS | 13,14-dihydro-15(R)-prostaglandin E1 | - | 1.08 | 3.31 | 0.0028 |
| M1783_POS | Poly THF n6 | - | 1.46 | −3.07 | 0.0173 |
| M1993_POS | Monoolein | - | 1.21 | 0.82 | 0.0291 |
| M2114_POS | Pilocarpine | C07474 | 1.18 | −4.77 | 0.0148 |
| M2597_POS | Acetic acid | - | 1.37 | −1.09 | 0.0444 |
| M2701_POS | 1-piperazinecarboxylic acid | - | 1.72 | −1.23 | 0.0035 |
| M3152_POS | Benzoic acid | - | 2.27 | 2.83 | 0.0001 |
| M3180_POS | 4-methylumbelliferyl α-D-glucoside | - | 1.21 | 1.38 | 0.0405 |
| M3317_POS | Di-4-coumaroylputrescine | - | 1.78 | 3.89 | 0.0023 |
| M0059_NEG | Malonic acid | C00383 | 1.12 | 1.29 | 0.0430 |
| M0103_NEG | Neochlorogenic acid | C17147 | 3.13 | 2.34 | 0.0069 |
| M0112_NEG | Fraxin | - | 1.85 | 3.11 | 0.0033 |
| M0130_NEG | Scopolin | C01527 | 2.82 | 2.06 | 0.0119 |
| M0173_POS | 4-guanidinobutyric acid | C01035 | 1.51 | 1.59 | 0.0012 |
| M0188_NEG | L-tyrosine | C00082 | 1.18 | −0.75 | 0.0008 |
| M0300_POS | L-isoleucine | C00407 | 2.83 | −0.65 | 0.0020 |
| M0408_POS | Allopurinol | - | 1.64 | 0.64 | 0.0340 |
| M0461_POS | N-benzylformamide | C15561 | 1.27 | −0.67 | 0.0159 |
| M0462_POS | 2-hydroxycinnamate | C01772 | 3.05 | −0.64 | 0.0380 |
| M0506_POS | Isoleucine | - | 7.20 | −0.62 | 0.0038 |
| M0866_NEG | 2H-1,4-benzoxazin-3(4H)-one | - | 1.20 | −0.82 | 0.0216 |
| M0928_POS | 2,3-dinor-8-iso prostaglandin F2alpha | C14794 | 2.76 | 0.81 | 0.0237 |
| M1055_POS | Dihydroagathic acid | - | 2.05 | 1.88 | 0.0024 |
| M1056_POS | Eicosatetraynoic acid | - | 3.11 | 1.92 | 0.0012 |
| M1096_NEG | Pimelic acid | C02656 | 1.04 | 2.38 | 0.0062 |
| M1221_NEG | N-acetyl-L-phenylalanine | C03519 | 1.10 | −0.89 | 0.0471 |
| M1261_NEG | Phenylacetaldehyde | C00601 | 1.24 | −0.88 | 0.0076 |
| M1384_POS | 2-pyrazinecarboxamide | - | 1.28 | 2.05 | 0.0157 |
| M1483_POS | 1-naphthalenepentanoic acid | - | 1.31 | 1.53 | 0.0133 |
| M1527_POS | 9-KODE | C14766 | 5.39 | 0.93 | 0.0051 |
| M1715_POS | 16-heptadecyne-1,2,4-triol | - | 2.51 | 2.67 | 0.0323 |
| M1838_POS | Avocadyne 1-acetate | - | 6.43 | 1.46 | 0.0342 |
| M2118_POS | 3-aminosalicylic acid | - | 1.42 | −1.59 | 0.0252 |
| M2696_POS | Propentofylline | D01630 | 1.09 | −1.64 | 0.0125 |
| M2934_POS | 3-hydroxycinnamic acid | - | 1.00 | −0.77 | 0.0114 |
| M3146_POS | 1H-pyrazolo[3,4-b]pyridine-5-carboxamide | - | 1.03 | −1.80 | 0.0146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Ma, X.; Wang, J.; Yao, L.; Li, B.; Meng, Y.; Si, E.; Yang, K.; Shang, X.; Zhang, X.; et al. Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Low Phosphorus Tolerance in Wheat Seedling. Int. J. Mol. Sci. 2023, 24, 14840. https://doi.org/10.3390/ijms241914840
Li P, Ma X, Wang J, Yao L, Li B, Meng Y, Si E, Yang K, Shang X, Zhang X, et al. Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Low Phosphorus Tolerance in Wheat Seedling. International Journal of Molecular Sciences. 2023; 24(19):14840. https://doi.org/10.3390/ijms241914840
Chicago/Turabian StyleLi, Pengcheng, Xiaole Ma, Juncheng Wang, Lirong Yao, Baochun Li, Yaxiong Meng, Erjing Si, Ke Yang, Xunwu Shang, Xueyong Zhang, and et al. 2023. "Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Low Phosphorus Tolerance in Wheat Seedling" International Journal of Molecular Sciences 24, no. 19: 14840. https://doi.org/10.3390/ijms241914840
APA StyleLi, P., Ma, X., Wang, J., Yao, L., Li, B., Meng, Y., Si, E., Yang, K., Shang, X., Zhang, X., & Wang, H. (2023). Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Low Phosphorus Tolerance in Wheat Seedling. International Journal of Molecular Sciences, 24(19), 14840. https://doi.org/10.3390/ijms241914840






