Antiviral Efficacy of RNase H-Dependent Gapmer Antisense Oligonucleotides against Japanese Encephalitis Virus
Abstract
:1. Introduction
2. Results
2.1. Design of LNA Gapmers Targeting 3′ UTR of JEV Genomic RNA
2.2. Inhibitory Effect of LNA Gapmers on JEV Proliferation in Vero Cells
2.3. RNase H-Mediated Antiviral Mechanism of LNA Gapmers with Sequence and Modification Specificity
2.4. Antiviral Efficacy of JEV RNA-Targeted LNA Gapmers in a Human Neuroblastoma Cell Line
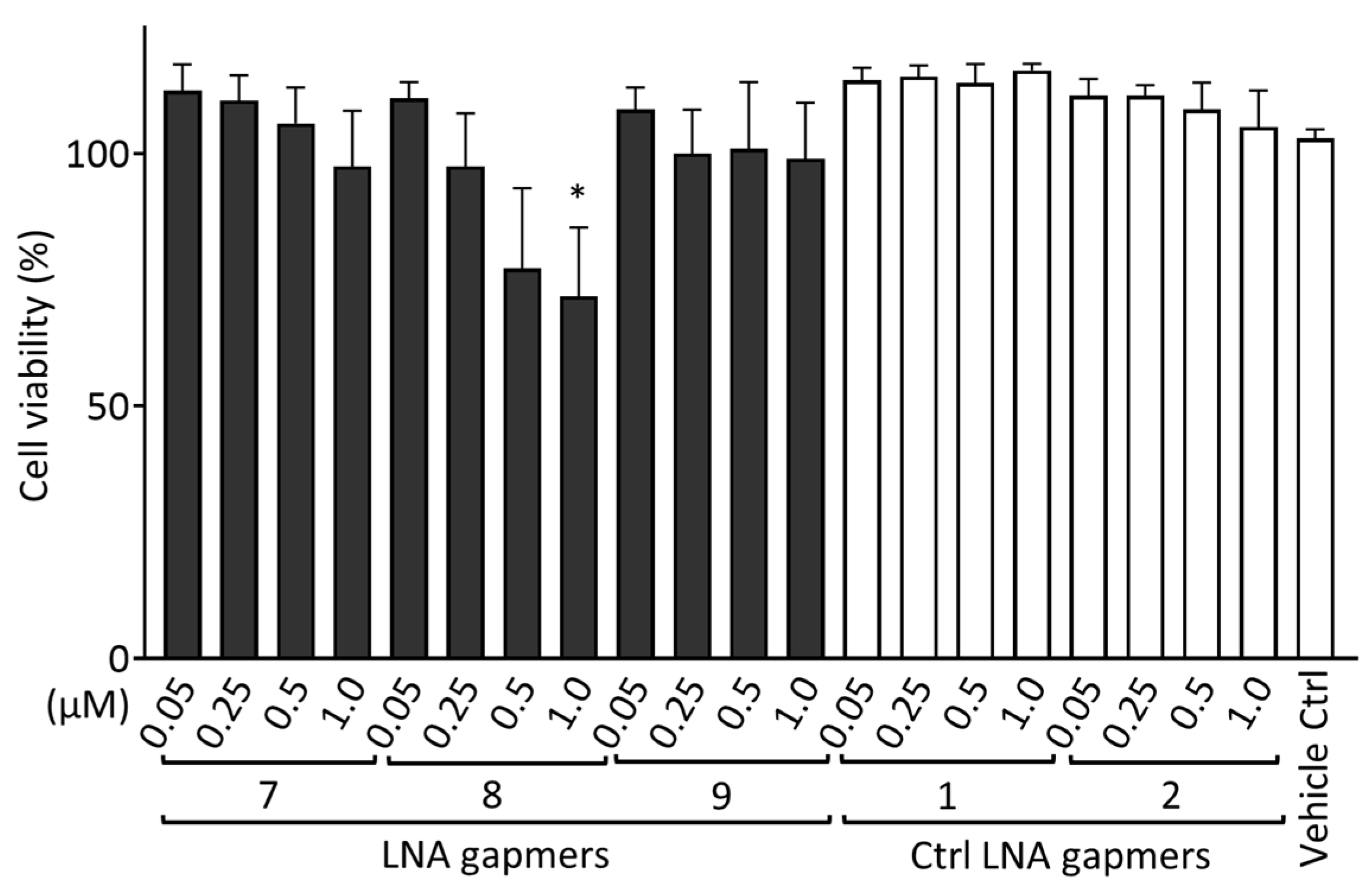
2.5. Cytotoxic Effect of LNA Gapmers
2.6. In Silico Analysis of the Sequence-Dependent Off-Target Effects of LNA Gapmers
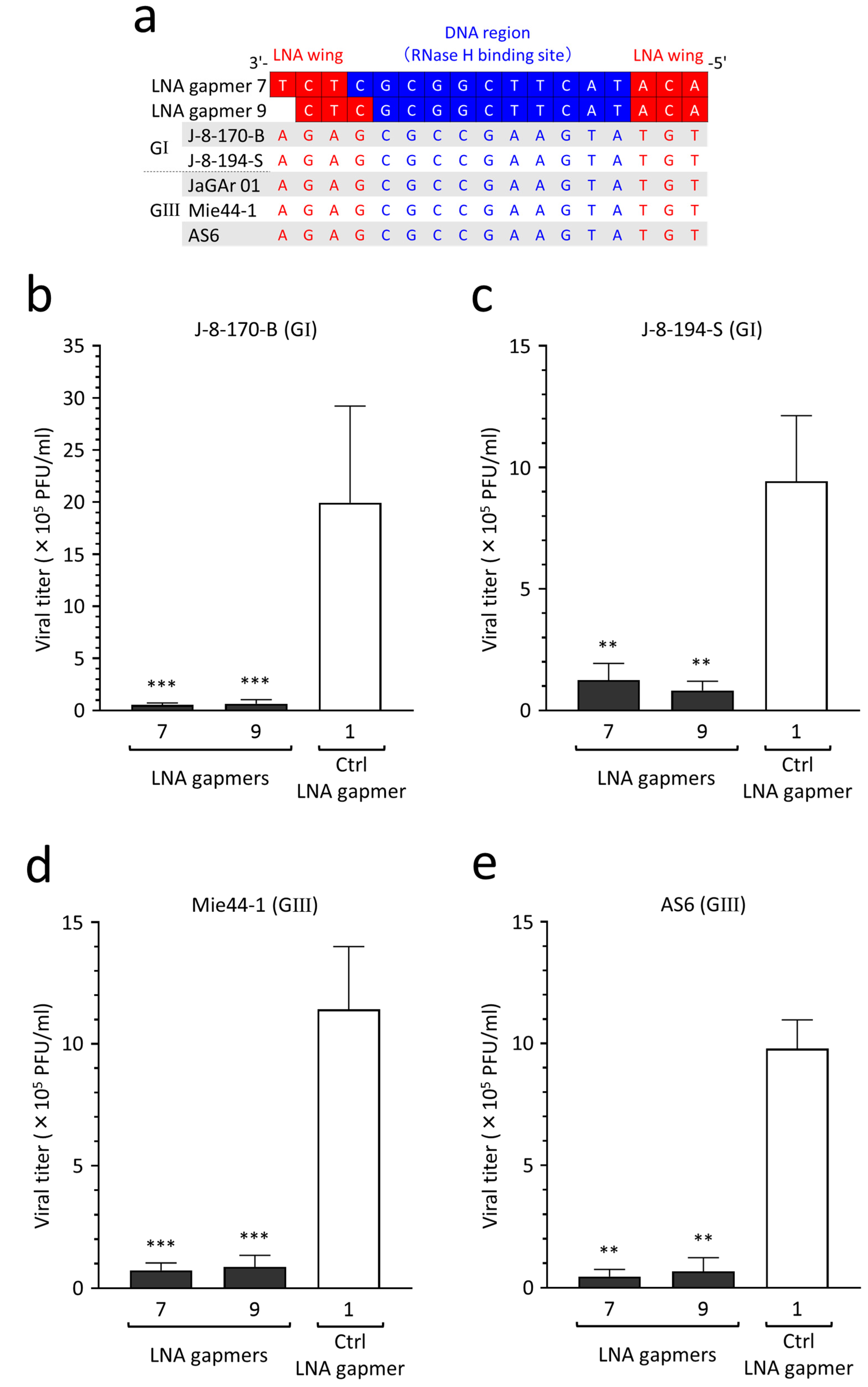
2.7. Potential Applicability of Antiviral LNA Gapmers to JEV Genotypes I–V
2.8. Antiviral Efficacy of LNA Gapmers against Different JEV Strains of Genotypes I and III
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. JEV RNA Secondary Structure Prediction
4.3. LNA Gapmer Design and Synthesis
4.4. Transfection
4.5. Plaque Assay
4.6. RNA Cleavage Assay
4.7. Sequencing and Quantitative Real-Time RT-PCR
4.8. Cytotoxicity Assay
4.9. Analysis for Potential Off-Target Binding of JEV RNA-Targeted LNA Gapmers to Human DNA and RNA
4.10. Conservation Analysis of LNA Gapmer Target Region in JEV Genotypes
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo, S.J.; Chae, S.U.; Lee, C.B.; Bae, S.K. Clinical Pharmacokinetics of Approved RNA Therapeutics. Int. J. Mol. Sci. 2023, 24, 746. [Google Scholar] [CrossRef]
- Moumné, L.; Marie, A.C.; Crouvezier, N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics 2022, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Tarn, W.-Y.; Cheng, Y.; Ko, S.-H.; Huang, L.-M. Antisense oligonucleotide-based therapy of viral infections. Pharmaceutics 2021, 13, 2015. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Voell, J.; Muñoz, P.; Kumar, P.; Brooks, K.M.; Zhang, J.; Iversen, P.; Heald, A.; Wong, M.; Davey, R.T. Safety, tolerability, and pharmacokinetics of radavirsen (AVI-7100), an antisense oligonucleotide targeting influenza a M1/M2 translation. Br. J. Clin. Pharmacol. 2018, 84, 25–34. [Google Scholar] [CrossRef]
- Heald, A.E.; Iversen, P.L.; Saoud, J.B.; Sazani, P.; Charleston, J.S.; Axtelle, T.; Wong, M.; Smith, W.B.; Vutikullird, A.; Kaye, E. Safety and pharmacokinetic profiles of phosphorodiamidate morpholino oligomers with activity against ebola virus and marburg virus: Results of two single-ascending-dose studies. Antimicrob. Agents Chemother. 2014, 58, 6639–6647. [Google Scholar] [CrossRef]
- Zhu, C.; Lee, J.Y.; Woo, J.Z.; Xu, L.; Nguyenla, X.; Yamashiro, L.H.; Ji, F.; Biering, S.B.; Van Dis, E.; Gonzalez, F.; et al. An intranasal ASO therapeutic targeting SARS-CoV-2. Nat. Commun. 2022, 13, 4503. [Google Scholar] [CrossRef]
- Hagey, R.J.; Elazar, M.; Pham, E.A.; Tian, S.; Ben-Avi, L.; Bernardin-Souibgui, C.; Yee, M.F.; Moreira, F.R.; Rabinovitch, M.V.; Meganck, R.M.; et al. Programmable antivirals targeting critical conserved viral RNA secondary structures from influenza A virus and SARS-CoV-2. Nat. Med. 2022, 28, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Lulla, V.; Wandel, M.P.; Bandyra, K.J.; Ulferts, R.; Wu, M.; Dendooven, T.; Yang, X.; Doyle, N.; Oerum, S.; Beale, R. Targeting the conserved stem loop 2 motif in the SARS-CoV-2 genome. J. Virol. 2021, 95, e00663-21. [Google Scholar] [CrossRef] [PubMed]
- Dhorne-Pollet, S.; Fitzpatrick, C.; Da Costa, B.; Bourgon, C.; Eléouët, J.F.; Meunier, N.; Burzio, V.A.; Delmas, B.; Barrey, E. Antisense oligonucleotides targeting ORF1b block replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Front. Microbiol. 2022, 13, 915202. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.M.; Fontana, P.; Mao, T.; Leger, V.; Zhang, Y.; Fu, T.M.; Lieberman, J.; Gehrke, L.; Shi, M.; Wang, L.; et al. Targeting stem-loop 1 of the SARS-CoV-2 5′ UTR to suppress viral translation and Nsp1 evasion. Proc. Natl. Acad. Sci. USA 2022, 119, e2117198119. [Google Scholar] [CrossRef]
- Stincarelli, M.A.; Rocca, A.; Antonelli, A.; Rossolini, G.M.; Giannecchini, S. Antiviral Activity of Oligonucleotides Targeting the SARS-CoV-2 Genomic RNA Stem-Loop Sequences within the 3′-End of the ORF1b. Pathogens 2022, 11, 1286. [Google Scholar] [CrossRef]
- Meganck, R.M.; Baric, R.S. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat. Med. 2021, 27, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Sun, H.; Nichols, J.G.; Crooke, S.T. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol. Ther. 2017, 25, 2075–2092. [Google Scholar] [CrossRef]
- Brentari, I.; Zadorozhna, M.; Denti, M.A.; Giorgio, E. RNA therapeutics for neurological diseases. Br. Med. Bull. 2023, 147, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Quemener, A.M.; Centomo, M.L.; Sax, S.L.; Panella, R. Small drugs, huge impact: The extraordinary impact of antisense oligonucleotides in research and drug development. Molecules 2022, 27, 536. [Google Scholar] [CrossRef]
- Nan, Y.; Zhang, Y.J. Antisense Phosphorodiamidate Morpholino Oligomers as Novel Antiviral Compounds. Front. Microbiol. 2018, 9, 750. [Google Scholar] [CrossRef]
- Szczesniak, I.; Baliga-Gil, A.; Jarmolowicz, A.; Soszynska-Jozwiak, M.; Kierzek, E. Structural and Functional RNA Motifs of SARS-CoV-2 and Influenza A Virus as a Target of Viral Inhibitors. Int. J. Mol. Sci. 2023, 24, 1232. [Google Scholar] [CrossRef]
- Veedu, R.N.; Wengel, J. Locked nucleic acid as a novel class of therapeutic agents. RNA Biol. 2009, 6, 321–323. [Google Scholar] [CrossRef]
- Lim, K.R.Q.; Maruyama, R.; Echigoya, Y.; Nguyen, Q.; Zhang, A.; Khawaja, H.; Sen Chandra, S.; Jones, T.; Jones, P.; Chen, Y.-W. Inhibition of DUX4 expression with antisense LNA gapmers as a therapy for facioscapulohumeral muscular dystrophy. Proc. Natl. Acad. Sci. USA 2020, 117, 16509–16515. [Google Scholar] [CrossRef]
- Maruyama, R.; Nguyen, Q.; Roshmi, R.R.; Touznik, A.; Yokota, T. Allele-Selective LNA Gapmers for the Treatment of Fibrodysplasia Ossificans Progressiva Knock Down the Pathogenic ACVR1R206H Transcript and Inhibit Osteogenic Differentiation. Nucleic Acids Ther. 2022, 32, 185–193. [Google Scholar] [CrossRef]
- Stepniak-Konieczna, E.; Konieczny, P.; Cywoniuk, P.; Dluzewska, J.; Sobczak, K. AON-induced splice-switching and DMPK pre-mRNA degradation as potential therapeutic approaches for Myotonic Dystrophy type 1. Nucleic Acids Res. 2020, 48, 2531–2543. [Google Scholar] [CrossRef] [PubMed]
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qi, Z.; Qian, X. Recent Advancements in Mosquito-Borne Flavivirus Vaccine Development. Viruses 2023, 15, 813. [Google Scholar] [CrossRef]
- Filgueira, L.; Lannes, N. Review of Emerging Japanese Encephalitis Virus: New Aspects and Concepts about Entry into the Brain and Inter-Cellular Spreading. Pathogens 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774E. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Qi, Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses 2022, 14, 1226. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Niu, Y.; Liang, G. The 5′ and 3′ Untranslated Regions of the Japanese Encephalitis Virus (JEV): Molecular Genetics and Higher Order Structures. Front. Microbiol. 2021, 12, 730045. [Google Scholar] [CrossRef] [PubMed]
- Anantpadma, M.; Stein, D.A.; Vrati, S. Inhibition of Japanese encephalitis virus replication in cultured cells and mice by a peptide-conjugated morpholino oligomer. J. Antimicrob. Chemother. 2010, 65, 953–961. [Google Scholar] [CrossRef]
- Nazmi, A.; Dutta, K.; Basu, A. Antiviral and neuroprotective role of octaguanidinium dendrimer-conjugated morpholino oligomers in Japanese encephalitis. PLoS Negl. Trop. Dis. 2010, 4, e892. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, C.M.; Kim, J.H.; Kim, J.Y.; Oh, J.W. Inhibition of Japanese encephalitis virus replication by peptide nucleic acids targeting cis-acting elements on the plus- and minus-strands of viral RNA. Antiviral. Res. 2009, 82, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.S.; Hahn, Y.S.; Rice, C.M.; Lee, E.; Dalgarno, L.; Strauss, E.G.; Strauss, J.H. Conserved elements in the 3′ untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol. 1987, 198, 33–41. [Google Scholar] [CrossRef]
- Bharucha, T.; Cleary, B.; Farmiloe, A.; Sutton, E.; Hayati, H.; Kirkwood, P.; Al Hamed, L.; van Ginneken, N.; Subramaniam, K.S.; Zitzmann, N.; et al. Mouse models of Japanese encephalitis virus infection: A systematic review and meta-analysis using a meta-regression approach. PLoS Negl. Trop. Dis. 2022, 16, e0010116. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935-2017. Vector Borne Zoonotic Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Pickett, B.E.; Sadat, E.L.; Zhang, Y.; Noronha, J.M.; Squires, R.B.; Hunt, V.; Liu, M.; Kumar, S.; Zaremba, S.; Gu, Z.; et al. ViPR: An open bioinformatics database and analysis resource for virology research. Nucleic Acids Res. 2012, 40, D593–D598. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef]
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese encephalitis: The vectors, ecology and potential for expansion. J. Travel Med. 2018, 25, S16–S26. [Google Scholar] [PubMed]
- Srivastava, K.S.; Jeswani, V.; Pal, N.; Bohra, B.; Vishwakarma, V.; Bapat, A.A.; Patnaik, Y.P.; Khanna, N.; Shukla, R. Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines. Vaccines 2023, 11, 742. [Google Scholar] [CrossRef]
- Holden, K.L.; Stein, D.A.; Pierson, T.C.; Ahmed, A.A.; Clyde, K.; Iversen, P.L.; Harris, E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3′ stem-loop structure. Virology 2006, 344, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Laxton, C.; Brady, K.; Moschos, S.; Turnpenny, P.; Rawal, J.; Pryde, D.C.; Sidders, B.; Corbau, R.; Pickford, C.; Murray, E. Selection, optimization, and pharmacokinetic properties of a novel, potent antiviral locked nucleic acid-based antisense oligomer targeting hepatitis C virus internal ribosome entry site. Antimicrob. Agents Chemother. 2011, 55, 3105–3114. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Kont, A.; Aburto, M.R.; Cryan, J.F.; O’Driscoll, C.M. Advances in the design of (nano) formulations for delivery of antisense oligonucleotides and small interfering RNA: Focus on the central nervous system. Mol. Pharm. 2021, 18, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, P.H.; Pontoppidan, M.; Bisgaard, T.S.; Berrera, M.; Dieckmann, A.; Ebeling, M.; Møller, M.R.; Hudlebusch, H.; Jensen, M.L.; Hansen, H.F.; et al. Identifying and avoiding off-target effects of RNase H-dependent antisense oligonucleotides in mice. Nucleic Acids Res. 2018, 46, 5366–5380. [Google Scholar] [CrossRef] [PubMed]
- Kuespert, S.; Heydn, R.; Peters, S.; Wirkert, E.; Meyer, A.L.; Siebörger, M.; Johannesen, S.; Aigner, L.; Bogdahn, U.; Bruun, T.H. Antisense Oligonucleotide in LNA-Gapmer Design Targeting TGFBR2-A Key Single Gene Target for Safe and Effective Inhibition of TGFβ Signaling. Int. J. Mol. Sci. 2020, 21, 1952. [Google Scholar] [CrossRef]
- Goyenvalle, A.; Jimenez-Mallebrera, C.; van Roon, W.; Sewing, S.; Krieg, A.M.; Arechavala-Gomeza, V.; Andersson, P. Considerations in the preclinical assessment of the safety of antisense oligonucleotides. Nucleic Acids Ther. 2023, 33, 1–16. [Google Scholar] [CrossRef]
- Yasuhara, H.; Yoshida, T.; Sasaki, K.; Obika, S.; Inoue, T. Reduction of off-target effects of gapmer antisense oligonucleotides by oligonucleotide extension. Mol. Diagn. Ther. 2022, 26, 117–127. [Google Scholar] [CrossRef]
- Hagedorn, P.H.; Brown, J.M.; Easton, A.; Pierdomenico, M.; Jones, K.; Olson, R.E.; Mercer, S.E.; Li, D.; Loy, J.; Høg, A.M.; et al. Acute Neurotoxicity of Antisense Oligonucleotides After Intracerebroventricular Injection Into Mouse Brain Can Be Predicted from Sequence Features. Nucleic Acids Ther. 2022, 32, 151–162. [Google Scholar] [CrossRef]
- Yoshida, T.; Naito, Y.; Yasuhara, H.; Sasaki, K.; Kawaji, H.; Kawai, J.; Naito, M.; Okuda, H.; Obika, S.; Inoue, T. Evaluation of off-target effects of gapmer antisense oligonucleotides using human cells. Genes Cells 2019, 24, 827–835. [Google Scholar] [CrossRef]
- Burdick, A.D.; Sciabola, S.; Mantena, S.R.; Hollingshead, B.D.; Stanton, R.; Warneke, J.A.; Zeng, M.; Martsen, E.; Medvedev, A.; Makarov, S.S. Sequence motifs associated with hepatotoxicity of locked nucleic acid—Modified antisense oligonucleotides. Nucleic Acids Res. 2014, 42, 4882–4891. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, Y.; Wang, B.; Du, J.; Jin, Q. Broad-spectrum antiviral activity of RNA interference against four genotypes of Japanese encephalitis virus based on single microRNA polycistrons. PLoS ONE 2011, 6, e26304. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Feng, X.; Gao, X.; Luo, Y.; Liu, C.; Liu, P.; Yang, G.; Ren, H.; Huang, R.; Feng, Y.; et al. Effective inhibition of different Japanese encephalitis virus genotypes by RNA interference targeting two conserved viral gene sequences in vitro and in vivo. Virus Genes 2018, 54, 746–755. [Google Scholar] [CrossRef]
- Shen, T.; Liu, K.; Miao, D.; Cao, R.; Zhou, B.; Chen, P. Lentivirus-mediated RNA interference against Japanese encephalitis virus infection in vitro and in vivo. Antiviral. Res. 2014, 108, 56–64. [Google Scholar] [CrossRef]
- Pyke, A.T.; Choong, K.; Moore, F.; Schlebusch, S.; Taylor, C.; Hewitson, G.; McMahon, J.; Nair, N.; Moore, P.; Finger, M.; et al. A Case of Japanese Encephalitis with a Fatal Outcome in an Australian Who Traveled from Bali in 2019. Trop. Med. Infect. Dis. 2020, 5, 133. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.; Tiemensma, M.; Currie, B.J.; Williams, D.T.; Baird, R.W.; Krause, V.L. Japanese Encephalitis in Australia—A Sentinel Case. N. Engl. J. Med. 2022, 387, 661–662. [Google Scholar] [CrossRef]
- Zeng, M.; Duan, Y.; Zhang, W.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Universal RNA Secondary Structure Insight Into Mosquito-Borne Flavivirus (MBFV) cis-Acting RNA Biology. Front. Microbiol. 2020, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hirakawa, Y.; Yamairi, F.; Kurita, T.; Murahashi, K.; Nishimura, H.; Iwazaki, N.; Yasuhara, H.; Tateoka, T.; Ohta, T.; et al. Altered Biodistribution and Hepatic Safety Profile of a Gapmer Antisense Oligonucleotide Bearing Guanidine-Bridged Nucleic Acids. Nucleic Acids Ther. 2022, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Corey, D.R. Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat. Neurosci. 2017, 20, 497–499. [Google Scholar] [CrossRef]
- Nagata, T.; Dwyer, C.A.; Yoshida-Tanaka, K.; Ihara, K.; Ohyagi, M.; Kaburagi, H.; Miyata, H.; Ebihara, S.; Yoshioka, K.; Ishii, T.; et al. Cholesterol-functionalized DNA/RNA heteroduplexes cross the blood-brain barrier and knock down genes in the rodent CNS. Nat. Biotechnol. 2021, 39, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Yu, L.; Cao, S.; Wang, K.; Yuan, J.; Wang, C.; Wang, K.; Cui, M.; Fu, Z.F. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J. Virol. 2015, 89, 5602–5614. [Google Scholar] [CrossRef] [PubMed]
- Chery, J.; Petri, A.; Wagschal, A.; Lim, S.Y.; Cunningham, J.; Vasudevan, S.; Kauppinen, S.; Näär, A.M. Development of Locked Nucleic Acid Antisense Oligonucleotides Targeting Ebola Viral Proteins and Host Factor Niemann-Pick C1. Nucleic Acids Ther. 2018, 28, 273–284. [Google Scholar] [CrossRef]
- Nerome, R.; Tajima, S.; Takasaki, T.; Yoshida, T.; Kotaki, A.; Lim, C.K.; Ito, M.; Sugiyama, A.; Yamauchi, A.; Yano, T.; et al. Molecular epidemiological analyses of Japanese encephalitis virus isolates from swine in Japan from 2002 to 2004. J. Gen. Virol. 2007, 88, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Lin, C.C.; Chien, P.T.; Hsiao, L.D.; Yang, C.M. Thrombin/Matrix Metalloproteinase-9-Dependent SK-N-SH Cell Migration is Mediated Through a PLC/PKC/MAPKs/NF-κB Cascade. Mol. Neurobiol. 2016, 53, 5833–5846. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
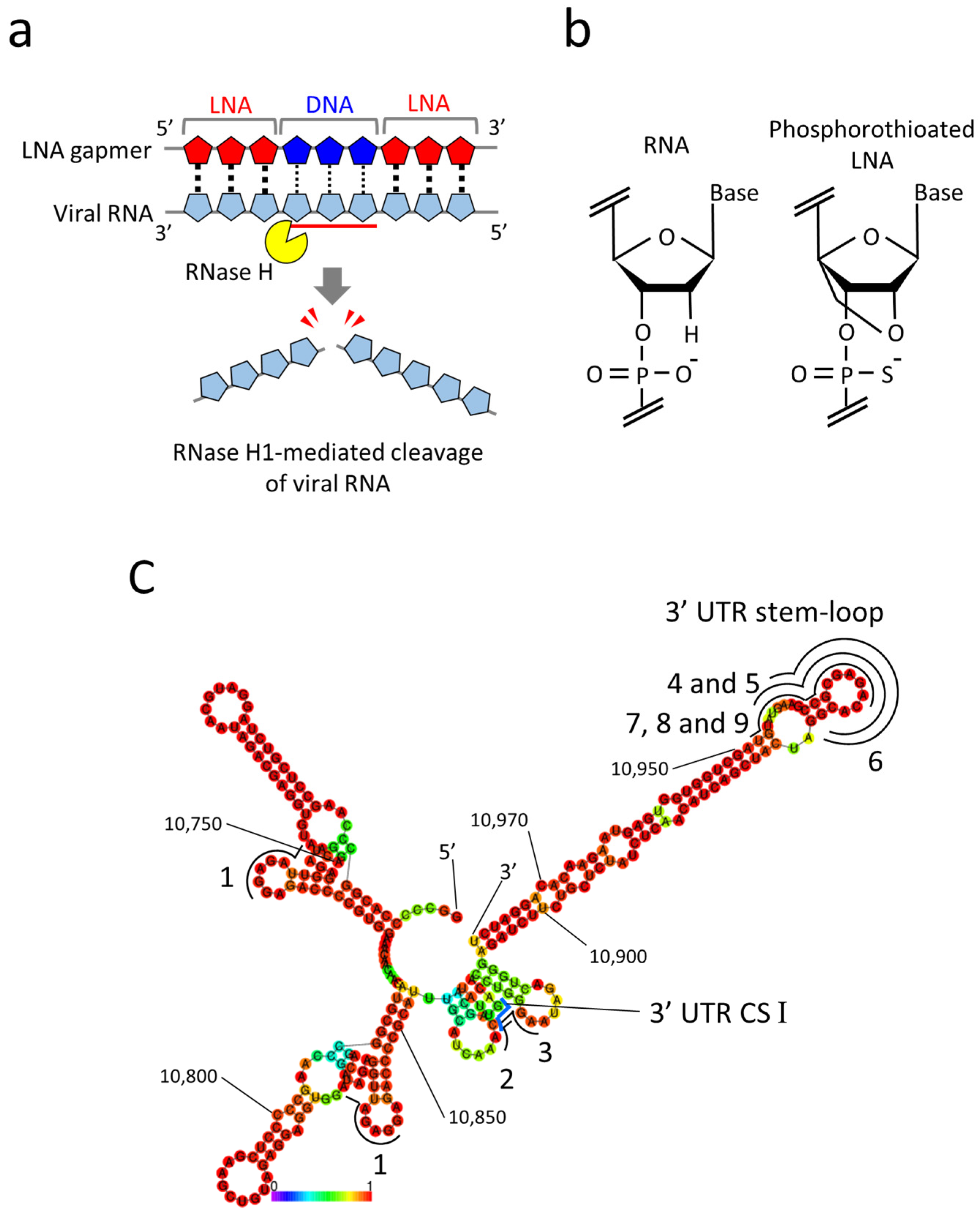
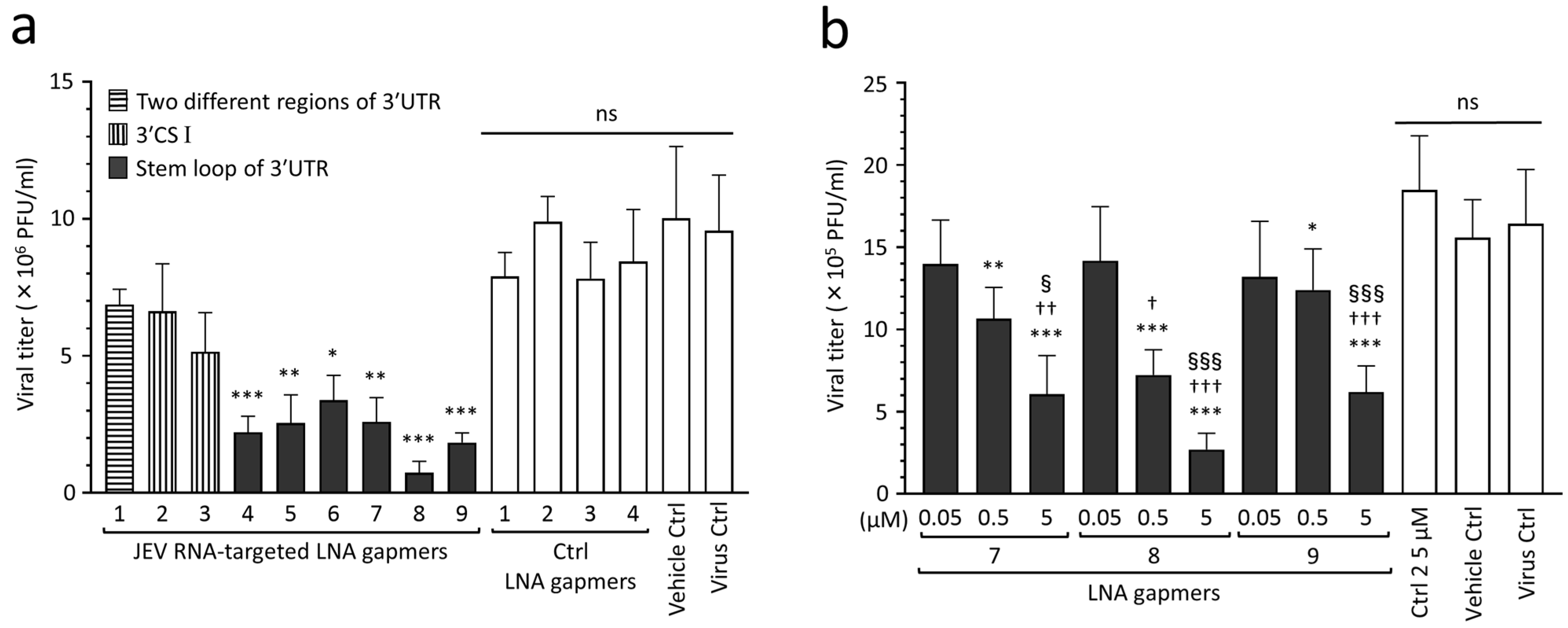
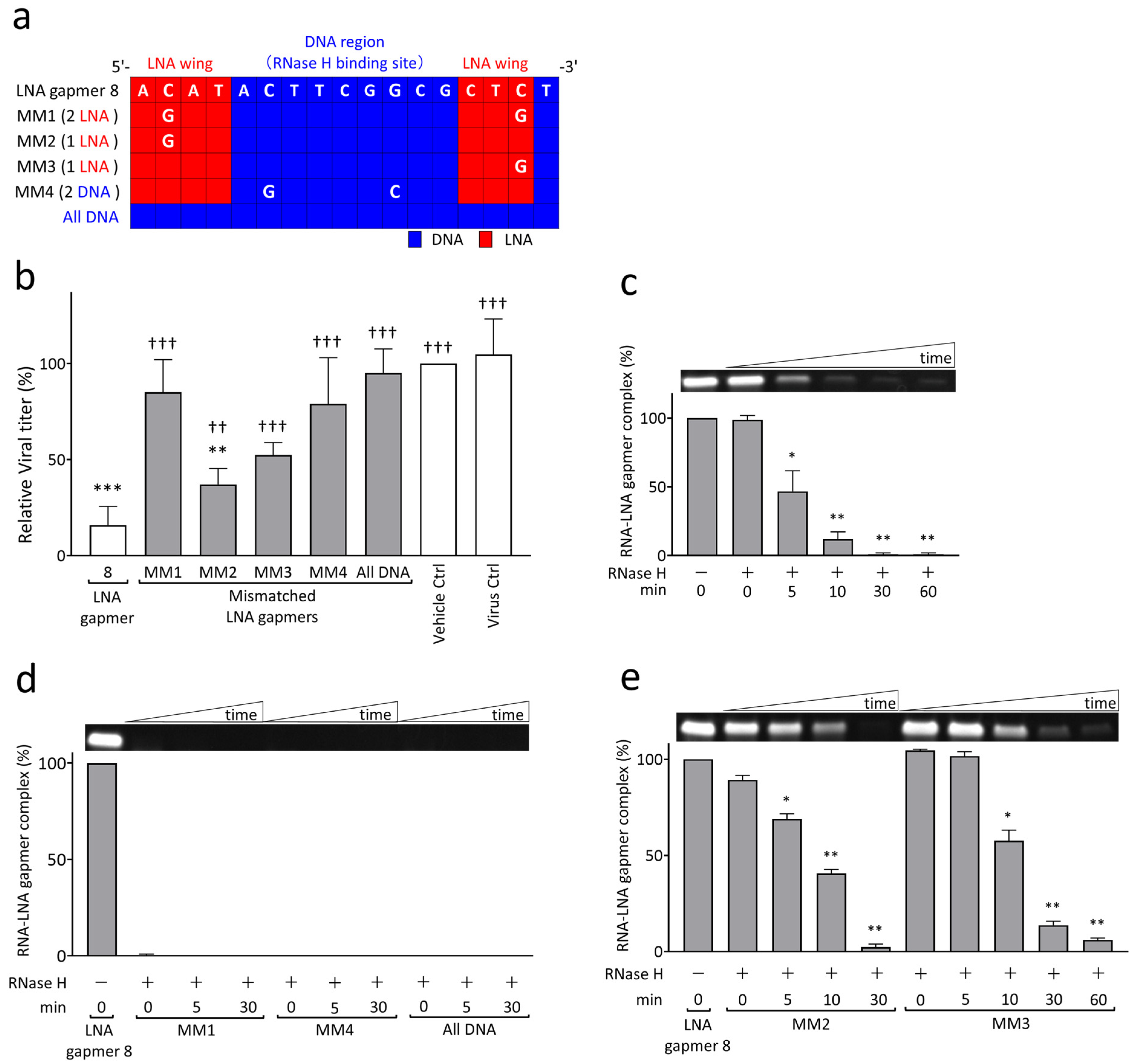
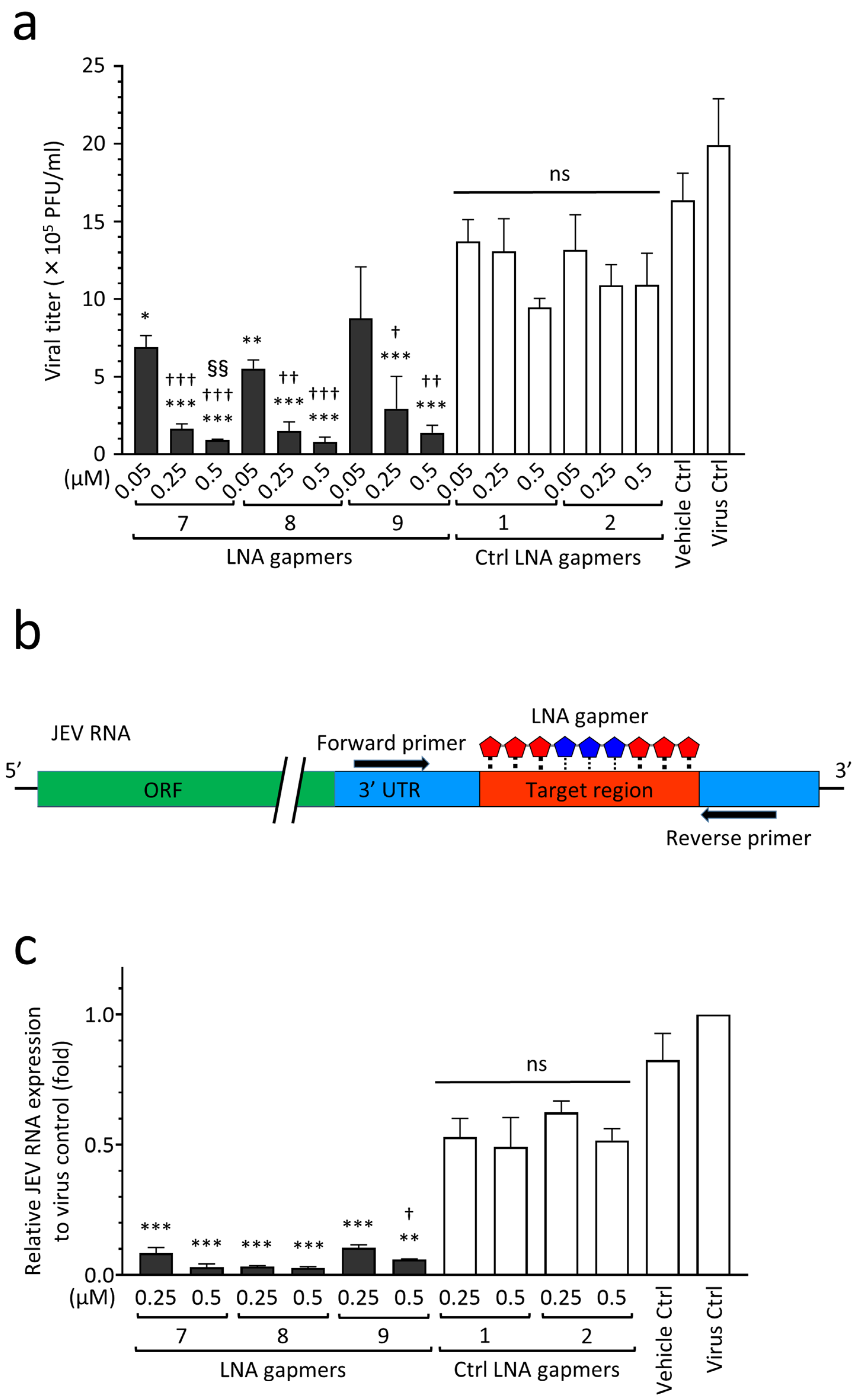

| Name | Sequence (5′→3′) | Target Regions of the JEV Genome✵(JaGAr 01, Accession No. AF069076.1) | Length (mer) |
|---|---|---|---|
| LNA gapmer 1 | mC ^ T ^ mC ^ t ^ a ^ a ^ c ^ c ^ t ^ c ^ t ^ a ^ G ^ T ^ mC | 10,749–10,763; 10,827–10,841 | 15 |
| LNA gapmer 2 | G ^ G ^ T ^ g ^ t ^ c ^ a ^ a ^ t ^ a ^ t ^ g ^ c ^ T ^ G ^ T | 10,863–10,878 | 16 |
| LNA gapmer 3 | T ^ mC ^ mC ^ c ^ a ^ g ^ g ^ t ^ g ^ t ^ c ^ a ^ a ^ T ^ A ^ T | 10,868–10,883 | 16 |
| LNA gapmer 4 | A ^ mC ^ T ^ t ^ c ^ g ^ g ^ c ^ g ^ c ^ t ^ c ^ t ^ G ^ T ^ G | 10,956–10,971 | 16 |
| LNA gapmer 5 | A ^ mC ^ T ^ t ^ c ^ g ^ g ^ c ^ g ^ c ^ t ^ c ^ t ^ G ^ T ^ g | 10,956–10,971 | 16 |
| LNA gapmer 6 | T ^ T ^ mC ^ g ^ g ^ c ^ g ^ c ^ t ^ c ^ t ^ g ^ t ^ G ^ mC ^ mC | 10,958–10,973 | 16 |
| LNA gapmer 7 | A ^ mC ^ A ^ t ^ a ^ c ^ t ^ t ^ c ^ g ^ g ^ c ^ g ^ c ^ T ^ mC ^ T | 10,952–10,968 | 17 |
| LNA gapmer 8 | A ^ mC ^ A ^ T ^ a ^ c ^ t ^ t ^ c ^ g ^ g ^ c ^ g ^ mC ^ T ^ mC ^ t | 10,952–10,968 | 17 |
| LNA gapmer 9 | A ^ mC ^ A ^ t ^ a ^ c ^ t ^ t ^ c ^ g ^ g ^ c ^ g ^ mC ^ T ^ mC | 10,952–10,967 | 16 |
| Control LNA gapmer 1 | A ^ mC ^ T ^ c ^ t ^ c ^ g ^ t ^ c ^ a ^ a ^ c ^ c ^ A ^ A ^ T | NA | 16 |
| Control LNA gapmer 2 | G ^ T ^ A ^ a ^ c ^ t ^ c ^ g ^ t ^ c ^ g ^ t ^ a ^ A ^ mC ^ A | NA | 16 |
| Control LNA gapmer 3 | mC ^ G ^ A ^ a ^ t ^ a ^ g ^ t ^ t ^ a ^ g ^ t ^ a ^ G ^ mC ^ G | NA | 16 |
| Control LNA gapmer 4 | G ^ A ^ mC ^ c ^ a ^ a ^ t ^ c ^ t ^ c ^ g ^ t ^ t ^ A ^ G ^ T | NA | 16 |
| Number of Regions Complementary to LNA Gapmers | ||||
|---|---|---|---|---|
| 0 MM or Gap | 1 MM or Gap | 2 MMs or Gaps | 3 MMs or Gaps | |
| Human genome | ||||
| LNA gapmers 7 and 8 | 0 | 1 | 77 | 4491 |
| LNA gapmer 9 | 0 | 4 | 287 | 15,069 |
| Ctrl LNA gapmer 1 | 0 | 31 | 1626 | 44,191 |
| Ctrl LNA gapmer 2 | 0 | 1 | 315 | 15,601 |
| Human RNA | ||||
| LNA gapmers 7 and 8 | 0 | 0 | 10 | 1673 |
| LNA gapmer 9 | 0 | 0 | 81 | 5081 |
| Ctrl LNA gapmer 1 | 0 | 0 | 178 | 8291 |
| Ctrl LNA gapmer 2 | 0 | 0 | 59 | 3585 |
| Primer Name | Sequence (5′→3′) | Position | Purpose |
|---|---|---|---|
| JaGAr 01_F | CCTGGGAATAGACTGGGAGAT | 10,877–10,897 | RT-qPCR |
| JaGAr 01_R | GTTCTTACTCACCACCAGCTACA | 10,946–10,968 | |
| GAPDH_F | GCCAGCCGAGCCACAT | NA | RT-qPCR |
| GAPDH_R | CTTTACCAGAGTTAAAAGCAGCCC | NA | |
| JE955f | TGYTGGTCGCTCCGGCYTA | 955–973 | Envelope protein |
| JE2536r | AAGATGCCACTTCCACAYCTC | 2516–2536 | |
| JE_LNA_1F | TCCAGGAAGACAGGGTCATC | 10,372–10,391 | LNA gapmer target region |
| JE_LNA_1R | CCTGTGTTCTTACTCACCACCAG | 10,951–10,973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, S.; Echigoya, Y.; Tago, A.; Segawa, T.; Sato, Y.; Itou, T. Antiviral Efficacy of RNase H-Dependent Gapmer Antisense Oligonucleotides against Japanese Encephalitis Virus. Int. J. Mol. Sci. 2023, 24, 14846. https://doi.org/10.3390/ijms241914846
Okamoto S, Echigoya Y, Tago A, Segawa T, Sato Y, Itou T. Antiviral Efficacy of RNase H-Dependent Gapmer Antisense Oligonucleotides against Japanese Encephalitis Virus. International Journal of Molecular Sciences. 2023; 24(19):14846. https://doi.org/10.3390/ijms241914846
Chicago/Turabian StyleOkamoto, Shunsuke, Yusuke Echigoya, Ayaka Tago, Takao Segawa, Yukita Sato, and Takuya Itou. 2023. "Antiviral Efficacy of RNase H-Dependent Gapmer Antisense Oligonucleotides against Japanese Encephalitis Virus" International Journal of Molecular Sciences 24, no. 19: 14846. https://doi.org/10.3390/ijms241914846
APA StyleOkamoto, S., Echigoya, Y., Tago, A., Segawa, T., Sato, Y., & Itou, T. (2023). Antiviral Efficacy of RNase H-Dependent Gapmer Antisense Oligonucleotides against Japanese Encephalitis Virus. International Journal of Molecular Sciences, 24(19), 14846. https://doi.org/10.3390/ijms241914846






