Prokaryotic Argonaute Proteins: A New Frontier in Point-of-Care Viral Diagnostics
Abstract
:1. Introduction
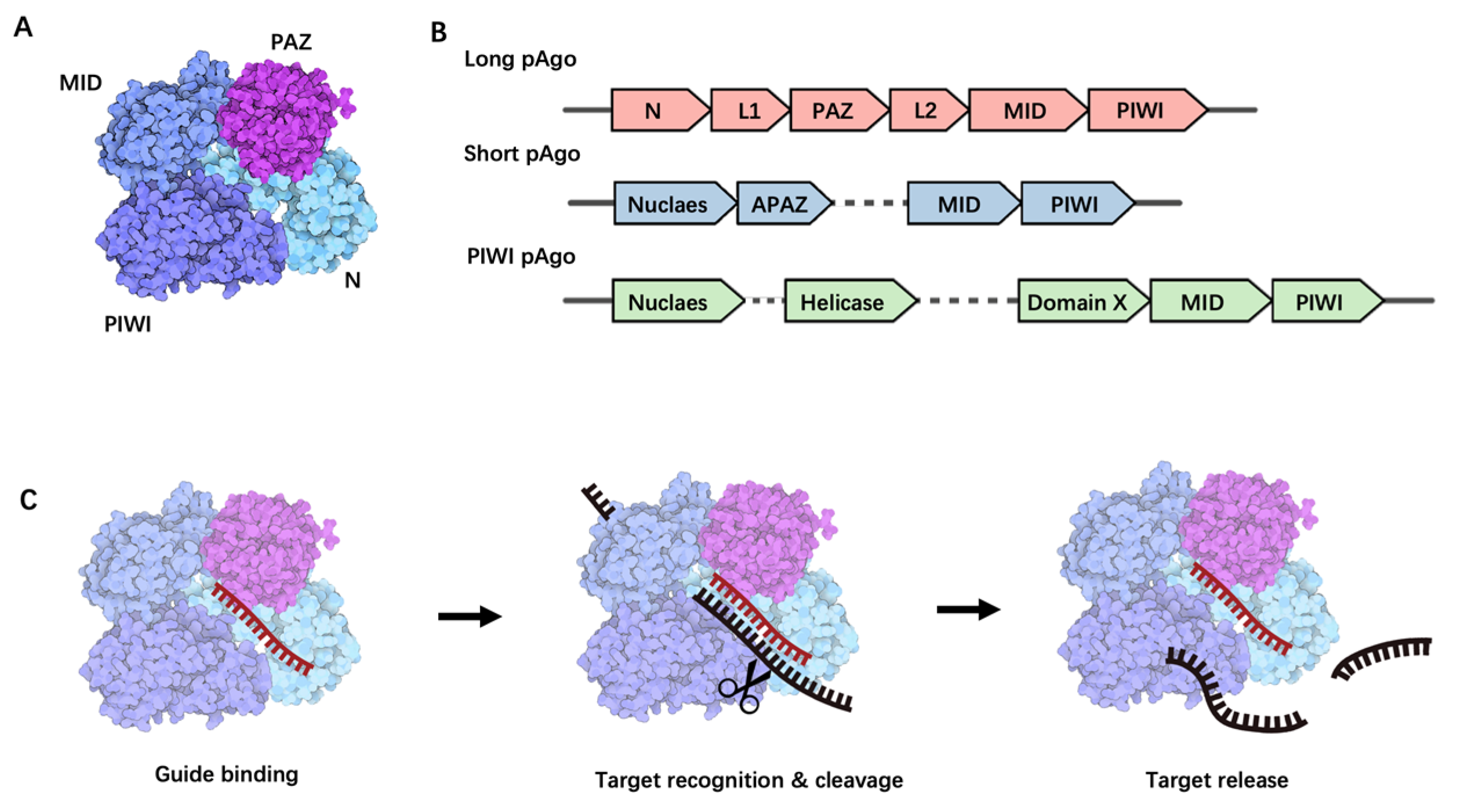
2. Argonaute Proteins: Structure and Function
2.1. Structural Overview
2.2. Biological Functions of Long pAgo
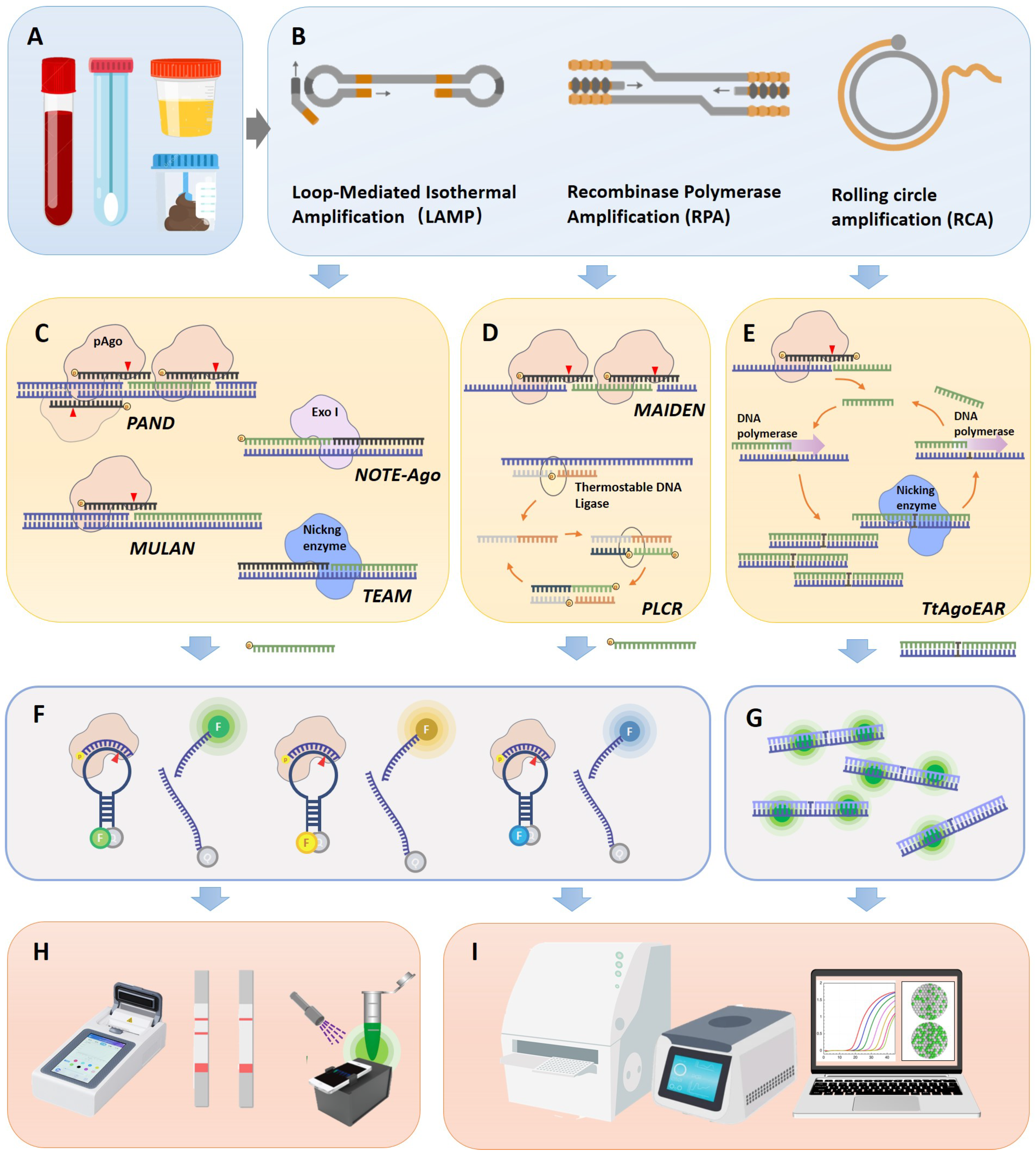
3. pAgo-Based POC Detection Technologies
3.1. PfAgo-Mediated Nucleic Acid Detection, PAND
3.2. Ultra-Short PCR and Pyrococcus furiosus Argonaute Combined Nucleic Acid Detection (USPCRP)
3.3. Ago-Directed Specific Target Enrichment and Detection (A-Star)
3.4. Multiplex Ago-Based Nucleic Acid Detection System (MULAN)
3.5. TtAgo-Assisted Exponential Isothermal Amplification for Multiplex Detection (TEAM)
3.6. Mesophilic Ago-Based Isothermal Detection Method (MAIDEN)
3.7. Tt Argonaute-Based Thermostable Exponential Amplification Reaction (TtAgoEAR)
3.8. Short Prokaryotic Argonaute/TIR-APAZ (SPARTA)-Based Nucleic Acid Detection Tool
| Detection Platform | pAgo Protein | Amplification | Limit of Detection (LoD) | Target/Type | Detection Time | Signal Reading | Features | Reference |
|---|---|---|---|---|---|---|---|---|
| PAND | PfAgo | PCR or tHDA | 1 copy/μL | HPV/DNA | 2 h | qPCR system | Multiplex target detection short qPCR instrument usage time | [45] |
| USPCRP | PfAgo | usPCR | 10 aM | SARS-CoV-2, MERS-CoV, SARS-CoV/RNA | 70 min | Fluorescence detector 1 | less than two enzymes include short target enrichment time, detection of extremely short targets | [48] |
| A-Star | PfAgo | PCR or RT-PCR | 0.01% mutant | KRAS G12D/DNA | N/A | qPCR system, Sanger sequencing | Improve the amplification efficiency of mutant genes, | [49] |
| MULAN | PfAgo | RT-LAMP | 5 copies/μL | SARS-CoV-2, influenza virus/RNA | 35 min | Blue-light, qPCR system Lateral flow dipstick | One-pot detection, multiple detection | [43] |
| TEAM | TtAgo | EXPAR | 1 aM | let-7/miRNA | 35 min | Fluorescence detector 1 | Multiple miRNA detection | [41] |
| TtAgoEAR | TtAgo | EXPAR | 20 aM | SARS-CoV-2, HOTTIP/RNA | 80 min | qPCR system | Adaptable to a lateral-flow-based readout | [44] |
| PLCR | PfAgo | LCR/RT-LCR | 1 aM | HPV, SARS-CoV-2/DNA or RNA | 100 min | fluorescence plate reader | Multiple detection | [40] |
| NOTE-Ago | PfAgo | PCR | 1 CFU/mL | Salmonella typh, Staphylococcus aureus/DNA | 2 h | 3D-printed fluorescent reader | Fluorescent visualization | [52] |
| RADAR | PfAgo | PCR or RT-PCR | 10–15 M | HPV, DNA | 2 h | Fuji FLA7000 scanner | Multiple detection, gene genotyping | [37] |
| MAIDEN | Mesophilic Ago | Reverse transcription | 1 nM | SARS-CoV-2, RNA | 60 min | qPCR system | Portable, single-tube detection | [29] |
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34, e00228-20. [Google Scholar] [CrossRef]
- Lessler, J.; Chaisson, L.H.; Kucirka, L.M.; Bi, Q.; Grantz, K.; Salje, H.; Carcelen, A.C.; Ott, C.T.; Sheffield, J.S.; Ferguson, N.M.; et al. Assessing the global threat from Zika virus. Science 2016, 353, aaf8160. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A., 2nd; Hewlett, A.; Kraft, C.S.; Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef]
- Vashist, S.K. Point-of-Care Diagnostics: Recent Advances and Trends. Biosensors 2017, 7, 62. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Kostyusheva, A.; Brezgin, S.; Babin, Y.; Vasilyeva, I.; Glebe, D.; Kostyushev, D.; Chulanov, V. CRISPR-Cas systems for diagnosing infectious diseases. Methods 2022, 203, 431–446. [Google Scholar] [CrossRef]
- Kropocheva, E.V.; Lisitskaya, L.A.; Agapov, A.A.; Musabirov, A.A.; Kulbachinskiy, A.V.; Esyunina, D.M. Prokaryotic Argonaute Proteins as a Tool for Biotechnology. Mol. Biol. 2022, 56, 854–873. [Google Scholar] [CrossRef]
- Ketting, R.F. The many faces of RNAi. Dev. Cell 2011, 20, 148–161. [Google Scholar] [CrossRef]
- Bohmert, K.; Camus, I.; Bellini, C.; Bouchez, D.; Caboche, M.; Benning, C. AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J. 1998, 17, 170–180. [Google Scholar] [CrossRef]
- Kuzmenko, A.; Oguienko, A.; Esyunina, D.; Yudin, D.; Petrova, M.; Kudinova, A.; Maslova, O.; Ninova, M.; Ryazansky, S.; Leach, D.; et al. DNA targeting and interference by a bacterial Argonaute nuclease. Nature 2020, 587, 632–637. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jung, Y.; Lim, D. Argonaute system of Kordia jejudonensis is a heterodimeric nucleic acid-guided nuclease. Biochem. Biophys. Res. Commun. 2020, 525, 755–758. [Google Scholar] [CrossRef]
- Swarts, D.C.; Makarova, K.; Wang, Y.; Nakanishi, K.; Ketting, R.F.; Koonin, E.V.; Patel, D.J.; van der Oost, J. The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 2014, 21, 743–753. [Google Scholar] [CrossRef]
- Olina, A.V.; Kulbachinskiy, A.V.; Aravin, A.A.; Esyunina, D.M. Argonaute Proteins and Mechanisms of RNA Interference in Eukaryotes and Prokaryotes. Biochemistry 2018, 83, 483–497. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Cho, W.C.; Zheng, Y.J. Argonaute proteins: Structural features, functions and emerging roles. J. Adv. Res. 2020, 24, 317–324. [Google Scholar]
- Hegge, J.W.; Swarts, D.C.; van der Oost, J. Prokaryotic Argonaute proteins: Novel genome-editing tools? Nat. Rev. Microbiol. 2018, 16, 5–11. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; van der Oost, J.; Koonin, E.V. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol. Direct 2009, 4, 29. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Iyer, L.M.; Aravind, L. Two novel PIWI families: Roles in inter-genomic conflicts in bacteria and Mediator-dependent modulation of transcription in eukaryotes. Biol. Direct 2013, 8, 13. [Google Scholar] [CrossRef]
- Yuan, Y.R.; Pei, Y.; Ma, J.B.; Kuryavyi, V.; Zhadina, M.; Meister, G.; Chen, H.Y.; Dauter, Z.; Tuschl, T.; Patel, D.J. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol. Cell. 2005, 19, 405–419. [Google Scholar] [CrossRef]
- Rashid, U.J.; Paterok, D.; Koglin, A.; Gohlke, H.; Piehler, J.; Chen, J.C. Structure of Aquifex aeolicus argonaute highlights conformational flexibility of the PAZ domain as a potential regulator of RNA-induced silencing complex function. J. Biol. Chem. 2007, 282, 13824–13832. [Google Scholar] [CrossRef]
- Kuzmenko, A.; Yudin, D.; Ryazansky, S.; Kulbachinskiy, A.; Aravin, A.A. Programmable DNA cleavage by Ago nucleases from mesophilic bacteria Clostridium butyricum and Limnothrix rosea. Nucleic Acids Res. 2019, 47, 5822–5836. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.C.; Hegge, J.W.; Hinojo, I.; Shiimori, M.; Ellis, M.A.; Dumrongkulraksa, J.; Terns, R.M.; Terns, M.P.; van der Oost, J. Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA. Nucleic Acids Res. 2015, 43, 5120–5129. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, I.; Chan, K.; Sachidanandam, R.; Newman, D.; Aravin, A. Bacterial Argonaute Samples the Transcriptome to Identify Foreign DNA. Mol. Cell. Biol. 2013, 51, 594–605. [Google Scholar] [CrossRef]
- Jolly, S.M.; Gainetdinov, I.; Jouravleva, K.; Zhang, H.; Strittmatter, L.; Bailey, S.M.; Hendricks, G.M.; Dhabaria, A.; Ueberheide, B.; Zamore, P.D. Thermus thermophilus Argonaute Functions in the Completion of DNA Replication. Cell 2020, 182, 1545–1559. [Google Scholar] [CrossRef]
- Swarts, D.C.; Jore, M.M.; Westra, E.R.; Zhu, Y.; Janssen, J.H.; Snijders, A.P.; Wang, Y.; Patel, D.J.; Berenguer, J.; Brouns, S.J.J.; et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature 2014, 507, 258–261. [Google Scholar] [CrossRef]
- Swarts, D.C.; Koehorst, J.J.; Westra, E.R.; Schaap, P.J.; van der Oost, J. Effects of Argonaute on Gene Expression in Thermus thermophilus. PLoS ONE 2015, 10, e0124880. [Google Scholar] [CrossRef]
- Enghiad, B.; Zhao, H. Programmable DNA-Guided Artificial Restriction Enzymes. ACS Synth. Biol. 2017, 6, 752–757. [Google Scholar] [CrossRef]
- Kaya, E.; Doxzen, K.W.; Knoll, K.R.; Wilson, R.C.; Strutt, S.C.; Kranzusch, P.J.; Doudna, J.A. A bacterial Argonaute with noncanonical guide RNA specificity. Proc. Natl. Acad. Sci. USA 2016, 113, 4057–4062. [Google Scholar] [CrossRef]
- Li, X.; Dong, H.; Guo, X.; Huang, F.; Xu, X.; Li, N.; Yang, Y.; Yao, T.; Feng, Y.; Liu, Q. Mesophilic Argonaute-based isothermal detection of SARS-CoV-2. Front. Microbiol. 2022, 13, 957977. [Google Scholar] [CrossRef]
- Hegge, J.W.; Swarts, D.C.; Chandradoss, S.D.; Cui, T.J.; van der Oost, J. DNA-guided DNA cleavage at moderate temperatures by Clostridium butyricum Argonaute. Nucleic Acids Res. 2019, 47, 5809–5821. [Google Scholar] [CrossRef]
- Sun, S.; Xu, D.; Zhu, L.; Hu, B.; Huang, Z. A Programmable, DNA-Exclusively-Guided Argonaute DNase and Its Higher Cleavage Specificity Achieved by 5′-Hydroxylated Guide. Biomolecules 2022, 12, 1340. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, W.; Wang, J.; Sheng, G.; Xiang, G.; Zhang, T.; Shi, W.; Li, C.; Wang, Y.; Zhao, F.; et al. Argonaute proteins from human gastrointestinal bacteria catalyze DNA-guided cleavage of single- and double-stranded DNA at 37 °C. Cell Discov. 2019, 5, 38. [Google Scholar] [CrossRef]
- Kropocheva, E.; Kuzmenko, A.; Aravin, A.A.; Esyunina, D.; Kulbachinskiy, A. A programmable pAgo nuclease with universal guide and target specificity from the mesophilic bacterium Kurthia massiliensis. Nucleic Acids Res. 2021, 49, 4054–4065. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wang, F.; Ma, L. A programmable pAgo nuclease with RNA target preference from the psychrotolerant bacteria Mucilaginibacter paludis. Nucleic Acids Res. 2021, 50, 5226–5238. [Google Scholar] [CrossRef]
- Chong, Y.; Liu, Q.; Huang, F.; Song, D.; Feng, Y. Characterization of a recombinant thermotolerant argonaute protein as an endonuclease by broad guide utilization. Bioresour. Bioprocess. 2019, 6, 21. [Google Scholar] [CrossRef]
- Willkomm, S.; Oellig, C.A.; Zander, A.; Restle, T.; Keegan, R.; Grohmann, D.; Schneider, S. Structural and mechanistic insights into an archaeal DNA-guided Argonaute protein. Nat. Microbiol. 2017, 2, 17035. [Google Scholar] [CrossRef]
- Xun, G.; Liu, Q.; Chong, Y.; Guo, X.; Li, Z.; Li, Y.; Fei, H.; Li, K.; Feng, Y. Argonaute with stepwise endonuclease activity promotes specific and multiplex nucleic acid detection. Bioresour. Bioprocess. 2021, 8, 46. [Google Scholar] [CrossRef]
- Panteleev, V.; Kropocheva, E.; Esyunina, D.; Kulbachinskiy, A. Strong temperature effects on the fidelity of target DNA recognition by a thermophilic pAgo nuclease. Biochimie 2023, 209, 142–149. [Google Scholar] [CrossRef]
- Swarts, D.C.; Szczepaniak, M.; Sheng, G.; Chandradoss, S.D.; Zhu, Y.; Timmers, E.M.; Zhang, Y.; Zhao, H.; Lou, J.; Wang, Y.; et al. Autonomous Generation and Loading of DNA Guides by Bacterial Argonaute. Mol. Cell 2017, 65, 985–998.e986. [Google Scholar] [CrossRef]
- Wang, L.; He, R.; Lv, B.; Yu, X.; Liu, Y.; Yang, J.; Li, W.; Wang, Y.; Zhang, H.; Yan, G.; et al. Pyrococcus furiosus Argonaute coupled with modified ligase chain reaction for detection of SARS-CoV-2 and HPV. Talanta 2021, 227, 122154. [Google Scholar] [CrossRef]
- Lin, Q.; Han, G.; Fang, X.; Chen, H.; Weng, W.; Kong, J. Programmable Analysis of MicroRNAs by Thermus thermophilus Argonaute-Assisted Exponential Isothermal Amplification for Multiplex Detection (TEAM). Anal. Chem. 2022, 94, 11290–11297. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hegge, J.W.; Mauk, M.G.; Chen, J.; Till, J.E.; Bhagwat, N.; Azink, L.T.; Peng, J.; Sen, M.; Mays, J.; et al. Highly specific enrichment of rare nucleic acid fractions using Thermus thermophilus argonaute with applications in cancer diagnostics. Nucleic Acids Res. 2020, 48, e19. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, H.; Guo, X.; Liu, D.; Li, Z.; Sun, J.; Huang, J.; Liu, T.; Zhao, P.; Xu, H.; et al. Argonaute-integrated isothermal amplification for rapid, portable, multiplex detection of SARS-CoV-2 and influenza viruses. Biosens. Bioelectron. 2022, 207, 114169. [Google Scholar] [CrossRef]
- Yuan, C.; Fang, J.; Fu, W. Thermus thermophilus Argonaute-Based Isothermal Amplification Assay for Ultrasensitive and Specific RNA Detection. Anal. Chem. 2023, 95, 8291–8298. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wang, L.; Wang, F.; Li, W.; Liu, Y.; Li, A.; Wang, Y.; Mao, W.; Zhai, C.; Ma, L. Pyrococcus furiosus Argonaute-mediated nucleic acid detection. Chem. Commun. 2019, 55, 13219–13222. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; He, R.; Yu, X.; Chen, S.; Liu, Y.; Wang, L.; Li, A.; Liu, L.; Zhai, C.; et al. PfAgo-based detection of SARS-CoV-2. Biosens. Bioelectron. 2021, 177, 112932. [Google Scholar] [CrossRef]
- Xun, G.; Lane, S.T.; Petrov, V.A.; Pepa, B.E.; Zhao, H. A rapid, accurate, scalable, and portable testing system for COVID-19 diagnosis. Nat. Commun. 2021, 12, 2905. [Google Scholar] [CrossRef]
- He, R.; Wang, L.; Wang, F.; Yang, J.; Yu, X.; Wang, Y.; Liu, Z.; Li, C.; Ma, L. Combination of ultrashort PCR and Pyrococcus furiosus Argonaute for DNA detection. Analyst 2021, 147, 35–39. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, X.; Xun, G.; Li, Z.; Chong, Y.; Yang, L.; Wang, H.; Zhang, F.; Luo, S.; Cui, L.; et al. Argonaute integrated single-tube PCR system enables supersensitive detection of rare mutations. Nucleic Acids Res. 2021, 49, e75. [Google Scholar] [CrossRef]
- Koopal, B.; Potocnik, A.; Mutte, S.K.; Aparicio-Maldonado, C.; Lindhoud, S.; Vervoort, J.J.M.; Brouns, S.J.J.; Swarts, D.C. Short prokaryotic Argonaute systems trigger cell death upon detection of invading DNA. Cell 2022, 185, 1471–1486.e1419. [Google Scholar] [CrossRef]
- Potocnik, A.; Swarts, D.C. Short prokaryotic Argonaute system repurposed as a nucleic acid detection tool. Clin. Transl. Med. 2022, 12, e1059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kou, J.; Han, X.; Qiao, J.; Zhang, W.; Man, S.; Ma, L. Argonaute-triggered visual and rebuilding-free foodborne pathogenic bacteria detection. J. Hazard. Mater. 2023, 454, 131485. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, X.; Ma, X.; Wang, R.; Chen, S.; Li, F.; Li, Z. One-Pot Isothermal LAMP-CRISPR-Based Assay for Klebsiella pneumoniae Detection. Microbiol. Spectr. 2022, 10, e0154522. [Google Scholar] [CrossRef]
- Khan, S.H.; Zaidi, S.K.; Gilani, M. PCR to CRISPR: Role of Nucleic Acid Tests (NAT) in detection of COVID-19. J. Pak. Med. Assoc. 2022, 72, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.I.; Aman, R.; Mahfouz, M. Onsite detection of plant viruses using isothermal amplification assays. Plant Biotechnol. J. 2022, 20, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, H.; Taguchi, Y.; Nakagawa, R.; Makino, A.; Okazaki, S.; Nakano, M.; Muramoto, Y.; Takahashi, C.; Takahashi, I.; Ando, J.; et al. Amplification-free RNA detection with CRISPR-Cas13. Commun. Biol. 2021, 4, 476. [Google Scholar] [CrossRef]
- Filius, M.; Cui, T.J.; Ananth, A.N.; Docter, M.W.; Hegge, J.W.; van der Oost, J.; Joo, C. High-Speed Super-Resolution Imaging Using Protein-Assisted DNA-PAINT. Nano Lett. 2020, 20, 2264–2270. [Google Scholar] [CrossRef]
- Shin, S.; Jung, Y.; Uhm, H.; Song, M.; Son, S.; Goo, J.; Jeong, C.; Song, J.J.; Kim, V.N.; Hohng, S. Quantification of purified endogenous miRNAs with high sensitivity and specificity. Nat. Commun. 2020, 11, 6033. [Google Scholar] [CrossRef]
| Protein Name | Host | Guide a | Target | Guide-Independent Activity | Iron | Reaction b Temperature | Reference |
|---|---|---|---|---|---|---|---|
| BlAgo | Brevibacillus laterosporus | P-DNA, OH-DNA | DNA | / | Mg2+, Mn2+ | 65 °C | [29] |
| CbAgo | Clostridium butyricum | P-DNA, OH-DNA | DNA | Nicking of Plasmid,chopping of dsDNA | Mg2+, Mn2+, Co2+ | 30–54 °C | [21,30] |
| CdAgo | Clostridium disporicum | P-DNA, OH-DNA | DNA | chopping of dsDNA, plasmid | Mn2+, weekly Mg2+, Co2+ | 37–55 °C | [31] |
| CpAgo | Clostridium perfringens | P-DNA, OH-DNA | DNA | / | Mg2+, Mn2+ | 37–50 °C | [32] |
| IbAgo | Intestinibacter bartlettii | P-DNA, OH-DNA | DNA | / | Mg2+, Mn2+ | 37–70 °C | [32] |
| KmAgo | Kurthia massiliensis | P-DNA, OH-DNA, P-RNA | RNA | Nicking of Plasmid | Mn2+, Mg2+, weakly Co2+ | 45–55 °C | [33] |
| LrAgo | Limnothrix rosea | P-DNA, OH-DNA | DNA | Nicking of Plasmid,chopping of dsDNA | Mn2+, Mg2+, weakly Co2+ | 50–54 °C | [21] |
| MbpAgo | Mucilaginibacter paludis | P-DNA, OH-DNA | RNA | / | Mn2+, Mg2+ | 30–55 °C | [34] |
| MfAgo | Methanocaldococcus fervens | P-DNA, OH-DNA, P-RNA | DNA | / | Mn2+, Mg2+, Co2+ | 80–90 °C | [35] |
| MjAgo | Methanocaldococcus jannaschii | P-DNA, OH-DNA | DNA | chopping of dsDNA, plasmid | Mg2+ | 85–95 °C | [36] |
| MpAgo | Marinitoga piezophila | OH-RNA, OH-DNA, P-DNA | DNA, RNA | / | Mn2+, Mg2+, weakly Ni2+ | 60 °C | [28] |
| PfAgo | Pyrococcus furiosus | P-DNA | DNA, | Nicking of Plasmid | Mn2+, Co2+ | 90–99.9 °C | [22,37] |
| Tce Ago | Thermobrachium celere | P-DNA, OH-DNA | DNA | / | Mn2+, Mg2+, Co2+, weekly Ca2+ | 40–60 °C | [38] |
| TtAgo | Thermus thermophilus | P-DNA | DNA, RNA | chopping of dsDNA | Mn2+, Mg2+ | 50–75 °C | [25,39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Liu, Y.; Zhao, W.; Ma, B.; Zhang, M.; Yu, X.; Ye, Z. Prokaryotic Argonaute Proteins: A New Frontier in Point-of-Care Viral Diagnostics. Int. J. Mol. Sci. 2023, 24, 14987. https://doi.org/10.3390/ijms241914987
Sun K, Liu Y, Zhao W, Ma B, Zhang M, Yu X, Ye Z. Prokaryotic Argonaute Proteins: A New Frontier in Point-of-Care Viral Diagnostics. International Journal of Molecular Sciences. 2023; 24(19):14987. https://doi.org/10.3390/ijms241914987
Chicago/Turabian StyleSun, Kai, Yan Liu, Wei Zhao, Biao Ma, Mingzhou Zhang, Xiaoping Yu, and Zihong Ye. 2023. "Prokaryotic Argonaute Proteins: A New Frontier in Point-of-Care Viral Diagnostics" International Journal of Molecular Sciences 24, no. 19: 14987. https://doi.org/10.3390/ijms241914987





