Fast and High-Efficiency Synthesis of Capsanthin in Pepper by Transient Expression of Geminivirus
Abstract
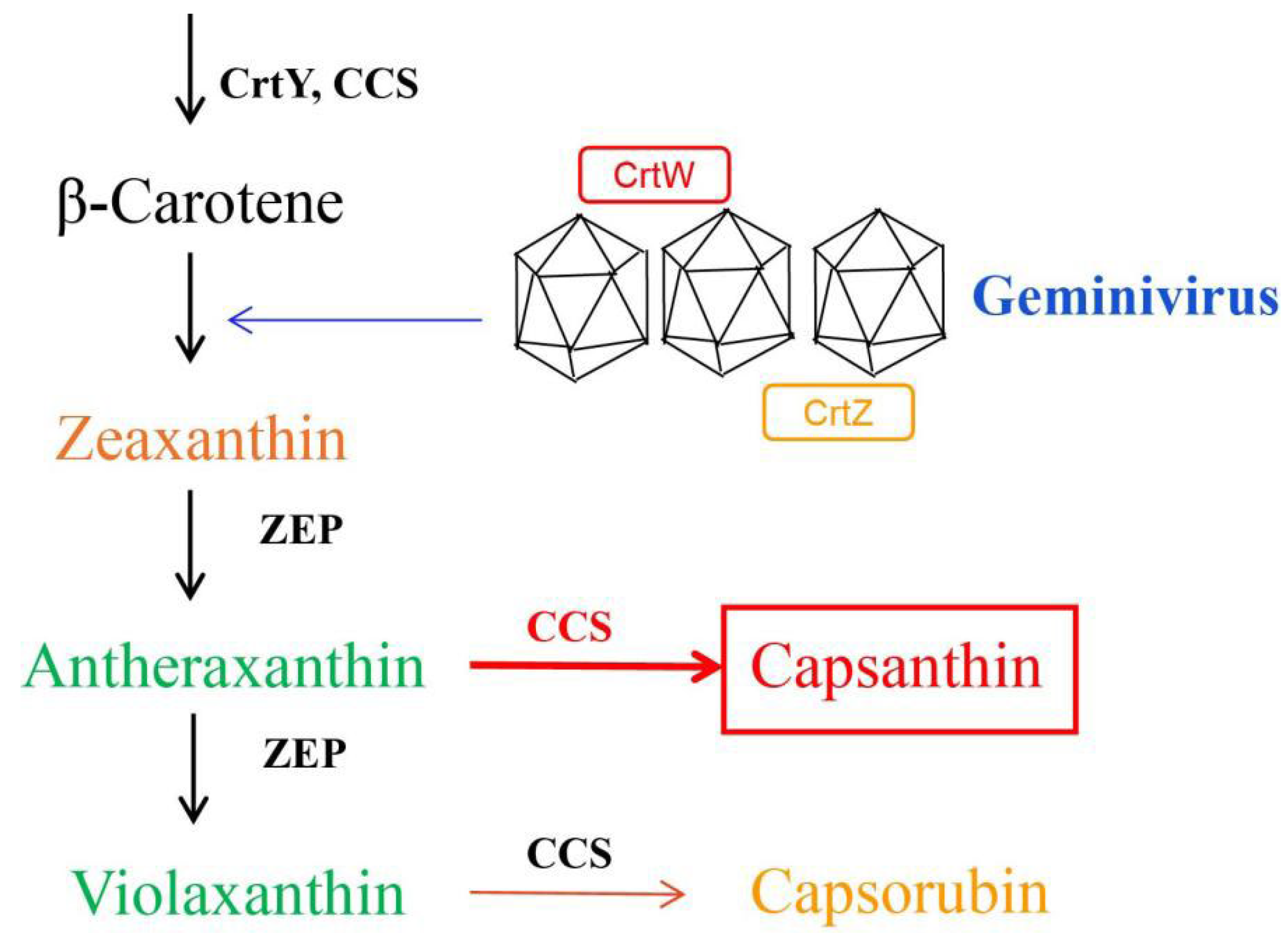
:1. Introduction
2. Results
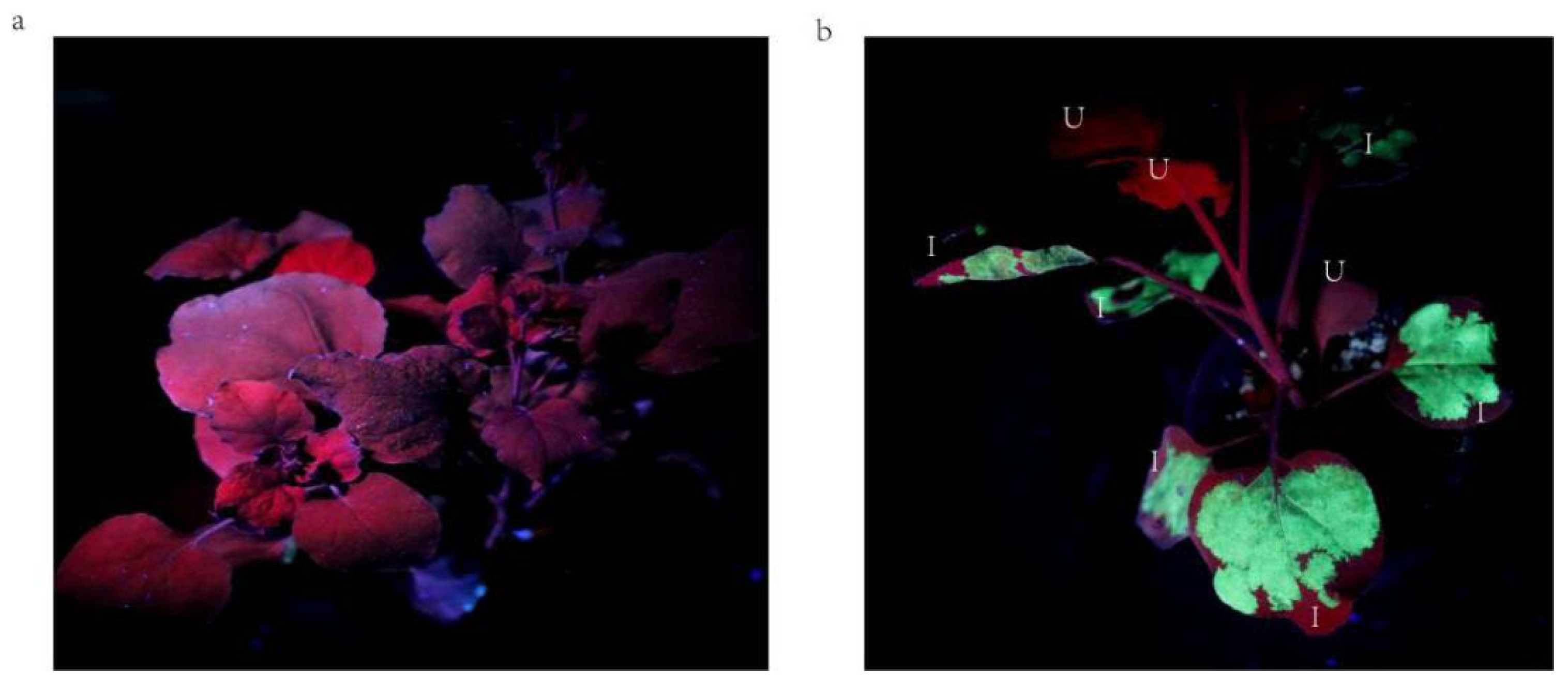
2.1. Analysis of Geminivirus Infection in N. benthamiana Plants
2.2. Codon Optimization and Gene Synthesis
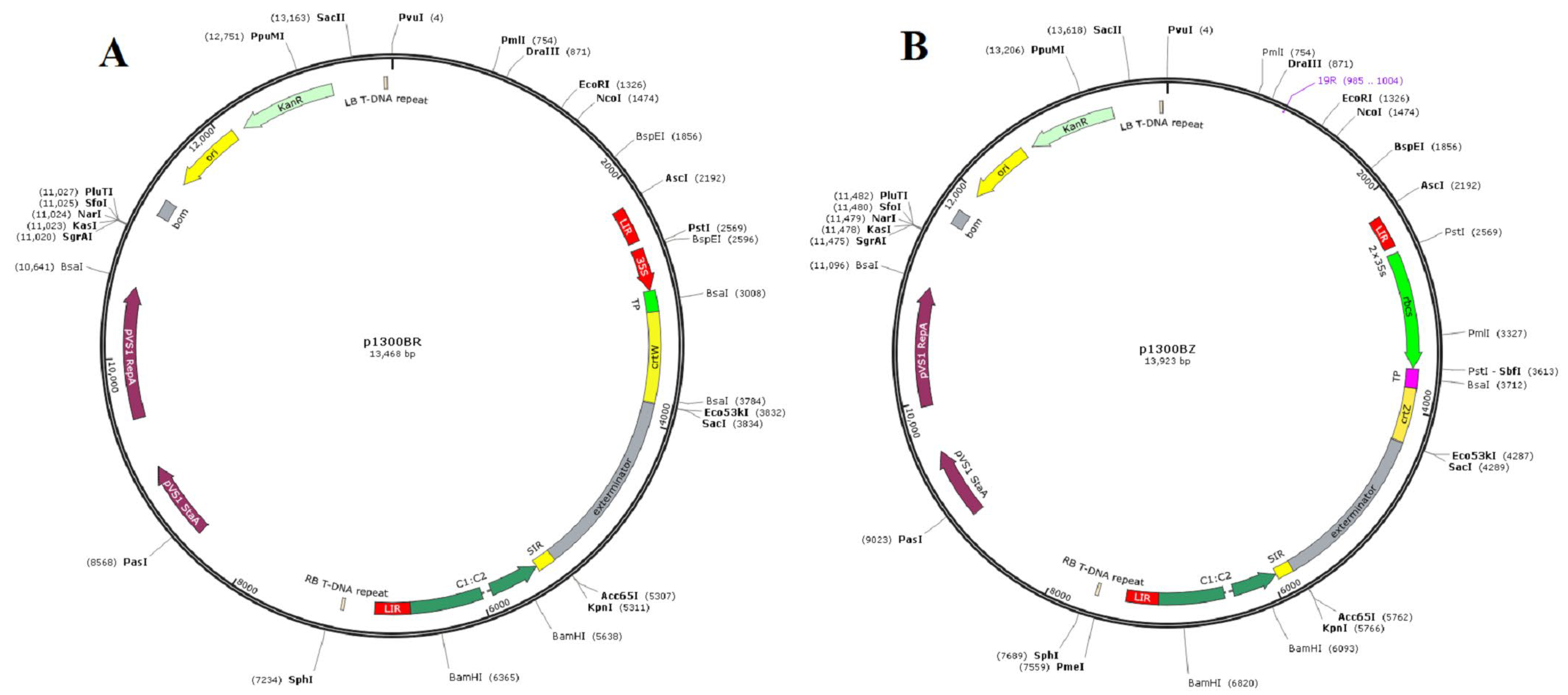
2.3. Efficient Expression Vectors to Produce Capsanthin in Peppers
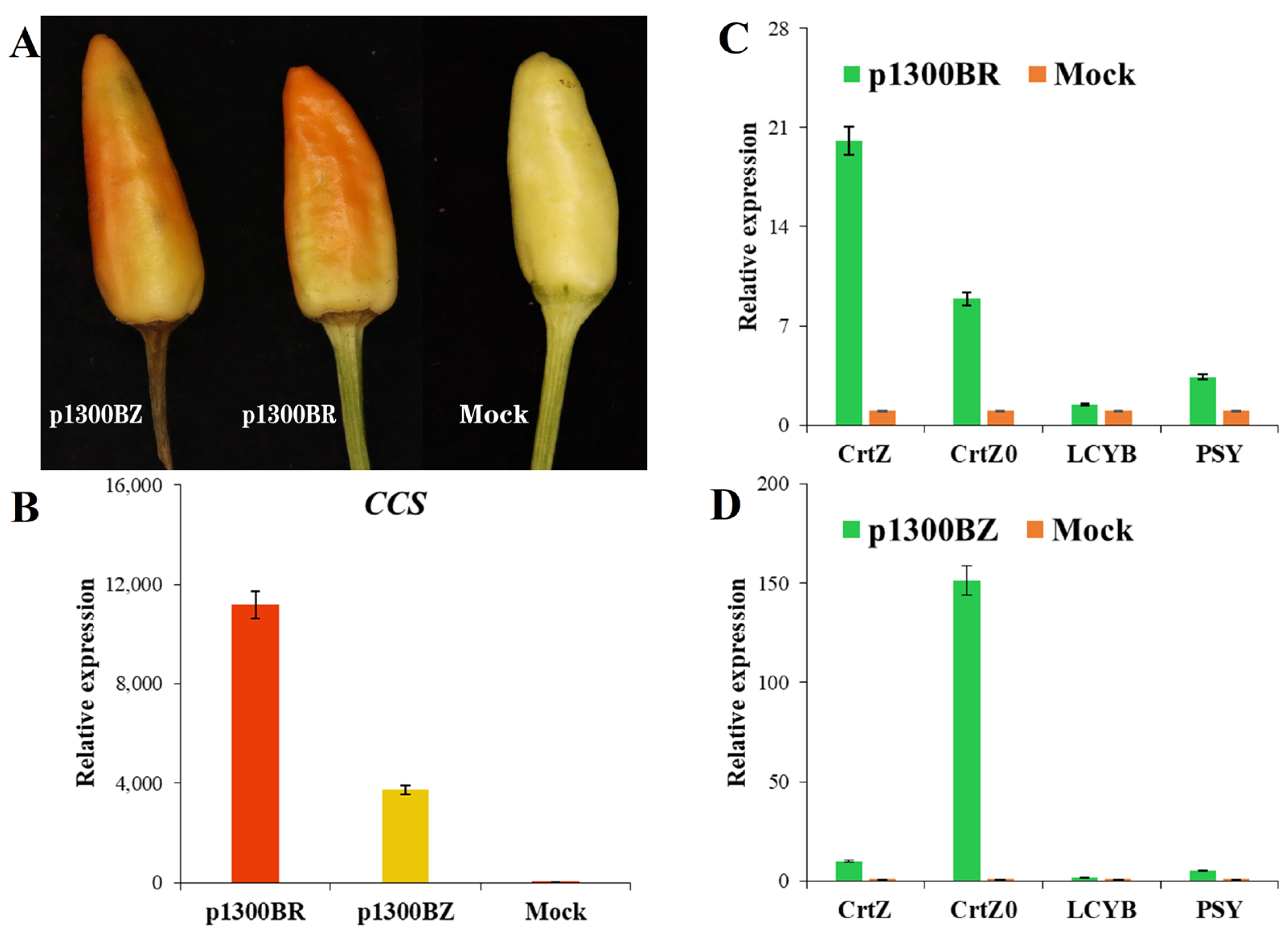
2.4. The CrtZ and CrtW Genes Speed up Color Change in Peppers
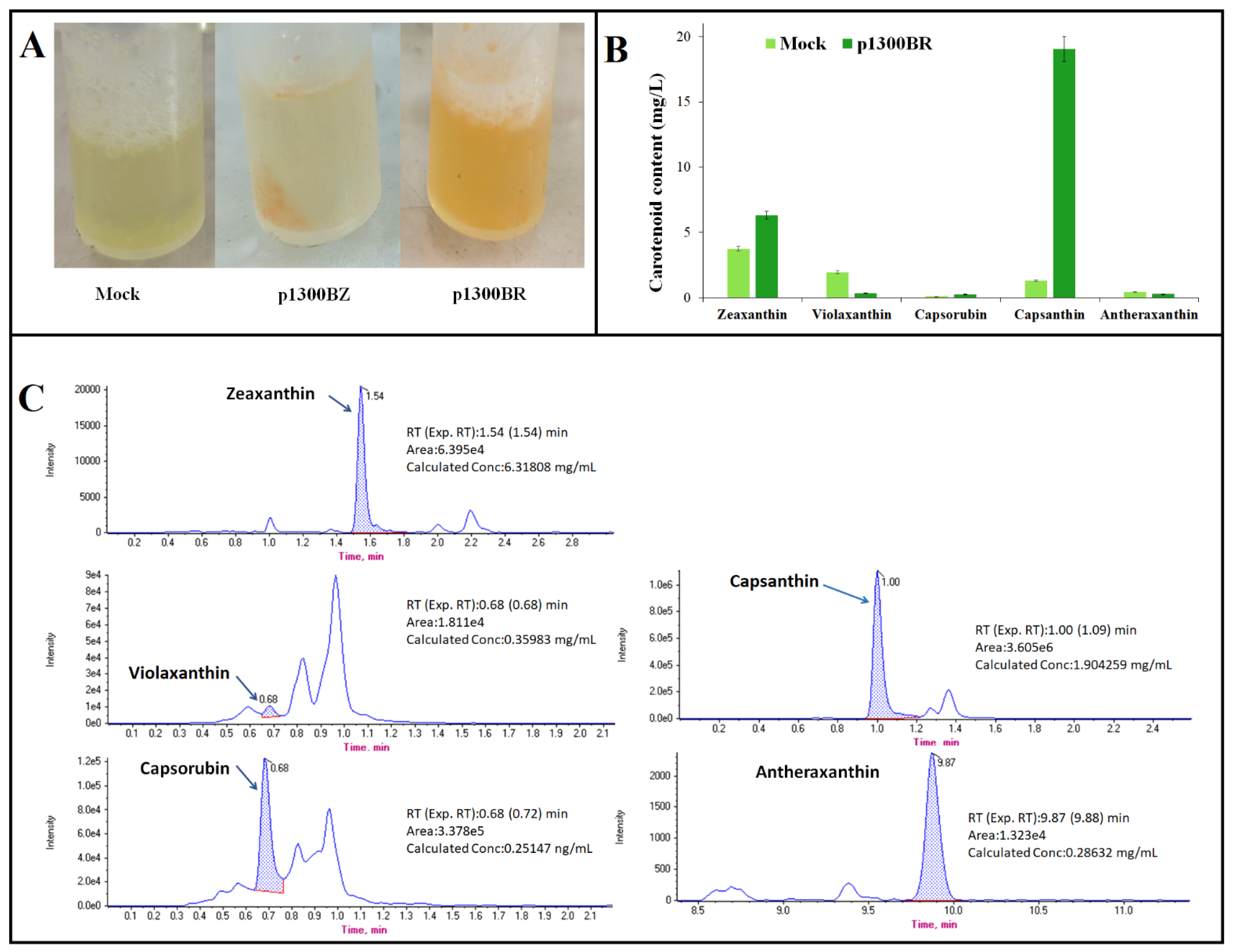
2.5. Effect of the CrtW Gene on Capsanthin Production
3. Discussion
3.1. Effect of Heterologous CrtZ and CrtW Genes on CCS in the Carotenoid Pathway
3.2. Realization of Chili Peppers as Reactors Using Geminiviruses
4. Materials and Methods
4.1. Codon Optimization and Gene Synthesis
4.2. Geminivirus Expression Vector Construction
4.3. Transient Expression on Tobacco and Pepper
4.4. Carotenoid Analysis
4.5. RNA Extraction and RT-qPCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duranova, H.; Valkova, V.; Gabriny, L. Chili Peppers (Capsicum Spp.): The Spice Not Only for Cuisine Purposes: An Update on Current Knowledge. Phytochem. Rev. 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Tian, S.-L.; Li, L.; Chai, W.-G.; Shah, S.N.M.; Gong, Z.-H. Effects of Silencing Key Genes in the Capsanthin Biosynthetic Pathway on Fruit Color of Detached Pepper Fruits. BMC Plant Biol. 2014, 14, 314. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Furubayashi, M.; Maoka, T.; Mitani, Y. Promiscuous Activity of Β-carotene Hydroxylase CrtZ on Epoxycarotenoids Leads to the Formation of Rare Carotenoids with 6-hydroxy-3-keto-ε-ends. FEBS Lett. 2022, 596, 1921–1931. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red Pepper (Capsicum Annuum) Carotenoids as a Source of Natural Food Colors: Analysis and Stability—A Review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef]
- Mohd Hassan, N.; Yusof, N.A.; Yahaya, A.F.; Mohd Rozali, N.N.; Othman, R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Kennedy, L.E.; Abraham, A.; Kulkarni, G.; Shettigar, N.; Dave, T.; Kulkarni, M. Capsanthin, a Plant-Derived Xanthophyll: A Review of Pharmacology and Delivery Strategies. AAPS PharmSciTech 2021, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Fray, R.G.; Grierson, D. Identification and Genetic Analysis of Normal and Mutant Phytoene Synthase Genes of Tomato by Sequencing, Complementation and Co-Suppression. Plant Mol. Biol. 1993, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D.; Pogson, B.J. VITAMIN SYNTHESIS IN PLANTS: Tocopherols and Carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Meng, C.; Yuan, Y.; Nath, U.K.; Zhao, Y.; Wang, Z.; Yang, S.; Li, L.; Niu, L.; Yao, Q.; et al. CaPSY1 Gene Plays Likely the Key Role in Carotenoid Metabolism of Pepper (Capsicum Annuum) at Ripening. Funct. Plant Biol. 2021, 48, 141. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, T.-J.; Zheng, H.; Zhou, C.-F.; Wang, Z.; Wang, R.; Lu, S. Manipulation of Carotenoid Metabolic Flux by Lycopene Cyclization in Ripening Red Pepper (Capsicum Annuum Var. Conoides) Fruits. J. Agric. Food Chem. 2019, 67, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Tomlekova, N.; Spasova-Apostolova, V.; Pantchev, I.; Sarsu, F. Mutation Associated with Orange Fruit Color Increases Concentrations of β-Carotene in a Sweet Pepper Variety (Capsicum Annuum L.). Foods 2021, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Otani, M.; Kitayama, K.; Ishikuro, H.; Hattan, J.; Maoka, T.; Harada, H.; Shiotani, Y.; Eguchi, A.; Nitasaka, E.; Misawa, N. Construction of Transgenic Ipomoea Obscura That Exhibits New Reddish Leaf and Flower Colors Due to Introduction of β-Carotene Ketolase and Hydroxylase Genes. Plant Biotechnol. 2021, 38, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, D.; Li, S.; Wang, J.; Bi, C.; Zhang, X. Combinatorial Modulation of Initial Codons for Improved Zeaxanthin Synthetic Pathway Efficiency in Escherichia Coli. Microbiologyopen 2019, 8, e930. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Tian, S.-L.; Li, Z.; Li, L.; Shah, S.N.M.; Gong, Z.-H. Analysis of Tandem Repeat Units of the Promoter of Capsanthin/Capsorubin Synthase (Ccs) Gene in Pepper Fruit. Physiol. Mol. Biol. Plants 2017, 23, 685–691. [Google Scholar] [CrossRef]
- Berry, H.M.; Rickett, D.V.; Baxter, C.J.; Enfissi, E.M.A.; Fraser, P.D. Carotenoid Biosynthesis and Sequestration in Red Chilli Pepper Fruit and Its Impact on Colour Intensity Traits. J. Exp. Bot. 2019, 70, 2637–2650. [Google Scholar] [CrossRef]
- Jang, S.-J.; Jeong, H.-B.; Jung, A.; Kang, M.-Y.; Kim, S.; Ha, S.-H.; Kwon, J.-K.; Kang, B.-C. Phytoene Synthase 2 Can Compensate for the Absence of PSY1 in the Control of Color in Capsicum Fruit. J. Exp. Bot. 2020, 71, 3417–3427. [Google Scholar] [CrossRef]
- Borovsky, Y.; Tadmor, Y.; Bar, E.; Meir, A.; Lewinsohn, E.; Paran, I. Induced Mutation in β-CAROTENE HYDROXYLASE Results in Accumulation of β-Carotene and Conversion of Red to Orange Color in Pepper Fruit. Theor. Appl. Genet. 2013, 126, 557–565. [Google Scholar] [CrossRef]
- Ye, R.W.; Stead, K.J.; Yao, H.; He, H. Mutational and Functional Analysis of the β-Carotene Ketolase Involved in the Production of Canthaxanthin and Astaxanthin. Appl. Environ. Microbiol. 2006, 72, 5829–5837. [Google Scholar] [CrossRef]
- Furubayashi, M.; Kubo, A.; Takemura, M.; Otani, Y.; Maoka, T.; Terada, Y.; Yaoi, K.; Ohdan, K.; Misawa, N.; Mitani, Y. Capsanthin Production in Escherichia Coli by Overexpression of Capsanthin/Capsorubin Synthase from Capsicum Annuum. J. Agric. Food Chem. 2021, 69, 5076–5085. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, C.L.; Misawa, N.; Perez-Fons, L.; Robertson, F.P.; Harada, H.; Bramley, P.M.; Fraser, P.D. The Formation and Sequestration of Nonendogenous Ketocarotenoids in Transgenic Nicotiana Glauca. Plant Physiol. 2017, 173, 1617–1635. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nishihara, M.; Nakatsuka, T.; Misawa, N.; Ogiwara, I.; Yamamura, S. Flower Color Alteration in Lotus Japonicus by Modification of the Carotenoid Biosynthetic Pathway. Plant Cell Rep. 2007, 26, 951–959. [Google Scholar] [CrossRef]
- Kildegaard, K.R.; Adiego-Pérez, B.; Doménech Belda, D.; Khangura, J.K.; Holkenbrink, C.; Borodina, I. Engineering of Yarrowia Lipolytica for Production of Astaxanthin. Synth. Syst. Biotechnol. 2017, 2, 287–294. [Google Scholar] [CrossRef]
- Ye, L.; Zhu, X.; Wu, T.; Wang, W.; Zhao, D.; Bi, C.; Zhang, X. Optimizing the Localization of Astaxanthin Enzymes for Improved Productivity. Biotechnol. Biofuels 2018, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, P.; Liu, X.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Combinatorial Expression of Different β-Carotene Hydroxylases and Ketolases in Escherichia Coli for Increased Astaxanthin Production. J. Ind. Microbiol. Biotechnol. 2019, 46, 1505–1516. [Google Scholar] [CrossRef]
- Matsufuji, H.; Ishikawa, K.; Nunomura, O.; Chino, M.; Takeda, M. Anti-Oxidant Content of Different Coloured Sweet Peppers, White, Green, Yellow, Orange and Red (Capsicum Annuum L.). Int. J. Food Sci. Technol. 2007, 42, 1482–1488. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ahn, J.; Lee, S.-J.; Moon, B.; Ha, T.-Y.; Kim, S. Phytochemicals and Antioxidant Activity of Fruits and Leaves of Paprika (Capsicum Annuum L., Var. Special) Cultivated in Korea. J. Food Sci. 2011, 76, C193–C198. [Google Scholar] [CrossRef]
- Ha, S.-H.; Kim, J.K.; Jeong, Y.S.; You, M.-K.; Lim, S.-H.; Kim, J.-K. Stepwise Pathway Engineering to the Biosynthesis of Zeaxanthin, Astaxanthin and Capsanthin in Rice Endosperm. Metab. Eng. 2019, 52, 178–189. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries; Humana Press: New York, NY, USA, 2018; pp. 57–71. [Google Scholar]
- Camara, B.; Moneger, R. Carotenoid Biosynthesis In Vitro Conversion of Antheraxanthin to Capsanthin by a Chromoplast Enriched Fraction of Capsicum Fruits. Biochem. Biophys. Res. Commun. 1981, 99, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Hugueney, P.; Bouvier, F.; Badillo, A.; D’Harlingue, A.; Kuntz, M.; Camara, B. Identification of a Plastid Protein Involved in Vesicle Fusion and/or Membrane Protein Translocation. Proc. Natl. Acad. Sci. USA 1995, 92, 5630–5634. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.H.; Keller, Y.; Bouvier, F.; Clary, D.; Camara, B. Functional Integration of Non-native Carotenoids into Chloroplasts by Viral-derived Expression of Capsanthin–Capsorubin Synthase in Nicotiana Benthamiana. Plant J. 1998, 14, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Uribe, L.; Guzman, I.; Rajapakse, W.; Richins, R.D.; O’Connell, M.A. Carotenoid Accumulation in Orange-Pigmented Capsicum Annuum Fruit, Regulated at Multiple Levels. J. Exp. Bot. 2012, 63, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.R.; Cho, M.-C.; Kim, B.-D.; Huh, J.H. A Splicing Mutation in the Gene Encoding Phytoene Synthase Causes Orange Coloration in Habanero Pepper Fruits. Mol. Cells 2010, 30, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Maoka, T.; Osawa, A.; Hattan, J.; Kanamoto, H.; Shindo, K.; Otomatsu, T.; Misawa, N. Construction of Transplastomic Lettuce (Lactuca Sativa) Dominantly Producing Astaxanthin Fatty Acid Esters and Detailed Chemical Analysis of Generated Carotenoids. Transgenic Res. 2014, 23, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Xu, J.-Y.; Li, Q.-Y.; Tang, J.-L.; Jia, S.-R.; Bi, C.-H.; Dai, Z.-B.; Zhu, X.-N.; Zhang, X.-L. Engineering CrtW and CrtZ for Improving Biosynthesis of Astaxanthin in Escherichia Coli. Chin. J. Nat. Med. 2020, 18, 666–676. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.W.; Lee, P.C. Complete Genome Sequence of Flavobacterium Kingsejongi WV39, a Type Species of the Genus Flavobacterium and a Microbial C40 Carotenoid Zeaxanthin Producer. J. Biotechnol. 2018, 266, 9–13. [Google Scholar] [CrossRef]
- Götz, T.; Sandmann, G.; Römer, S. Expression of a Bacterial Carotene Hydroxylase Gene (CrtZ) Enhances UV Tolerance in Tobacco. Plant Mol. Biol. 2002, 50, 129–142. [Google Scholar] [CrossRef]
- Makino, T.; Harada, H.; Ikenaga, H.; Matsuda, S.; Takaichi, S.; Shindo, K.; Sandmann, G.; Ogata, T.; Misawa, N. Characterization of Cyanobacterial Carotenoid Ketolase CrtW and Hydroxylase CrtR by Complementation Analysis in Escherichia Coli. Plant Cell Physiol. 2008, 49, 1867–1878. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Lu, C.-Z.; Zheng, Y.; Ma, H.-Z.; Jin, J.; Jia, B.; Yuan, Y.-J. Directed Evolution of the Fusion Enzyme for Improving Astaxanthin Biosynthesis in Saccharomyces Cerevisiae. Synth. Syst. Biotechnol. 2023, 8, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G.; Misawa, N. Carotenoid Production in Escherichia Coli: Case of Acyclic Carotenoids. Adv. Exp. Med. Biol. 2021, 1261, 201–208. [Google Scholar] [PubMed]
- Kanamoto, H.; Nakamura, K.; Misawa, N. Carotenoid Production in Oleaginous Yeasts. Adv. Exp. Med. Biol. 2021, 1261, 153–163. [Google Scholar] [PubMed]
- Houhou, F.; Martí, M.; Cordero, T.; Aragonés, V.; Sáez, C.; Cebolla-Cornejo, J.; Pérez de Castro, A.; Rodríguez-Concepción, M.; Picó, B.; Daròs, J. Carotenoid Fortification of Zucchini Fruits Using a Viral RNA Vector. Biotechnol. J. 2022, 17, e2100328. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, V. Plant Carotenoids Evolution during Cultivation, Postharvest Storage, and Food Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1561–1604. [Google Scholar] [CrossRef]
- Jain, H.; Chahal, S.; Singh, I.; Sain, S.K.; Siwach, P. The Rising Threat of Geminiviruses: Molecular Insights into the Disease Mechanism and Mitigation Strategies. Mol. Biol. Rep. 2023, 50, 3835–3848. [Google Scholar] [CrossRef]
- Rentería-Canett, I.; Xoconostle-Cázares, B.; Ruiz-Medrano, R.; Rivera-Bustamante, R.F. Geminivirus Mixed Infection on Pepper Plants: Synergistic Interaction between PHYVV and PepGMV. Virol. J. 2011, 8, 104. [Google Scholar] [CrossRef]
- Mor, T.S.; Moon, Y.-S.; Palmer, K.E.; Mason, H.S. Geminivirus Vectors for High-Level Expression of Foreign Proteins in Plant Cells. Biotechnol. Bioeng. 2003, 81, 430–437. [Google Scholar] [CrossRef]
- Tian, J.; Pei, H.; Zhang, S.; Chen, J.; Chen, W.; Yang, R.; Meng, Y.; You, J.; Gao, J.; Ma, N. TRV–GFP: A Modified Tobacco Rattle Virus Vector for Efficient and Visualizable Analysis of Gene Function. J. Exp. Bot. 2014, 65, 311–322. [Google Scholar] [CrossRef]
- Hattan, J.; Furubayashi, M.; Maoka, T.; Takemura, M.; Misawa, N. Reconstruction of the Native Biosynthetic System of Carotenoids in E. Coli—Biosynthesis of a Series of Carotenoids Specific to Paprika Fruit. ACS Synth. Biol. 2023, 12, 1072–1080. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Ali, M.M.; Yi, X.; Zhang, L.; Wang, S. Fast and High-Efficiency Synthesis of Capsanthin in Pepper by Transient Expression of Geminivirus. Int. J. Mol. Sci. 2023, 24, 15008. https://doi.org/10.3390/ijms241915008
Lin Z, Ali MM, Yi X, Zhang L, Wang S. Fast and High-Efficiency Synthesis of Capsanthin in Pepper by Transient Expression of Geminivirus. International Journal of Molecular Sciences. 2023; 24(19):15008. https://doi.org/10.3390/ijms241915008
Chicago/Turabian StyleLin, Zhimin, Muhammad Moaaz Ali, Xiaoyan Yi, Lijuan Zhang, and Shaojuan Wang. 2023. "Fast and High-Efficiency Synthesis of Capsanthin in Pepper by Transient Expression of Geminivirus" International Journal of Molecular Sciences 24, no. 19: 15008. https://doi.org/10.3390/ijms241915008
APA StyleLin, Z., Ali, M. M., Yi, X., Zhang, L., & Wang, S. (2023). Fast and High-Efficiency Synthesis of Capsanthin in Pepper by Transient Expression of Geminivirus. International Journal of Molecular Sciences, 24(19), 15008. https://doi.org/10.3390/ijms241915008






